Abstract
Background
The immune response to melanoma is rarely curative suggesting the emergence of immunosuppression. FOXP3-expressing regulatory T cells (Treg cells) function to suppress immune responses. The objective of this study was to determine if melanoma evades immune surveillance, in part, by inducing Treg cells.
Material and methods
Peripheral blood mononuclear cells (PBMCs) were isolated and exposed to melanoma-conditioned media (MCM) or control media for one week. The induction of Treg cells in these PBMCs was determined by measuring the proportion of CD25+FOXP3+ T cells in all CD4+ T cells by flow cytometry. FOXP3 expression was determined by mean fluorescence intensity (MFI) and Western blot. Supernatant cytokines were determined by ELISA.
Results
Normal PBMCs exposed to MCM revealed higher proportions of Treg cells than those exposed to control media after six days (3.4% v. 1.3%, respectively, P < 0.02). The expression of FOXP3 in Treg cells from PBMCs exposed to MCM increased over time by MFI and Western blot but was not significantly different than those exposed to control media. The level of IL-10 and TGF-β in supernatants after six days growth was higher in MCM than control media but this did not reach statistical significance.
Conclusion
Exposure of PBMCs to melanoma results in induction of FOXP3+ Treg cells.
Keywords: melanoma, regulatory T cells, FOXP3, cytokines
Introduction
Melanoma possesses the ability to elicit a profound immune response, as demonstrated by spontaneous regression of primary and metastatic disease [1–3]. The critical components of this immune response have been extensively investigated and exploited for immunotherapy in the form if IL-2, INF-α, tumor vaccines and adoptive cell transfer [4–8]. While some patients experience tremendous regression of their disease with these treatments, most immune therapies ultimately fail suggesting the development of a counter-regulatory immune suppressive mechanism.
Regulatory T cells (Treg cells) are an immunosuppressive population of T cells expressing high levels of CD25 (IL-2Rα), constituting 2–3% of the total CD4+ T cell population in the CD25 high group [9]. Treg cells provide peripheral immune tolerance in normal individuals and elimination of Treg cells results in severe autoimmunity [10]. Although the precise mechanism of Treg cell-induced immune suppression is not understood, Treg cells act in part by secreting immunosuppressive cytokines and suppressing the activation and proliferation of T cells in both cell contact-dependant and contact-independent mechanisms [11]. Increased frequencies of peripheral Treg cells have been reported in a variety of malignancies including lung, breast, pancreatic, gastric, esophageal, colorectal, gallbladder, ovarian and cervical cancers and they may partially suppress the antitumor immune response in these tumors [12–16].
FOXP3 is a transcription factor in the forkhead/winged-helix family of transcriptional regulators and is predominantly found in CD4+CD25+ Treg cells. Mutations in FOXP3 in humans lead to severe autoimmunity in the form of IPEX (immune dysfunction, polyendocrinopathy, enteropathy, X-linked), a lethal multisystem autoimmune disease characterized by a variety of mutations along the full length of the FOXP3 gene resulting in elimination of Treg cells [17]. FOXP3 expression is both necessary and sufficient for the development and function of Treg cells, making it one of the most Treg cell-specific markers [18, 19].
Given our understanding of the immunosuppression associated with melanoma and the growing understanding of the activity of Treg cells, we hypothesized that melanoma directly induces Treg cells, as defined by the CD4+CD25+FOXP3+ phenotype. We sought to determine if melanoma could induce Treg formation in normal peripheral blood mononuclear cells (PBMCs) in an in vitro system. To do this, we exposed normal PBMCs to melanoma-conditioned media. We also investigated possible mechanisms of Treg induction and activity, including FOXP3 expression and immunosuppressive cytokine secretion.
