Abstract
Objective To compare the effect of intramuscular olanzapine with intramuscular haloperidol plus promethazine on rapid tranquillisation of agitated or violent people with mental illness.
Design Pragmatic, allocation concealed, randomised controlled trial.
Setting Emergency services of a general hospital psychiatry department in Vellore, south India.
Participants 300 adults with agitated or violent behaviour as a result of mental illness; 150 randomised to intramuscular olanzapine and 150 randomised to intramuscular haloperidol plus promethazine.
Interventions Open treatment with intramuscular olanzapine or intramuscular haloperidol plus promethazine.
Main outcome measures Primary outcome was proportion of patients who were tranquil or asleep at 15 minutes and 240 minutes. Secondary outcomes were proportion of patients who were tranquil, asleep, restrained, absconding, or clinically improved at 15, 30, 60, 120, and 240 minutes; additional medical interventions and adverse effects over four hours; and compliance with oral drugs and adverse effects over two weeks.
Results Of 300 people randomised to receive either intramuscular olanzapine or intramuscular haloperidol plus promethazine, follow-up data were available for primary outcomes for 298 (99%). Both treatments resulted in similar proportions of people being tranquil or asleep at 15 minutes (olanzapine 131/150 (87%), haloperidol plus promethazine 136/150 (91%); relative risk 0.96, 95% confidence interval 0.34 to 1.47) and 240 minutes (olanzapine 144/150 (96%), haloperidol plus promethazine 145/150 (97%); relative risk 0.99, 0.95 to 1.03). However, more people given olanzapine than those given haloperidol plus promethazine required additional drugs over four hours (65/150 (43%) v 31/150 (21%); relative risk 2.07, 1.43 to 2.97). Adverse effects were uncommon with both treatments.
Conclusions Intramuscular olanzapine and intramuscular haloperidol plus promethazine were effective at rapidly tranquillising or sedating agitated or violent patients with mental illness but the combination resulted in fewer additional medical interventions within four hours of intervention.
Trial registration Clinical trials NCT00455234.
Introduction
About 15 million people in India are estimated to have serious mental disorders.1 Agitated or violent behaviour, mostly as a result of serious mental illness and substance misuse,2 3 4 constitutes around 10% of the reasons for use of emergency services worldwide. As rates of mental illness are similar worldwide5 it is reasonable to presume that the management of aggressive or violent behaviour is an important problem and a mental health priority in low and middle income countries, where most of the world's people live, and particularly in countries with large populations, such as India.
Non-pharmacological strategies are recommended to manage violence in the emergency psychiatry setting, but rapid tranquillisation with drugs to reduce agitation and prevent injury to people and damage to property may be unavoidable.6 7 8 Guidelines and clinical practice vary widely on the choice of drugs to manage violence in psychiatric emergencies6 7 8 9; some recommendations are largely consensus statements and are influenced by local practice rather than by reliable evidence.
Intramuscular haloperidol and promethazine combined is widely used for rapid tranquillisation in low and middle income countries such as Brazil and India.10 11 Promethazine is an antihistamine with sedative properties that prevents acute dystonic reactions otherwise common with the intramuscular use of haloperidol.12 Two pragmatic trials designed by the TREC Collaborative Group (tranquilização rápida-ensaio clínico [rapid tranquillisation-clinical trial]), carried out in India11 and Brazil,13 proved the efficacy and safety of this combination in 250 agitated or violent people compared with 251 people given intramuscular benzodiazepines. Guidelines from the UK's National Institute for Health and Clinical Excellence6 and a commentary14 commended the methodological quality of the trials and noted the efficacy of the combined treatment. However, NICE stopped short of recommending its routine use in the United Kingdom owing to insufficient data, particularly on safety, and the preference in the United Kingdom for calming rather than for sedation as an outcome of intervention.
Intramuscular olanzapine is one of the drugs recommended by NICE for treating violence.6 The evidence for the efficacy and safety of intramuscular olanzapine in treating acute agitation comes from randomised controlled trials in people with schizophrenia (two trials, n=316),15 16 17 mania (one trial, n=99),18 and dementia (one trial, n=137),19 compared with haloperidol,15 16 17 lorazepam,18 19 and placebo.18 19 Patients received 1-3 injections of olanzapine (2.5-10 mg/injection), haloperidol (7.5 mg/injection), lorazepam (2 mg/injection), or placebo. A variety of rating scales were used to evaluate outcome over 24 hours, and blood pressure and electrocardiograms were also monitored. In these trials olanzapine and haloperidol were superior to placebo. Olanzapine (2.5-5 mg) was superior to lorazepam at 24 hours but not at two hours. In one trial of patients with acute schizophrenia, olanzapine (10 mg) showed reduced agitation on a rating scale significantly more than haloperidol (7.5 mg) at 15, 30, and 45 minutes.16 17 Haloperidol produced more extrapyramidal side effects than olanzapine.16 17 18 19 None of the drugs showed problems with hypotension or electrocardiographic abnormalities.20
The problems in extrapolating the results from these studies to real life are that the studies were sponsored and authored by the drug industry; used rating scales to evaluate outcomes, which, although validated, are not routinely used in busy emergency settings, at least in low and middle income countries; and excluded people with comorbid alcohol or drug dependence, those with violence towards others, and those who needed restraints. Moreover, all the participants signed informed consent and some were randomised to placebo, suggesting that the participants had milder degrees of severity of agitated or violent behaviour, different from those seen in emergency situations.14 21
We did a randomised trial to compare intramuscular olanzapine with a commonly used, relatively inexpensive, and effective intervention of combined intramuscular haloperidol and promethazine. As in the earlier TREC trials,11 13 we used a pragmatic design that excluded few patients, was done in real life conditions with little interference to normal clinical practice, used outcomes chosen by the emergency team, was independent of industry sponsorship, and yet retained the ability to minimise bias by centralised randomisation, allocation concealment, and reliable blinding.22 We aimed to provide results of practical relevance for the pharmacological management of agitated and violent people in psychiatric emergency settings.
