Abstract
One of the requirements for tumor growth is the ability to recruit a blood supply, a process known as angiogenesis. Angiogenesis begins early in the progression of cervical disease from mild to severe dysplasia and on to invasive cancer. We have previously reported that expression of human papillomavirus type 16 E6 and E7 (HPV 16 E6E7) proteins in primary foreskin keratinocytes (HFKs) decreases expression of two inhibitors and increases expression of two angiogenic inducers [(Toussaint-Smith et al., Oncogene 23:2988-2995 (2004)]. Here we report that HPV-induced early changes in the keratinocyte phenotype are sufficient to alter endothelial cell behavior both in vitro and in vivo. Conditioned media from HPV 16 E6E7 expressing HFKs as well as from human cervical keratinocytes containing the intact HPV 16 were able to stimulate proliferation and migration of human microvascular endothelial cells. In addition, introduction of the conditioned media into immunocompetent mice using a Matrigel plug model resulted in a clear angiogenic response. These novel data support the hypothesis that HPV proteins contribute not only to the uncontrolled keratinocyte growth seen following HPV infection but also to the angiogenic response needed for tumor formation.
Keywords: human papillomavirus, keratinocytes, angiogenesis
Introduction
To grow, solid tumors must gain access to nutrients and oxygen via the recruitment of a blood supply, in a process called angiogenesis (Bouck et al., 1996; Hanahan and Folkman, 1996). Angiogenesis involves a reprogramming of the transcription profile within the tumor cells so that angiogenic inducers predominate over angiogenic inhibitors (the angiogenic switch). As a lesion progresses, from hyperplasia to dysplasia and ultimately invasive carcinoma, there is an increase in microvessel density as well as their proximity to the epithelium (Dobbs et al., 1997; Guidi et al., 1995; Smith-McCune et al., 1997). This progressive alteration is seen in human cervical lesions progressing from dysplasia to invasive carcinoma and is recapitulated in the human papillomavirus type 16 (HPV 16) transgenic mouse model (Arbeit et al., 1996; Smith-McCune et al., 1997).
Multiple factors either inhibit or promote angiogenesis (Bouck et al., 1996; Hanahan and Folkman, 1996). The importance of the proangiogenic factor VEGF in this multistep process has been described in both the progression of disease in HPV 16 transgenic mice and in cervical carcinogenesis in women (Smith-McCune et al., 1997). Hyperplastic lesions in the mouse epidermis and mild cervical dysplasia (CIN I) in women display slight increases in expression of VEGF (by in situ hybridization) and frequency of microvessels. Dysplastic mouse epidermal lesions and severe cervical dysplasia (CIN III) in women display more significant increases in expression of VEGF and frequency of microvessels, with the latter closely apposed to the altered epithelium.
Human papillomaviruses (HPVs) cause cervical cancer and some head and neck cancer (Bosch et al., 2002; Herrero et al., 2003; Munoz et al., 2003; Smith et al., 1998; zur Hausen, 2000). The virus presumably enters through a break in the epithelium and initially infects the proliferating epithelial cells (keratinocytes). In these cells, the virus undergoes the non-productive stage of its life cycle where it is maintained as a low copy number extrachromosomal genome. When the infected cell moves to the differentiated compartment, infectious virus is produced. In contrast, at the late stage in the progression of HPV disease from low grade squamous intraepithelial lesion (LSIL) to high grade SIL to carcinoma in situ, the viral genome integrates, only expression of the E6 and E7 is maintained and expression of both is required for cell survival (Butz et al., 2003; DeFilippis et al., 2003; Hall and Alexander, 2003; Jiang and Milner, 2002).