Materials & Methods
Cells and Culture Conditions
Peripheral blood was collected from healthy human volunteers without a known history of melanoma, and the PBMC fraction was isolated using a Ficoll-Paque density gradient (Amersham Biosciences AB, Uppsala, Sweden) following an institutional review board-approved protocol. After three washes with Dulbecco phosphate-buffered saline (DPBS) the cells were grown in various media. Control media consisted of Roswell Park Memorial Institute (RPMI) 1640 media supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine and 10% human serum. Melanoma-conditioned media (MCM) was generated by bringing a metastatic melanoma cell line (A375; American Type Culture Collection, Manassas, VA) to 80–90% confluence in control media, removing the media and washing the cells with DPBS, adding fresh control media, and collecting the conditioned media 24 hours later. This media was then centrifuged at 350g, passed through a 0.22μm filter and stored at −80°C until used. Additional melanoma-conditioned media was created using other melanoma cell lines. For use in experiments, the MCM was diluted to 20% in control media to ensure the presence of adequate levels of growth factors and micronutrients. Isolated PBMCs were incubated in a 24 well plate (Costar, Corning, NY) at 2 × 106 cells per well in control media or MCM at 37°C in 5% CO2.
Cell Staining and Flow Cytometry
After incubation in media for up to one week, cells were harvested, washed with DBPS and stained with murine allophycocyanin (APC)-labeled anti-human CD3, tricolor (TC)-labeled anti-human CD4 (Invitrogen, Carlsbad, CA) and fluorescein isothiocyanate (FITC)-labeled anti-human CD25 (Beckman Coulter, Inc., Fullerton, CA) antibodies by incubating them for 15 minutes at room temperature. The cells were then washed and fixed using Fixation/Permeabilization solution purchased from eBioscience (San Diego, CA) according to the manufacturer’s instructions. The cells were then incubated for 15 minutes at 4°C with normal rat serum and permeabilization buffer (eBioscience) to prevent nonspecific binding with Fc receptors before incubation with rat phycoerythrin (PE)-labeled anti-human FOXP3 antibody (eBioscience) for 30 minutes at 4°C. Cells were then washed and resuspended in 1% paraformaldeyde (PFA) in DPBS and stored at 4°C in the dark until flow cytometric analysis was performed. Two hundred thousand events were collected by flow cytometry on a FACSCaliber flow cytometer (Beckton, Dickinson and Company, Franklin Lakes, NJ) with gating based on forward versus side scatter for live lymphocytes, then on CD3+/CD4+ for CD4+ T cells, then on CD25+/FOXP3+ for Treg cells in which the mean fluorescent intensity (MFI) of FL2 (FOXP3-PE channel) was determined. The gating and calculation of the proportion of Treg cells and MFI of FOXP3 was performed using FlowJo software (Tree Star, Inc., Ashland, OR).
Western Blot Analysis
Total cell lysates were extracted from cultures of PBMCs grown for various days in control media versus MCM. Lysates were solubilized in tris-buffered saline Tween (TBST) and boiled at 100°C for 5 minutes before undergoing SDS-polyacrylamide gel electrophoresis (PAGE) with equal volumes of cell lysate per lane. Proteins were then transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA) which was blocked with 5% nonfat dry milk in TBST overnight at 4°C. Immunostaining was performed with 1μg/ml rabbit anti-human FOXP3 antibody for 1 hour at room temperature and with 1μg/ml goat anti-rabbit horseradish peroxidase conjugated secondary antibody before development with the appropriate detection kit according to the manufacturer’s instructions (Abcam, Inc., Cambridge, MA). Membranes were also probed for GAPDH as additional loading control.
In Vitro Cytokine Analysis
After six days in culture, levels of the supernatant cytokines TGF-β, IL-2, IL-4 and IL-10 were assayed by ELISA (ELISATech, Denver, CO).
Statistics
The student’s t-test was used to compare the percentage of Treg cells and the MFI of FOXP3 in control media versus MCM. Observations on cytokine data between the two groups was also compared by the student’s t-test. JMP software (Cary, NC) was used for all calculations, with a P < 0.05 deemed statistically significant.
Results
Increased Treg Cells in Melanoma-Conditioned Media versus Control Media
Normal PBMCs cultured in MCM demonstrated an increased proportion of Treg cells over time compared to control media. Fig. 1 depicts the gating strategy used to identify CD4+CD25+FOXP3+ Treg cells. The percentage of CD25+FOXP3+ Treg cells of all CD4+ T cells increased in MCM and went down in control media over time (Fig. 2A, representative time-course experiment), while the MFI of FOXP3 in Treg cells increased after day two in both conditions over time. After six days, the proportion of Treg cells was significantly higher in PBMCs cultured in MCM versus control (3.4% v. 1.3%, respectively, *P < 0.02; Fig. 2B). The expression of FOXP3 in Treg cells, as measured by the MFI in the Treg gate, was not significantly different in PBMCs grown in MCM versus control media after six days (468 units v. 344 units, respectively, P = 0.29). The induction of FOXP3 by MCM on normal PBMCs over time was confirmed by Western blot (Fig. 3).