Methods
Our study was carried out in the psychiatric unit of Christian Medical College, Vellore (population 400 000), Tamil Nadu, south India. The unit has 100 beds; is situated in the suburban, residential campus of the medical college; and uses a family oriented model of care, where patients and families are admitted to residential units similar to the family environment. Wards are not closed and there are no rooms used for seclusion. Family members actively participate in the assessment and management of their relative. Around 350 outpatients are seen daily by 10-15 doctors. The department serves a catchment area that covers large parts of the country, but people with acute psychiatric emergencies are largely referred by general practitioners or acquaintances from the neighbouring towns and villages, or from emergency services of this and other hospitals. Patients are mostly brought in by family members, who stay with them in the emergency room until discharge (around 50% are discharged in four hours).11 Treatment is provided on a voluntary basis but non-consenting patients are admitted under provisions of the Mental Health Act, 1987.23
Between September 2005 and July 2006 we assessed consecutive patients for trial entry if the attending doctor thought that intramuscular sedation was clearly indicated because of agitation, aggression, or violent behaviour, and if the doctor thought that neither intervention posed an additional risk for the patient. In keeping with prevailing clinical practice in India, we obtained consent from a responsible relative if patients refused or lacked the capacity to consent to treatment. Relatives were fully informed about the voluntary nature of participation and interventions and their written consent was obtained; we excluded patients without a responsible relative.
Interventions
We randomised patients to intramuscular haloperidol (10 mg) and promethazine (25 mg or 50 mg), combined in the same syringe, or to intramuscular olanzapine (10 mg). All doses were at the discretion of the attending doctor, although the recommended dose was 10 mg haloperidol plus 50 mg promethazine, or 10 mg olanzepine. The dose of haloperidol plus promethazine was determined by prevailing clinical practice and the previous TREC trial.11 The dose of olanzapine was based on published results, which showed that 10 mg is an optimal dose for rapid control of agitation,15 and a confirmatory pilot study on 10 patients.
Randomisation and allocation concealment
Randomisation was according to a computer generated random numbers list in varying sized blocks of fewer than 10 prepared by the UK collaborator (CEA). He sent an allocation table independent of block size to a team member (JP) with no clinical responsibilities in carrying out the trial. Randomisation codes were kept from study staff until data entry and analysis had been completed. JP and a pharmacist (RN) prepared consecutively numbered, sealed, opaque cardboard boxes, identical in appearance and weight. A form attached to the outside of the box contained questions to be completed by the attending doctor while blind to the box's contents. As determined by the randomisation list the boxes contained either two ampoules of haloperidol (5 mg each) plus one ampoule of promethazine (50 mg), or one vial of olanzapine (10 mg) and distilled water for dilution (5 ml); one disposable syringe; a needle; and forms for follow-up. The contents were packed in thermacol to prevent identification of the intervention; PT confirmed this by pretesting the boxes. Staff with clinical involvement in the study had no indication of the drugs in the boxes until they were opened.
Procedures
After consent had been obtained the duty nurse opened the next consecutively numbered box, stored in the emergency drug cupboard. The duty doctor (and NSR) filled in identifying details of the patient on admission using the form supplied with the box, including the initial diagnosis according to the World Health Organization's international classification of diseases, 10th revision,24 and the severity of aggression and violence using the severity subscale of the clinical global impressions scale.25 The doctor then broke the seal (with NSR absent) to deliver the intervention. Once the seal was broken the patient was considered to be randomised. After the study intervention all subsequent interventions were carried out by, and at the discretion of, the primary treating team.
The patient was then followed up at 15 minutes by NSR; at 30, 60, and 120 minutes by the duty doctor or nurse (with telephone prompts by NSR); and at 240 minutes and two weeks by NSR. Data were also obtained from the case notes and from interviews with relatives and the treatment team.
Outcomes
We used outcomes similar to the earlier TREC trial, but we conferred with members of the department, particularly junior doctors and nurses, to confirm the clinical relevance of the outcomes. After these discussions the primary outcome was changed from “tranquil” or “asleep” four hours after the intervention (used as the primary outcome in the earlier trial) to include this assessment at 15 minutes. Participants were considered to be tranquil when they were calm and not exhibiting agitated, aggressive, or dangerous behaviour. They were considered to be asleep if, on observation, they appeared to be asleep and were not aroused by ambient disturbances; the depth of sleep was recorded as light or deep but dichotomised for data analysis into asleep or not asleep.