The involvement of HPV proteins in the angiogenic switch and in the vascularization seen in the HPV 16 transgenic mice and the HPV-positive cervical lesions in women has not been established since the characterization of angiogenesis has been conducted long after the initial HPV infection (Dobbs et al., 1997; Guidi et al., 1995; Smith-McCune et al., 1997). This same limitation exists for experiments monitoring angiogenic factors in HPV-positive carcinoma cell lines (Bequet-Romero and Lopez-Ocejo, 2000). Because of the potential effect of HPV proteins on angiogenic factors, we have designed experiments to determine whether HPV plays a role in angiogenesis. Specifically, we have used cells at early passage after the introduction of HPV genes, to test the hypothesis that HPV proteins induce an angiogenic switch in primary keratinocytes which is sufficient to render conditioned media from such keratinocytes angiogenic. We previously analyzed the activity of HPV 16 E6 and E7 when expressed as a cassette from a heterologous retroviral promoter and showed that together they alter the level of expression of two angiogenic inhibitors, maspin and thrombospondin-1, and two angiogenic inducers, VEGF and IL-8 (Toussaint-Smith et al., 2004). Here we show that the change in keratinocyte phenotype is sufficient to render conditioned media from these cells angiogenic, using both in vitro and in vivo assays. Further, we have shown this to be the case not only when E6 and E7 are expressed in a retroviral vector but also when HPV genes are expressed at physiological levels from their homologous promoters.
Results
Conditioned media from human foreskin keratinocytes (HFKs) transduced with HPV 16 E6E7 and from cervical keratinocytes stably transfected with intact HPV 16 genomes increase proliferation and migration of endothelial cells (ECs) in vitro
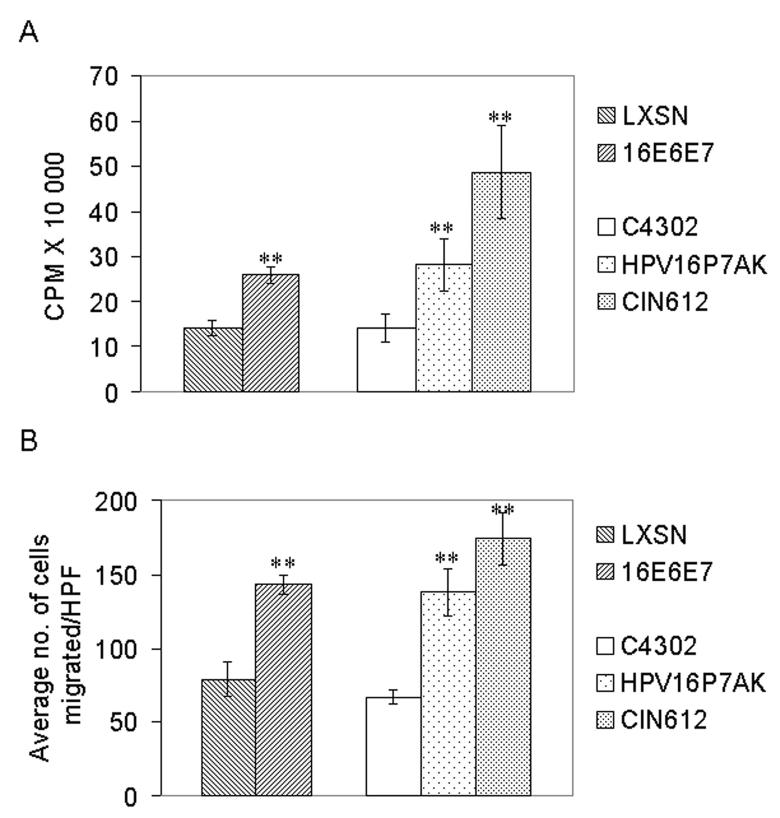
During angiogenesis, ECs have to penetrate the basement membrane, migrate, proliferate, and differentiate to form tubules (Hanahan and Folkman, 1996). In vitro proliferation and migration assays were used to determine whether HPV-induced changes in the conditioned media from HFKs transduced with human papillomavirus type 16 E6 and E7 (HPV 16 E6E7) and passaged for a short time were sufficient to alter EC behavior. In these experiments, conditioned media from LXSN (retroviral parent) transduced HFKs served as a negative control and VEGF served as a positive control. The conditioned media from HPV 16 E6E7-transduced HFKs induced human microvascular endothelial cells (HMVECs) to proliferate (Figure 1A) and to migrate (Figure 1B). Statistical analysis combining the results of three independent experiments indicated that the approximately two-fold difference in both proliferation and migration between LXSN and HPV 16 E6E7 was significant (p<0.01). The addition of VEGF resulted in a greater than 2 to 3-fold increase in proliferation and in migration compared to media control (data not shown).