FIG. 1.
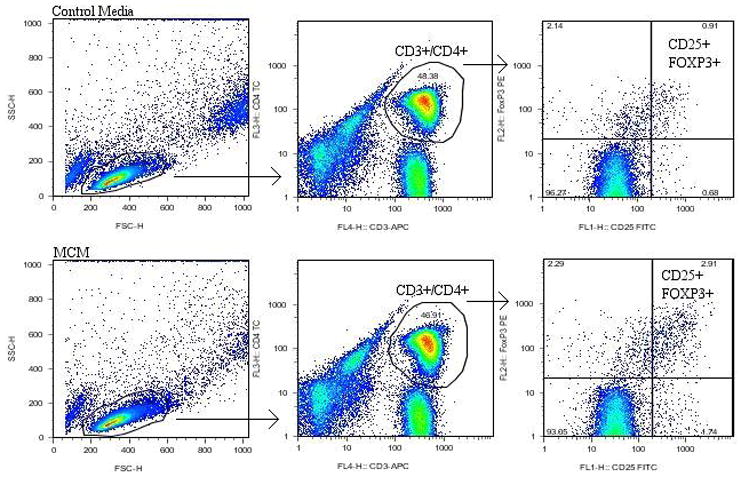
Gating strategy used for CD4+CD25+FOXP3+ Treg cells. Representative examples of flow cytometry plots and gating strategies of Treg cells from PBMCs grown in control media and MCM. Treg cells were those in the CD25+/FOXP3+ double-positive quadrant after gating for live lymphocytes and CD3+/CD4+ T cells, respectively. The quadrant labels showed the proportion of single or double-positive cells.
FIG. 2.
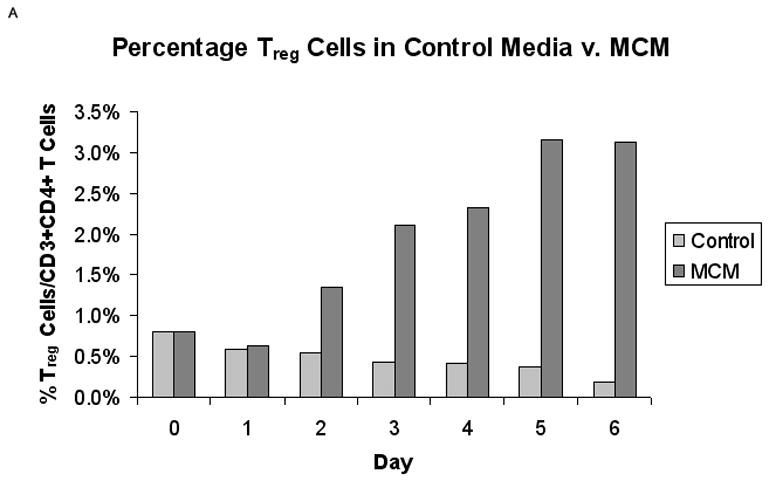
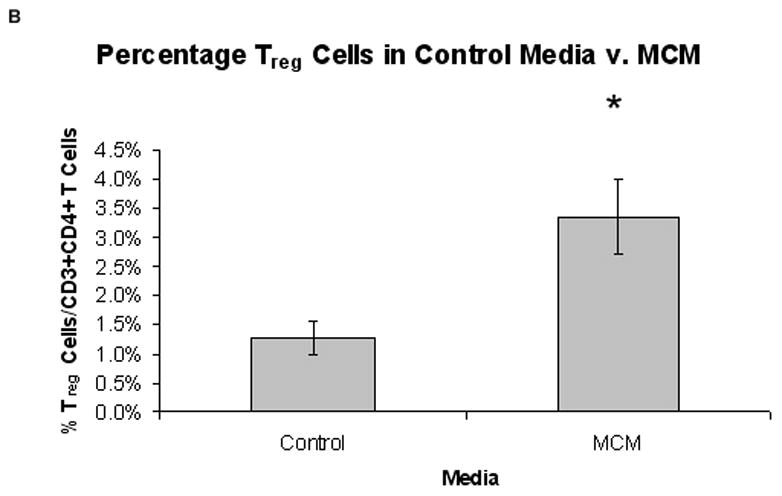
Proportion of Treg cells from normal PBMCs in MCM versus control media (n = 5). (A) Representative graph of the percentage of CD25+/FOXP3+ Treg cells in control media versus MCM over time. (B) There was a significantly higher percentage of Treg cells in MCM versus control after six days (3.4% v. 1.3%, respectively, *P < 0.02). Graph represents mean values ± SEM of the percentage of CD25+FOXP3+ Treg cells in CD3+CD4+ T cells from PBMCs grown in control media versus MCM after six days.