As this study used several raters, we assessed inter-rater reliability both before and during the trial. Training and rating sessions for raters were carried out before the start of the trial using videotaped episodes of incidents of violence obtained after the consent of relatives or patients. The ratings were carried out over two sessions: in the first session 30 people rated the severity of incidents involving four patients. In the second session 34 people rated the severity of incidents involving eight patients; 32 rated the improvement of four patients on the severity subscale of the clinical global impressions scale; and 32 rated five patients as tranquil or asleep. Over the period of training the inter-rater agreement improved to between 98% and 100% for the broad categories of “improved” or “not improved” and for “tranquil” and “asleep.”
Secondary outcomes were proportion of patients tranquil or asleep, asleep, restrained, absconding, or clinically improved on the improvement subscale of the clinical global impressions scale25 at 15, 30, 60, 120, and 240 minutes; the proportion requiring the doctor to be called back; the use of additional medical interventions; and adverse effects over four hours. Compliance with oral drugs and adverse effects were further assessed at two weeks. We rated all participants for extrapyramidal adverse effects at all assessment points on the Simpson–Angus extrapyramidal side effects rating scale26 and the Barnes akathisia scale.27 Any other clinically important adverse effect, especially dystonia, was also noted. These assessments were carried out only on participants who were awake, as extrapyramidal symptoms are usually not apparent during sleep or, in the case of dystonia or akathisia, are likely to prevent sleep. Additional measures, such as blood pressure and electrocardiograms, and investigations were undertaken as indicated by routine emergency room practice.
Blinding
The treatment team and investigators were blind to the study only until treatment. Concealment of allocation has been shown to be important in excluding selection biases.22 This was a pragmatic trial evaluating real life interventions that are not given blind and, after assignment, to help in decisions about management members of the team knew what injection was given. The first author (NSR) or, in a few instances, co-investigators undertook blind ratings at 15 minutes and 240 minutes for all patients for all outcomes. Additionally a minimum of 30% of the assessments for improvement at 30, 60, and 120 minutes were also rated blind by NSR. All participants were, however, blind to intervention type throughout the trial. The first author also guessed the allocated interventions at the end of 240 minutes to assess the reliability of blinding and remained blinded to allocation until data analysis.
Sample size
As both interventions are of proved efficacy even a small advantage for either intervention could prove beneficial in emergency settings. We estimated that 300 patients (150 in each arm) would be needed to detect a difference between groups of at least 20% 15 minutes and 240 minutes after drug administration, at a 5% level of significance and 80% probability.
Statistical analysis
We used double data entry and analysed data using SPSS version 11.0. We assessed the adequacy of randomisation by comparing baseline sociodemographic and clinical characteristics of participants. We calculated relative risks, the number needed to treat, and their 95% confidence intervals using intention to treat analysis for the proportion of patients tranquillised, asleep, improved (stipulated in the trial protocol as “much and very much improved” using the improvement subscale of the clinical global impressions scale), requiring restraints, requiring the doctor to be recalled, and requiring additional drugs for control of aggression. For intention to treat analysis we assumed any participant not available at any of the assessment points to have not improved, not to be tranquil, and not to be asleep.
We also used repeated measures analysis of variance to compare mean scores for improvement between groups across various time points, with being asleep entered as a covariate at the follow-up points. We compared mean times to tranquillisation and sedation in the two groups using the Mann-Whitney U test, as the data did not have a normal distribution. We used the κ statistic to evaluate agreement between the blinded guesses for treatment allocation and between blinded assessments of improvement undertaken by the first author with those of the duty nurses and doctors recorded between 30 and 120 minutes.
Ethical issues
Previous trials of intramuscular olanzapine15 16 17 were limited to consenting (and presumably less severely disturbed) participants. This also limited the relevance of the results to real life, where many patients who are severely disturbed lack the capacity to consent or refuse consent. NICE guidelines called for additional trials to confirm the efficacy of olanzapine in those who are more severely disturbed or violent.6 One commentator of an earlier olanzapine trial noted the difficulty in generating reliable evidence for the emergency management of violence in psychiatric settings. This was because of the perceived difficulty of carrying out randomised trials in non-consenting patients,21 but referred to the Helsinki declaration26 and other ethical guidelines to justify trials in nonconsenting patients provided no other context exists in which to answer the question and if all trial participants get clear therapeutic benefit from participation in the trial.
In this trial all participants were considered to have equal chances of benefiting from participation. Consent was obtained from responsible family members, as is usual practice in India when dealing with acute psychiatric disturbances, and family members were present with participants throughout the trial.
Results
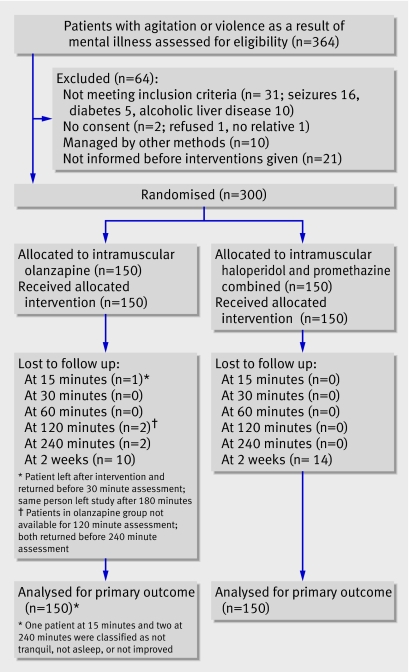
Overall, 364 eligible patients presenting with aggressive or violent behaviour as a result of mental illness were screened between 17 September 2005 and 20 July 2006. Of these, 64 were excluded (fig 1). One hundred and fifty patients were randomised to intramuscular olanzapine and 150 were randomised to intramuscular haloperidol plus promethazine.