Figure 1.
(A) Proliferation of ECs in response to conditioned media from cells expressing HPV 16 proteins. HMVECs were seeded at 5×104 cells/well onto collagen coated 6-well plates. After serum starvation, conditioned media from cells transduced with LXSN or L(16E6E7)SN or from HPV16P7AK or CIN612 cells were added overnight and then replaced with media containing 3H-TdR for five hours. Cells were lysed and incorporated radiolabel counted in a beta-counter. (B) Migration of ECs in response to conditioned media from cells expressing HPV 16 proteins. Serum starved HMVECs were plated on a collagen-coated transwell membrane. Conditioned media were placed in the bottom chamber. After 3 hrs, at least 5 high power fields (HPF) of cells on the bottom of the membrane were counted. For (A,B) the results (average ± standard deviation) are representative of three experiments conducted in triplicate. Statistical analysis combining the results of three independent experiments indicated that, in both the proliferation and the migration assays, the difference between LXSN and 16E6E7 and between C4302 and HPV16P7AK/CIN612 is significant (**p<0.01).
The experiments thus far indicated that conditioned media from keratinocytes retrovirally transduced with HPV 16 E6E7 and passaged for a short time were sufficient to induce an alteration in EC behavior. Since natural infection introduces the entire HPV genome, we determined whether conditioned media from cells stably transfected with the intact HPV genome and passaged for a short time (HPV16P7AK) could also induce ECs to proliferate and migrate. In this case, the viral proteins are produced at physiological levels. In these experiments, conditioned media from HPV 31-positive CIN612 cells, a cell line which was established from a severely dysplastic (CINIII) cervical biopsy and which is tumorigenic in nude mice (L. Laimins, personal communication), served as a positive control and C4302, early passage primary cervical cells, served as a negative control. The results showed that conditioned media from HPV16P7AK induced HMVECs to proliferate (Figure 1A) and to migrate (Figure 1B) approximately two-fold more than media from C4302. Statistical analysis combining the results of three independent experiments indicated that the difference between C4302 and HPV16P7AK was significant (p<0.01). As expected due to their tumorigenic phenotype, conditioned media from CIN612 cells could also alter EC behavior.
In mice, conditioned media from keratinocytes transduced with HPV 16 E6E7 or stably transfected with the intact HPV 16 genome induce angiogenesis
The magnitude of an angiogenic response depends upon a complex set of interactions within the microenvironment (Coussens et al., 1999; Mueller and Fusenig, 2004; Zhu et al., 2002). To test whether the conditioned media from early passage HPV 16 E6E7 transduced human foreskin keratinocytes could induce an angiogenic response in vivo, the Matrigel plug assay was used. Matrigel is derived from a tumor extract and can be injected into mice to form a plug. This well established in vivo assay is a rapid and efficient method to determine the angiogenic potential of different compounds placed into the plug prior to injection (Munchhof et al., 2006; Walker, 2001). The bloody appearance of the Matrigel plug provides a qualitative representation of the growth of blood vessels into the plug in response to the angiogenic stimuli. The assay of hemoglobin extracted from the plug allows quantification of the angiogenic response. Matrigel was mixed with conditioned media from CIN612, HPV 16 E6E7- expressing HFKs or HPV16 P7AK and injected into the ventral groin of immunocompetent mice. Seven days after injection the plug was removed, photographed, and the hemoglobin was extracted from the plug and quantified. Conditioned media from CIN612, 16 E6E7-expressing HFKs and HPV16P7AK stimulated an angiogenic response, quantitated by the hemoglobin extracted from the Matrigel (Figure 2). The activity seen with the conditioned media from the HPV-expressing cells was HPV-dependent since conditioned media from the control retrovirally transduced keratinocytes (LXSN) and C4302 did not induce an angiogenic response (Figure 2).