FIG. 3.
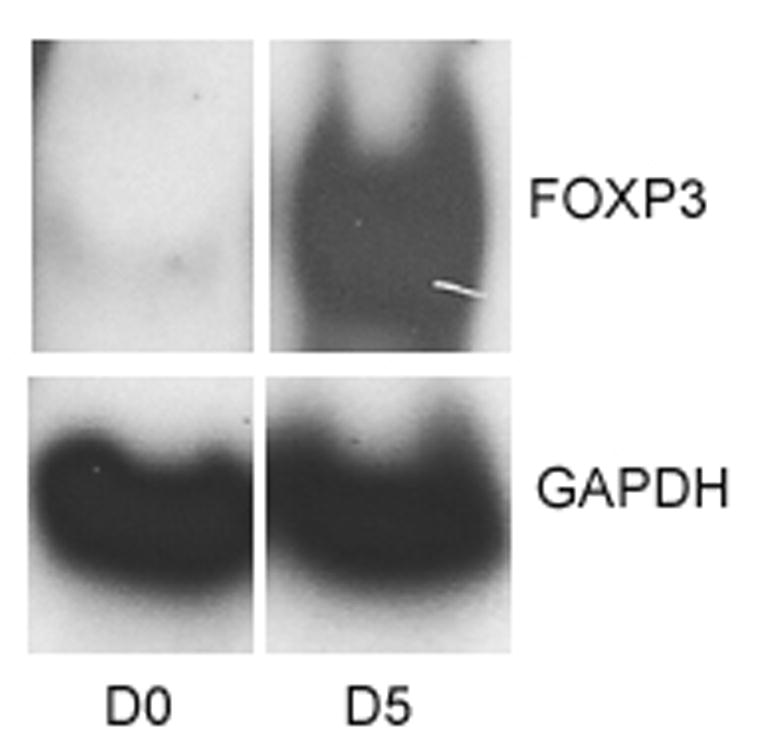
MCM increases expression of FOXP3 with time. Typical Western blot analysis showed increasing amounts of FOXP3 expression in PBMCs over time cultured in MCM. Lanes were loaded with equal volume of cell lysate and probed for GAPDH as loading controls.
Cytokine Production
Supernatant cytokine production from normal PBMCs grown in MCM or control media is shown in Fig. 4. Although there appears to be more TGF-β and IL-10 in the MCM group compared to control media, this did not reach statistical significance (3410 pg/ml v. 2862 pg/ml, respectively for TGF-β, P = 0.70; 114 pg/ml v. 62 pg/ml, respectively for IL-10, P = 0.37; Fig. 4). Levels of IL-2 and IL-4 were undetectable in all supernatants tested.
FIG. 4.