Patient flow through trial
Follow-up was 100% for the primary outcome of proportion of patients tranquil or asleep at 15 minutes and 240 minutes for people allocated to haloperidol plus promethazine. Follow-up was 99% at 15 minutes and 99% at 240 minutes for those allocated to olanzapine. At two weeks 276 (92%) patients were followed-up.
The groups were similar for age, sex distribution, diagnosis, and severity of illness at study entry (table 1). The clinical and demographic characteristics were similar to those of patients in the earlier study of rapid tranquillisation (TREC trial).11
Table 1.
Baseline demographic and clinical details of 300 patients randomised to intramuscular olanzapine or intramuscular haloperidol plus promethazine for agitation or violence as a result of mental illness. Values are numbers (percentages) of patients unless stated otherwise
| Characteristics | Olanzapine group (n=150) | Haloperidol plus promethazine group (n=150) |
|---|---|---|
| Male | 91 (61) | 97(65) |
| Clinical diagnosis (ICD-10): | ||
| Schizophrenia | 14 (9) | 11 (7) |
| Mania | 90 (60) | 98 (65) |
| Depression | 17 (11) | 14 (9) |
| Acute psychosis | 17 (11) | 13 (9) |
| Mental retardation with psychosis | 2 (1) | 1 (1) |
| Substance induced psychosis | 8 (5) | 12 (8) |
| Current treatment: | ||
| Antipsychotics | 42 (28) | 37 (25) |
| Lithium | 9 (6) | 5 (3) |
| Anticonvulsants | 3 (2) | 6 (3) |
| Benzodiazepines | 8 (5) | 3 (2) |
| Anticholinergics | 23 (15) | 17 (11) |
| Antidepressants | 8 (5) | 3 (2) |
| Clinical global impression—severity: | ||
| Mildly ill | 8 (5) | 4 (2) |
| Moderately ill | 70 (47) | 73 (49) |
| Markedly ill | 55 (37) | 68 (45) |
| Severely ill | 16 (11) | 14 (9) |
| Among the most extremely ill | 1(0.7) | 1 (0.7) |
| Mean (SD) clinical global impression—severity | 4.55 (0.78) | 4.63 (0.72) |
| Mean (SD) age (years) | 30.6 (10.5) | 30.4 (9.5) |
ICD-10=international classification of diseases, 10th revision.
Overall, 148 of 150 people randomised to haloperidol plus promethazine received 10 mg haloperidol and 50 mg promethazine and two received 5 mg haloperiodol and 25 mg olanzapine. Two of 150 people randomised to olanzapine received 5 mg and 148 received 10 mg.
The first author correctly guessed allocation in 50% of those randomised to olanzapine and 51% of those randomised to haloperidol plus promethazine (κ=0.13).
In addition to open ratings done by the duty nurses, blinded ratings of improvement on the clinical global impressions scale were undertaken between 30 and 120 minutes for 126 patients (42%) by the first author (NSR). There was agreement on dichotomised categories of improved or not improved for 118 (94%). In the eight instances of disagreement, the differences in categories differed by 1 point on the 7 point subscale of the clinical global impressions scale for seven patients and by 2 points for one patient (estimated overall κ=0.53, after adjusting for prevalence).
Outcomes
Table 2 details the outcomes for the assessment points within the four hours after intervention and at two weeks. Intramuscular olanzapine and intramuscular haloperidol plus promethazine were equally effective for the primary outcome of causing tranquillisation or sedation at 15 minutes and 240 minutes after intervention, with 87% of patients in the olanzapine arm and 91% in the combined drug arm being either tranquil or asleep at 15 minutes; these increased to 96% and 97% at four hours. Haloperidol plus promethazine, however, sedated patients more rapidly, with 14% more patients being asleep at 15 minutes (number needed to treat (NNT) for one extra patient to sleep=8, 95% confidence interval 4 to 36). The proportion of patients asleep favoured haloperidol plus promethazine at all assessment points. At four hours 16% more people given the combined drug were asleep (NNT=7, 4 to 18). Ratings on the improvement subscale did not differ significantly 15 minutes after intervention, but significantly greater proportions of patients given haloperidol plus promethazine compared with olanzapine were rated as improved from 30 minutes to four hours after intervention.
Table 2.
Outcomes for patients randomised to intramuscular olanzapine or intramuscular haloperidol plus promethazine for agitation or violence as a result of mental illness. Values are numbers (percentages) of patients unless stated otherwise
| Outcomes | Olanzapine group (n=150) | Haloperidol plus promethazine group (n=150) | Relative risk (95% CI) | Risk difference (95% CI) |
|---|---|---|---|---|
| Tranquil or asleep after injection (minutes): | ||||
| 15* | 131 (87) | 136 (91) | 0.96 (0.34 to 1.47) | 3.3 (−3.7 to 10.4) |
| 30 | 140 (93) | 144 (96) | 0.97 (0.92 to 1.03) | 2.7 (−2.4 to 7.7) |
| 60 | 141 (94) | 149 (99) | 0.95 (0.91 to 0.99) | 5.3 (1.3 to 9.4) |
| 120 | 141 (94) | 146 (97) | 0.97 (0.92 to 1.01) | 3.3 (−1.26 to 7.9) |
| 240* | 144 (96) | 145 (97) | 0.99 (0.95 to 1.03) | 0.67 (−3.6 to 4.9) |
| Asleep after injection (minutes): | ||||
| 15* | 65 (43) | 86 (57) | 0.76 (0.60 to 0.95) | 14.0 (2.8 to 25.2) |
| 30 | 95 (63) | 114 (76) | 0.83 (0.72 to 0.97) | 12.6 (2.4 to 23.0) |
| 60 | 99 (66) | 120 (80) | 0.83 (0.72 to 0.95) | 14.0 (4.1 to 23.9) |
| 120 | 91 (61) | 120 (80) | 0.76 (0.65 to 0.88) | 19.3 (9.2 to 29.4) |
| 240* | 88 (59) | 112 (75) | 0.79 (0.67 to 0.93) | 16.0 (5.5 to 26.5) |
| Improved† after injection (minutes): | ||||
| 15 | 98 (65) | 109 (73) | 0.90 (0.77 to 1.05) | 2.8 (−7.7 to 13.3) |
| 30 | 110 (73) | 127 (85) | 0.87 (0.77 to 0.98) | 11.3 (2.2 to 20.5) |
| 60 | 120 (80) | 138 (92) | 0.87 (0.79 to 0.95) | 12.0 (4.3 to 19.7) |
| 120 | 118 (79) | 136 (91) | 0.87 (0.79 to 0.99) | 12.0 (4.0 to 20.1) |
| 240 | 131 (87) | 141 (94) | 0.91 (0.86 to 1.00) | 6.7 (0.2 to 13.2) |
| In restraints after injection (minutes): | ||||
| 15 | 45 (30) | 43 (29) | 1.05 (0.74 to 1.49) | 1.3 (−9.0 to 11.6) |
| 30 | 42 (28) | 43 (29) | 0.98 (0.68 to 1.40) | 0.7 (−9.5 to 10.9) |
| 60 | 38 (25) | 37 (25) | 1.03 (0.69 to 1.52) | 0.7 (−9.1 to 10.5) |
| 120 | 34 (23) | 27 (18) | 1.26 (0.80 to 1.89) | 4.7 (−4.4 to13.8) |
| 240 | 24 (16) | 15 (10) | 1.60 (0.88 to 2.93) | 6.0 (−1.6 to 13.6) |
| Other outcomes in 4 hours: | ||||
| Doctor called back | 49 (33) | 23 (15) | 2.13 (1.37 to 3.31) | 17.3 (7.9 to 26.8) |
| Additional drugs used | 65 (43) | 31 (21) | 2.07 (1.43 to 2.97) | 20.0 (10.0 to 30.0) |
| Adverse effects | 3 (2.0) | 1 (0.6) | 3.00 (0.32 to 28.05) | 1.3 (−1.3 to 3.9) |
| Absconded | 5 (3.4) | 0 | — | 1.0 (−0.1 to 2.7) |
| After 4 hours: | ||||
| Admitted | 47 (31) | 38 (25) | 1.24 (0.86 to 1.78) | 6.0 (−4.2 to 16.2) |
| Discharged | 65 (43) | 70 (47) | 0.93 (0.72 to 1.19) | 3.3 (−8.0 to 14.6) |
| Further observation | 36 (24) | 42 (28) | 0.93 (0.58 to 1.26) | 4.0 (−5.9 to 13.9) |
| Lost to follow-up | 2 (1) | 0 | — | 1.3 (−0.5 to 3.2) |
| At 2 weeks: | ||||
| Lost to follow-up | 10 (7) | 14 (9) | 0.71 (0.33 to 1.6) | 2.7 (−3.5 to 8.8) |
| No serious adverse effects | 149 (99) | 150 (100) | 0.99 (0.98 to 1.0) | 0.7 (−0.6 to 2.0) |
| Taking oral drugs | 138 (92) | 133 (89) | 1.04 (0.96 to 1.12) | 3.3 (−3.3 to 10.0) |
*Primary outcomes.
†Rated on clinical global impression—improvement subscale.
Seventy eight patients were restrained during the four hours after intervention. Although the differences in proportions restrained between groups were not statistically significant, it may be clinically significant that 6% more people given olanzapine were restrained in the four hours after intervention.
Olanzapine rapidly calmed patients but the effects did not last long, and 17% more patients given olanzapine compared with haloperidol plus promethazine required doctors to be recalled to assess aggression (NNT=6, 4 to 13). Additional drugs to control aggression over the four hours (intramuscular haloperidol and promethazine, oral lorazepam, other oral antipsychotics) were prescribed for 20% more people given olanzapine than those given haloperidol plus promethazine (NNT=6, 3 to 10). Haloperidol plus promethazine both calmed and sedated patients rapidly and this effect lasted throughout the four hours. The interventions did not differ in proportion of patients requiring additional drugs in the first hour (olanzapine 37 (25%), combined drug 30 (20%); relative risk 1.23, 95% confidence interval 0.81 to 1.89). Between one hour and four hours, however, 49 (33%) of those given olanzapine needed additional drugs compared with 20 (13%) given haloperidol plus promethazine (relative risk 2.45, 1.53 to 3.91; NNT=6, 4 to 10). Four people given olanzapine and one given haloperidol plus promethazine were never tranquil or asleep and were rated as clinically not improved throughout the four hours.