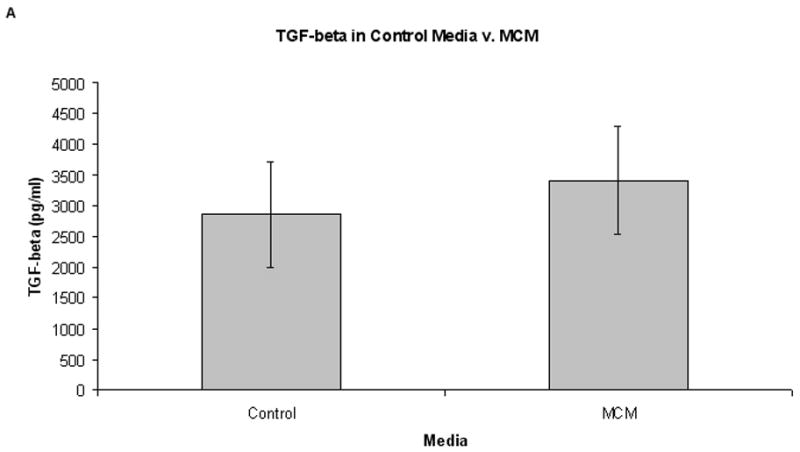
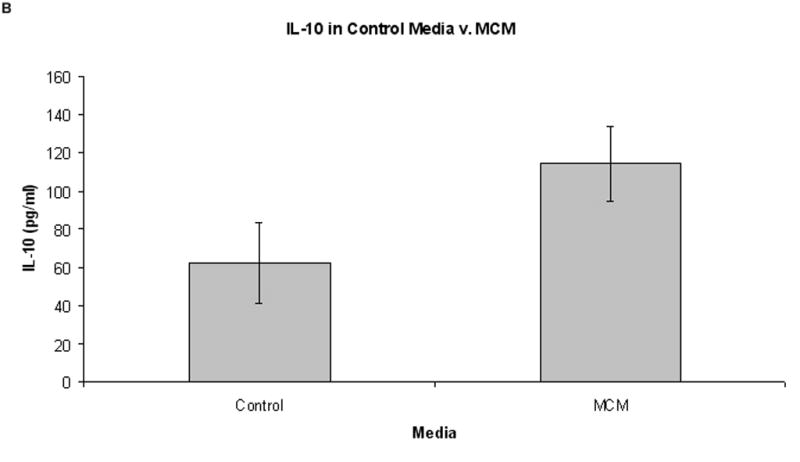
Cytokine levels in control media versus MCM after six days growth (n = 6). Graphs represent mean values ± SEM of levels of TGF-β and IL-10 as determined by ELISA. (A) There was an increased level of TGF-β in supernatants from PBMCs grown in MCM versus control media after six days (3410 pg/ml and 2862 pg/ml, respectively, P = 0.70), but this did not reach statistical significance. (B) Concurrently, there was no significant difference in the level of IL-10 in MCM v. control media after six days growth (114 pg/ml and 49 pg/ml, respectively, P = 0.37).
Discussion
In this study, we have demonstrated that melanoma induces immunosuppressive FOXP3+ regulatory T cells. Specifically, this study shows the ability of melanoma-conditioned media to result in a higher proportion of Treg cells compared to control media. The method of induction remains unclear and may involve stimulation or activation of Treg cells already present, induction of de novo Treg cells or expansion of the existing population of Treg cells. FOXP3 expression as determined by Western blot and MFI also seemed to increase within Treg cells over time in MCM, though the difference in MFI from PBMCs grown in control media did not reach statistical significance.
The method by which MCM induces Treg cells remains to be elucidated. This induction may involve interactions with antigen-presenting cells or with other T cells. In addition, other investigators have identified soluble factors in a variety of malignancies implicated in inducing Treg cells, including TGF-β, CCL22 and H-ferritin [20–22]. Furthermore, the induction mechanism within Treg cells is poorly understood, but may involve a FOXP3-dependant pathway. One proposed activity of FOXP3 in Treg induction is as a transcriptional repressor of the IL-2 gene through interactions with nuclear factor of activated T cells (NFAT) [23]. Additional questions regarding the development and upregulation of Treg cells remain to be answered. Specifically, does exposure to melanoma pre-condition existing Treg cells for later induction or influence naïve lymphocytes to become Treg cells after additional tumor exposure? We surmise that further exposure of PBMCs from melanoma patients to melanoma either in vitro with conditioned media or in vivo with advanced disease would result in enhanced induction of Treg cells. Further investigation of MCM may reveal induction mechanisms.