The mean (standard deviation) time for haloperidol plus promethazine to produce tranquillisation was less than that with olanzapine (12.8 (16.7) minutes v 20.5 (34.5) minutes); the same was true for time to sedation (26.2 (32.2) minutes v 34.9 (42.2) minutes). These differences, although of potential clinical importance, were not statistically significant (P=0.4 and P=0.2).
Scores for improvement on the clinical global impressions scale were subjected to repeated measures analysis of variance (table 3). The differences on the ratings over time and between interventions remained significant when being asleep was entered as a covariate at each assessment point.
Table 3.
Clinical global impression—improvement scores over four hours for 300 patients randomised to intramuscular olanzapine or intramuscular haloperidol plus promethazine for agitation or violence as a result of mental illness. Values are means (standard deviations)
| Assessment point (minutes) | Improvement scores* | |
|---|---|---|
| Olanzapine group (n=150) | Haloperidol plus promethazine group (n=150) | |
| 15 | 2.10 (1.028) | 1.83 (1.013) |
| 30 | 1.92 (1.096) | 1.57 (0.922) |
| 60 | 1.75 (1.018) | 1.34 (0.622) |
| 120 | 1.69 (1.037) | 1.33 (0.739) |
| 240 | 1.51 (0.841) | 1.24 (0.552) |
*Repeat measures analysis of variance: difference in clinical global impression—improvement scores over time, F=1925.15, P<0.001; difference in scores between interventions, F=19.70, P<0.001. With being asleep entered as covariate: difference in clinical global impression—improvement scores over time, F=10.9, P=0.001; difference in scores between interventions, F=4.08, P=0.044.
No patient given haloperidol plus promethazine developed dystonia in the four hours after intervention. No patient scored positive for any item on the Simpson-Angus scale for extrapyramidal side effects. Two patients in the olanzapine group developed akathisia and one patient reported nausea after four hours. One patient in the haloperidol plus promethazine group who was dehydrated as a result of psychotic excitement developed transient hypotension within 15 minutes of the injection; this had resolved by the 30 minute assessment, using intravenous saline.
No significant differences were found in the proportion of patients accepting oral drugs in either group at two weeks' follow-up. No patient in the haloperidol plus promethazine arm experienced any serious adverse events during the two weeks of follow-up. One patient in the olanzapine arm who had been prescribed clozapine subsequently developed intestinal obstruction, which resolved with conservative management.
Discussion
Intramuscular olanzapine and intramuscular haloperidol and promethazine combined are effective in the rapid tranquillisation of agitated or violent people as a result of mental illness. Important differences were, however, found in the effects of the two interventions. Olanzapine produced a calming effect within an hour of administration, but this seemed to wear off and additional medical attention and interventions were required, particularly after an hour. Haloperidol plus promethazine had a rapid calming effect but also put most patients to sleep rapidly, and this was maintained better over four hours than with olanzapine, reducing the need for additional medical involvement and intervention. The addition of promethazine facilitated sedation and prevented significant extrapyramidal adverse effects, particularly dystonia, otherwise seen when haloperidol is used alone for rapid tranquillisation.15 16 17 18 Although serious adverse effects were not seen with either intervention, this trial was not adequately powered to confirm the safety of either intervention.
Sleep is often not considered a desirable end point of rapid tranquillisation,6 and calming patients without producing sleep is considered the ideal. In an analysis of the industry sponsored trials of olanzapine,27 where 1-3 doses of the drug were used for tranquillisation, olanzapine was considered to produce a calming effect distinct from sedation. We did not find that olanzapine reliably produces this effect, as over 60% of patients given olanzapine were asleep during the hour after intervention. In addition, in settings where medical resources are scant or clinics are busy, the longer duration of action of haloperidol plus promethazine exposes people to dangerous behaviour for shorter periods, provides fewer occasions for doctors to be recalled, and results in less need for additional drugs over the first few hours. Thus haloperidol plus promethazine may be favoured over olanzapine.
Haloperidol and promethazine are both on the World Health Organization's list of essential drugs.28 The combined drug costs nearly a third less than injectable olanzapine in India. If the costs of the additional drugs used and the utilisation of additional resources were totalled, the difference is likely to favour haloperidol plus promethazine.
In India, as in Brazil,13 it is common to place violent people in physical restraints in addition to using drugs. The interventions did not result in statistically significant differences in proportion of patients restrained, although fewer people given haloperidol plus promethazine were restrained at all assessment points during the four hours after intervention.
Strengths and limitations of the study
The major strengths of this trial are the use of central random allocation to minimise selection bias, adequate measures to preserve allocation concealment, adequacy of blinding for the principal outcomes, sufficient power to detect significant differences in the main outcomes owing to an adequate sample size, multiple measures of outcome, and the pragmatic nature of the design that mimicked routine clinical practice. These strengths should provide sufficient internal and external validity to the results.
We did not use a placebo arm as the control because we thought it unethical when intervention with either drug has been shown to have therapeutic advantage and when using a placebo could have resulted in harm to the patient or to others.
Because concurrent drug use was uncontrolled, the use of additional drugs early in the trial may have influenced ratings. As more people randomised to olanzapine received additional drugs, however, subtracting the effects of this from the outcomes would only increase the superiority of haloperidol plus promethazine.
Assessments between 30 minutes and 120 minutes were done by people not blind to treatment allocation, although allocation itself was randomised and concealed. The primary outcomes were, however, rated blind to treatment allocation, the reliability of blinding was satisfactory, and outcomes were assessed in different ways with satisfactory inter-rater reliability pre-established. Moreover, outcomes such as being asleep are sufficiently objective that observer bias was likely to have been minimal.