We have also shown a trend of immunosuppressive cytokines secreted by PBMCs in response to melanoma exposure. The precise origin of these cytokines in culture is unclear, but Treg cells are a potential source as they have been shown to secrete IL-10 and TGF-β in tumor models [24]. Thus, either directly from Treg cells or from other PBMCs affected by Treg cells, these results reveal a potential mechanism of Treg-induced immunosuppression. Other possible mechanisms include cell-contact dependant interactions or antigen specific responses as found in other model systems [25]. Future inquiries will investigate more precisely the cytokine expression profile of melanoma-induced Treg cells and their suppressive activity on effector cells.
Although we found that media conditioned by melanoma resulted in an increased percentage of Treg cells in normal PBMCs over control media, Treg cells are induced by other conditions as well. In addition to induction by cancer cells, other in vitro studies with benign cells have shown the capacity to induce Treg cells [26]. In addition, they have been found to be induced by inflammation resulting from a large variety of disease states [27]. Thus, given the role of Treg cells to confer natural tolerance which occurs to prevent autoimmunity, Treg cells mitigate the immune response in many disease states, including malignancy.
Other investigators have demonstrated an increased proportion of Treg cells in lymph nodes with metastatic melanoma [28]. Furthermore, the failure of an objective clinical response in patients undergoing immune therapies has been mechanistically linked with elevated percentages of Treg cells in these patients [29, 30]. Although the percentage of Treg cells was relatively small in our study (ranging from 1.3% to 3.7% of CD4+ T cells), Treg cells are quite active in small concentrations and have been shown to inhibit cellular proliferation and cytokine secretion in effector cells in in vitro studies at concentrations as low at 3% of CD4+ T cells [31]. In addition, the immunosuppressive activity of Treg cells follows a dose response curve and small increases in their concentration greatly enhance their immune suppression [31]. These findings, taken together with the results of our study, point toward a mechanism of immunosuppression in melanoma involving Treg cells.
We have shown that melanoma has the potential to mitigate the antitumor immune response by the induction of Treg cells. As much of tumor immunity is generated from the expression of self-antigens, Treg cells may act in melanoma to suppress tumor-induced immunity. A better understanding of the mechanisms by which melanoma evades the immune response is crucial to developing more effective immunotherapies, as most have not resulted in durable clinical responses. Currently, clinical trials are underway or proposed to investigate the control of the Treg response by their elimination or blockade to subvert tumor-induced immunosuppression by using anti-CD25 antibodies, anti-CTLA-4 antibodies, IL-2-toxin chimeric proteins, glucocorticoid-induced TNF-like receptor (GITR) ligands and CD123/OX-40 ligands [32]. In addition to the therapeutic role of Treg cell elimination, they may serve as a diagnostic or prognostic marker for melanoma patients, as has been shown with other tumors [33].
In summary, our results support the hypothesis that melanoma evades the immune response, in part, by induction of immunosuppressive FOXP3+ regulatory T cells from normal PBMCs. This may be through induction of FOXP3 and the Treg cells may subsequently act partly through secretion of immunosuppressive cytokines. Further study is warranted to understand the mechanistic details of the induction and activity of Treg cells in melanoma.
Acknowledgments
We are grateful to the University of Colorado Center for AIDS Research Immunology Core (grant number P30 AI 054907) for their expertise in flow cytometry and assistance in identifying regulatory T cells.