We intentionally avoided using specific aggression scales as these are cumbersome and time consuming, are not used routinely in clinical care, and provide continuous measures of outcome that are difficult to interpret clinically. The outcomes used in the study are the ones most likely to be used in routine clinical practice and were chosen after discussion with emergency room doctors as being the most appropriate to guide clinical practice.
The study was powered only for primary outcomes, for which the interventions did not differ, and we used several secondary outcomes, some of which favoured haloperidol plus promethazine over olanzapine; these must be interpreted with caution.
The combined data from the two TREC trials at Vellore and the one in Brazil provide data on efficacy for 400 people randomised to intramuscular haloperidol and promethazine combined. Although the three trials were not individually powered to detect differences in adverse events with interventions, data now exist for 400 people given this combination. No patient developed dystonia or other extrapyramidal adverse effects with the combined drug. One patient in the present trial with pre-existing dehydration as a result of psychosis developed transient hypotension, which resolved uneventfully. One patient in an earlier TREC trial in Rio de Janeiro13 developed a seizure, a potential danger with antipsychotics. A fourth TREC trial in Rio de Janeiro29 was prematurely stopped (316 patients randomised to haloperidol and promethazine combined or haloperidol alone) owing to the development of dystonia in those given haloperidol alone.
Conclusions
Olanzapine and haloperidol plus promethazine were effective in controlling aggressive or violent behaviour as a result of mental illness by producing rapid tranquillisation and sedation. Patients given haloperidol plus promethazine required less medical attention or additional drugs in the four hours after intervention than those given olanzapine. In busy and chaotic emergency situations this advantage may mean that haloperidol plus promethazine is preferable.
What is already known on this topic
Guidelines for rapid tranquillisation of aggressive people with mental illness are often influenced by local practice, have limited evidence, and vary for recommended drugs
Haloperidol plus promethazine was shown to be effective in pragmatic trials carried out in Brazil and India
What this study adds
Around 90% of patients given olanzapine or haloperidol plus promethazine were tranquillised or sedated within 15 minutes and 96% by four hours
Additional interventions were used within four hours for 43% of those given olanzapine and 21% of those given haloperidol plus promethazine
Neither intervention produced serious adverse effects but the trial was not designed to evaluate the safety of these interventions
We thank the doctors and nurses who helped assess the clinical outcomes during the trial and the staff of the department of psychiatry at the Christian Medical College. Members of the Rapid Tranquillisation-India II Collaborative Group were Joncy Philip, Ravi Nesan, Jaishankar, Stalin Selvaraj, Rajesh Gopalakrishnan, Naveen Thomas, Titus Samson Premkumar, Susan Jeyabalan, Thangadurai P, Basanth Kumar, Anto Praveen Rajkumar, Satyaraj, Helen Charles, Sheeba Chandy, Devakumari K P, Karunakaran K P, P Raja R S K, Kasthuri Narasimhan, Parameswari R, Beulah Sam, Bhavani, Geetha R, Ida Priscilla.
Contributors: PT conceived the idea (based on the methods of previous trials on rapid tranquillisation designed by Giselle Huf and Evandro SF Coutinho from Brazil and CEA from the United Kingdom), secured funding, wrote the protocol, supervised the preparation and conduct of the trial, analysed data, and wrote the paper. NSR helped write the protocol, carried out the inter-rater reliability exercises, organised study materials, collected data for primary outcomes, collated the data, helped analyse data, and helped write the paper. JA contributed to the protocol, helped in preparations for the trial, and approved the final manuscript. CEA helped design the rapid tranquillisation trials, randomised allocation, and contributed to writing the paper. JP and RN prepared the study interventions according to the randomised allocation schedules. The other members of the TREC-India II Collaborative Group participated in finalising the protocol, administering interventions, recording data, and approving the manuscript. PT is the guarantor.
Funding: This trial was funded by an intramural grant from the fluid research fund (22F484) of the Christian Medical College, Vellore. The study drugs were purchased from commercial sources, were supplied by the hospital pharmacy services, and were provided free of cost to participants.
Competing interests: None declared.
Ethical approval: This study was approved by the institutional review board of the Christian Medical College, Vellore.
References
- 1.Reddy MV, Chandrasekhar CR. Prevalence of mental and behavioural disorders in India: a metaanalysis. Ind J Psychiatry 1998;40:149-57. [PMC free article] [PubMed] [Google Scholar]
- 2.McAllister-Williams RH, Ferrier IN. Rapid tranquillisation: time for a reappraisal of options for parenteral therapy. Br J Psychiatry 2002;180:485-9. [DOI] [PubMed] [Google Scholar]
- 3.Tardiff K, Koenigsberg HW. Assaultive behavior among psychiatric outpatients. Am J Psychiatry 1985;142:960-3. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan HI, Sadock BJ, Grebb JA. Kaplan and Sadock's synopsis of psychiatry Baltimore, MD: Williams & Wilkins, 1994
- 5.Jablensky A, Sartorius N, Ernberg G, Anker M, Korten A, Cooper J, et al. Schizophrenia: manifestations, incidence and course in different cultures. A world health organization ten-country study. Psychol Med Monogr Suppl 1992;20:1-97. [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Health and Clinical Excellence (NICE). Violence: clinical practice guidelines. The short-term management of disturbed/violent behaviour in in-patient psychiatric settings and emergency departments London: Royal College of Nursing, 2005
- 7.Hillard JR. Emergency treatment of acute psychosis. J Clin Psychiatry 1998;59(suppl 1):57-61. [PubMed] [Google Scholar]
- 8.Expert Consensus Guideline Group. Treatment of schizophrenia. The expert consensus guideline series. J Clin Psychiatry 1999;60(suppl 11):3-80. [PubMed] [Google Scholar]
- 9.Binder RL, McNiel DE. Emergency psychiatry: contemporary practices in managing acutely violent patients in 20 psychiatric emergency rooms. Psychiatr Serv 1999;50:1553-4. [DOI] [PubMed] [Google Scholar]
- 10.Huf G, da Silva Freire Coutinho E, Fagundes HM Jr, Oliveira ES, Lopez JR, Gewandszajder M, et al. Current practices in managing acutely disturbed patients at three hospitals in Rio de Janeiro-Brazil: a prevalence study. BMC Psychiatry 2002;2:4 www.biomedcentral.com/1471-244X/2/4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander J, Tharyan P, Adams CE, John T, Mol C, Philip J. Rapid tranquilisation of violent or agitated patients in a psychiatric emergency setting: a pragmatic randomised trial of intramuscular lorazepam versus haloperidol plus promethazine. Br J Psychiatry 2004;185:63-9. [DOI] [PubMed] [Google Scholar]
- 12.Van Harten PN, Hoel HW, Kahn RS. Acute dystonia induced by drug treatment. BMJ 1999;319:623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.TREC Collaborative Group. Rapid tranquillisation for agitated patients in emergency psychiatric rooms: a randomised trial of midazolam versus haloperidol plus promethazine. BMJ 2003;327:708-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAllister-Williams RH. Intramuscular haloperidol-promethazine sedates violent or agitated patients more quickly than intramuscular lorazepam. Evid Based Ment Health 2005;8:7. [DOI] [PubMed] [Google Scholar]
- 15.Brier A, Meehan K, Birkett M, David S, Ferchland I, Sutton V, et al. A double-blind, placebo-controlled dose response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Arch Gen Psychiatry 2002;59:441-8. [DOI] [PubMed] [Google Scholar]
- 16.Wright P, Birkett M, David SR, Meehan K, Ferchland I, Alaka KJ, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatry 2001;158:1149-51. [DOI] [PubMed] [Google Scholar]
- 17.Wright P, Lindborg SR, Birkett M, Meehan K, Jones B, Alaka K, et al. Intramuscular olanzapine and intramuscular haloperidol in acute schizophrenia: antipsychotic efficacy and extrapyramidal safety during the first 24 hours of treatment. Can J Psychiatry 2003;48:716-21. [DOI] [PubMed] [Google Scholar]
- 18.Meehan K, Zhang F, David S, Tohen M, Janicak P, Small J, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol 2001;21:389-97. [DOI] [PubMed] [Google Scholar]
- 19.Meehan KM, Wang H, David SR, Nisivoccia JR, Jones B, Beasley CM Jr, et al. Comparison of rapidly acting intramuscular olanzapine, lorazepam, and placebo: a double-blind, randomized study in acutely agitated patients with dementia. Neuropsychopharmacology 2002;26:494-504. [DOI] [PubMed] [Google Scholar]
- 20.David SR, Beasley CM Jr, Alaka K. Analysis of the QTC interval in acutely agitated patients with schizophrenia, bipolar mania, or dementia treated with intramuscular (IM) olanzapine vs. IM placebo or IM haloperidol. Eur Neuropsychopharmacol 2001;11:276 [Google Scholar]
- 21.Kerwin R. Olanzapine was more effective than lorazepam at 2 hours but not at 24 hours in bipolar mania with acute agitation. Evid Based Ment Health 2002;5:18. [DOI] [PubMed] [Google Scholar]
- 22.Juni P, Altman DG, Egger M. Assessing the quality of controlled clinical trials. BMJ 2001;323:42-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ministry of Law and Justice. The Mental Health Act, 1987 Gazette of India: Extraordinary. New Delhi: Ministry of Law and Justice (Legislative Department), 1987. http://nhrc.nic.in/Publications/Disability/annexure3.html
- 24.World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines Geneva: WHO, 1992
- 25.Guy W. ECDEU assessment manual for psychopharmacology, revised edn. Rockville, MD: National Institute of Mental Health, 1976
- 26.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. 2002. (accessed 24 Jan 2007).www.wma.net/e/policy/b3.htm [DOI] [PubMed]
- 27.Battaglia J, David SR, Alaka K, Meehan K, Wright P. Calming versus sedative effects of IM olanzapine in agitated patients. Schiz Res 2002;53(suppl 1):183. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. The WHO model list of essential drugs (EDL 1999). (accessed 3 Jan 2005).www.who.int/medicines/organization/par/edl/infedlmain.shtml
- 29.Huf G, Coutinho ESF, Adams CE, TREC Collaborative Group. Rapid tranquillisation in psychiatric emergency settings in Brazil: pragmatic randomised controlled trial of intramuscular haloperidol versus intramuscular haloperidol plus promethazine. BMJ 2007. doi: 10.1136/bmj.39339.448819.AE [DOI] [PMC free article] [PubMed] [Google Scholar]