Footnotes
This work was supported, in part, by a 5 K12 CA86913 clinical oncology research career development program award.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morton DL, Wanek L, Nizze JA, Elashoff RM, Wong JH. Improved long-term survival after lymphadenectomy of melanoma metastatic to regional nodes. Analysis of prognostic factors in 1134 patients from the John Wayne Cancer Clinic. Annals of surgery. 1991;214:491–499. doi: 10.1097/00000658-199110000-00013. discussion 499–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manola J, Atkins M, Ibrahim J, Kirkwood J. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol. 2000;18:3782–3793. doi: 10.1200/JCO.2000.18.22.3782. [DOI] [PubMed] [Google Scholar]
- 3.Saleh F, Renno W, Klepacek I, Ibrahim G, Dashti H, Asfar S, Behbehani A, Al-Sayer H, Dashti A. Direct evidence on the immune-mediated spontaneous regression of human cancer: an incentive for pharmaceutical companies to develop a novel anti-cancer vaccine. Current pharmaceutical design. 2005;11:3531–3543. doi: 10.2174/138161205774414556. [DOI] [PubMed] [Google Scholar]
- 4.Jack A, Boyes C, Aydin N, Alam K, Wallack M. The treatment of melanoma with an emphasis on immunotherapeutic strategies. Surgical oncology. 2006;15:13–24. doi: 10.1016/j.suronc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Lotze MT, Chang AE, Seipp CA, Simpson C, Vetto JT, Rosenberg SA. High-dose recombinant interleukin 2 in the treatment of patients with disseminated cancer. Responses, treatment-related morbidity, and histologic findings. Jama. 1986;256:3117–3124. [PubMed] [Google Scholar]
- 6.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, Wunderlich JR, Parkhurst MR, Kawakami Y, Seipp CA, Einhorn JH, White DE. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nature medicine. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. The New England journal of medicine. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 9.Baecher-Allan C, Wolf E, Hafler DA. Clinical immunology. Vol. 115. Orlando, Fla: 2005. Functional analysis of highly defined, FACS-isolated populations of human regulatory CD4+ CD25+ T cells; pp. 10–18. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 11.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. The Journal of experimental medicine. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 13.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 14.Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9:4404–4408. [PubMed] [Google Scholar]
- 15.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 16.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature medicine. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 17.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Current opinion in rheumatology. 2003;15:430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Ziegler SF. FOXP3: of mice and men. Annual review of immunology. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 19.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature immunology. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 20.Liyanage UK, Goedegebuure PS, Moore TT, Viehl CT, Moo-Young TA, Larson JW, Frey DM, Ehlers JP, Eberlein TJ, Linehan DC. Increased prevalence of regulatory T cells (Treg) is induced by pancreas adenocarcinoma. J Immunother. 2006;29:416–424. doi: 10.1097/01.cji.0000205644.43735.4e. [DOI] [PubMed] [Google Scholar]
- 21.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature medicine. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 22.Gray CP, Arosio P, Hersey P. Association of increased levels of heavy-chain ferritin with increased CD4+ CD25+ regulatory T-cell levels in patients with melanoma. Clin Cancer Res. 2003;9:2551–2559. [PubMed] [Google Scholar]
- 23.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 24.Jarnicki AG, Lysaght J, Todryk S, Mills KH. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 25.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. The Journal of experimental medicine. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark RA, Kupper TS. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood. 2006 doi: 10.1182/blood-2006-02-002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levings MK, Allan S, d’Hennezel E, Piccirillo CA. Functional dynamics of naturally occurring regulatory T cells in health and autoimmunity. Advances in immunology. 2006;92:119–155. doi: 10.1016/S0065-2776(06)92003-3. [DOI] [PubMed] [Google Scholar]
- 28.Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 29.Javia LR, Rosenberg SA. CD4+CD25+ suppressor lymphocytes in the circulation of patients immunized against melanoma antigens. J Immunother. 2003;26:85–93. doi: 10.1097/00002371-200301000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cesana GC, DeRaffele G, Cohen S, Moroziewicz D, Mitcham J, Stoutenburg J, Cheung K, Hesdorffer C, Kim-Schulze S, Kaufman HL. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol. 2006;24:1169–1177. doi: 10.1200/JCO.2005.03.6830. [DOI] [PubMed] [Google Scholar]
- 31.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 32.Frumento G, Piazza T, Di Carlo E, Ferrini S. Targeting tumor-related immunosuppression for cancer immunotherapy. Endocrine, metabolic & immune disorders drug targets. 2006;6:233–237. doi: 10.2174/187153006778250019. [DOI] [PubMed] [Google Scholar]
- 33.Ikemoto T, Yamaguchi T, Morine Y, Imura S, Soejima Y, Fujii M, Maekawa Y, Yasutomo K, Shimada M. Clinical roles of increased populations of Foxp3+CD4+ T cells in peripheral blood from advanced pancreatic cancer patients. Pancreas. 2006;33:386–390. doi: 10.1097/01.mpa.0000240275.68279.13. [DOI] [PubMed] [Google Scholar]