Figure 2.
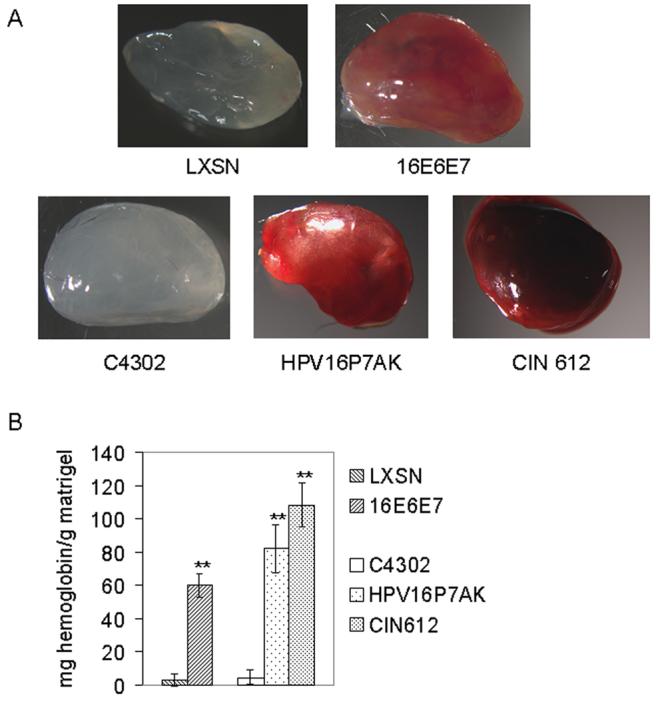
In vivo angiogenesis assays using conditioned media from keratinocytes expressing HPV 16 proteins. Conditioned media were mixed with Matrigel (BD Matrigel™) and injected into the ventral groin of mice. Seven days later the mice were sacrificed, the Matrigel plug was removed and photographed, and then hemoglobin was extracted and quantitated. Conditioned media from C4302 and LXSN serve as negative controls for CIN612 and HPV16P7AK, and 16E6E7, respectively. The results shown are representative of three independent experiments, each conducted in triplicate. The average ± standard deviation for the hemoglobin measured in one such experiment is shown (**p = <0.01).
Characterization of lysates and conditioned media from human foreskin keratinocytes (HFKs) transduced with HPV 16 E6E7 and from cervical keratinocytes stably transfected with extrachromosal HPV 16 genomes
We previously reported that retroviral transduction of HFKs with HPV 16 E6E7 resulted in altered levels of expression of two angiogenic inhibitors, thrombospondin-1 and maspin, and two angiogenic inducers, VEGF and IL8 (Toussaint-Smith et al., 2004). Here we compared the levels of these four angiogenic factors in retrovirally transduced HFKs to the levels in cervical keratinocytes expressing HPV proteins at physiological levels [from cervical keratinocytes stably transfected with, and maintaining extrachromosal HPV 16 genomes (HPV16P7AK) or HPV 31 (CIN612)].
Semi-quantitative RT-PCR (Figure 3A) showed that the levels of transcription of the two angiogenic inhibitors, thrombospondin-1 and maspin, in HFKs retrovirally transduced with HPV 16 E6E7 were slightly but significantly decreased, 74% and 64%, respectively, relative to HFKs transduced with the retroviral control. Thrombospondin-1 and maspin mRNA levels in HPV16P7AKs were 67% and 70%, respectively, of the levels in C4302. The levels of expression of these angiogenic inhibitors were also reduced in CIN612 cells compared to C4302. In contrast, the levels of the two angiogenic inducers, IL-8 and VEGF, were increased 1.7 to 2.5-fold in both the retrovirally transduced cells and those containing the intact HPV genomes, with the exception that only a 1.3-fold increase in VEGF was seen in CIN612 cells.
Figure 3.
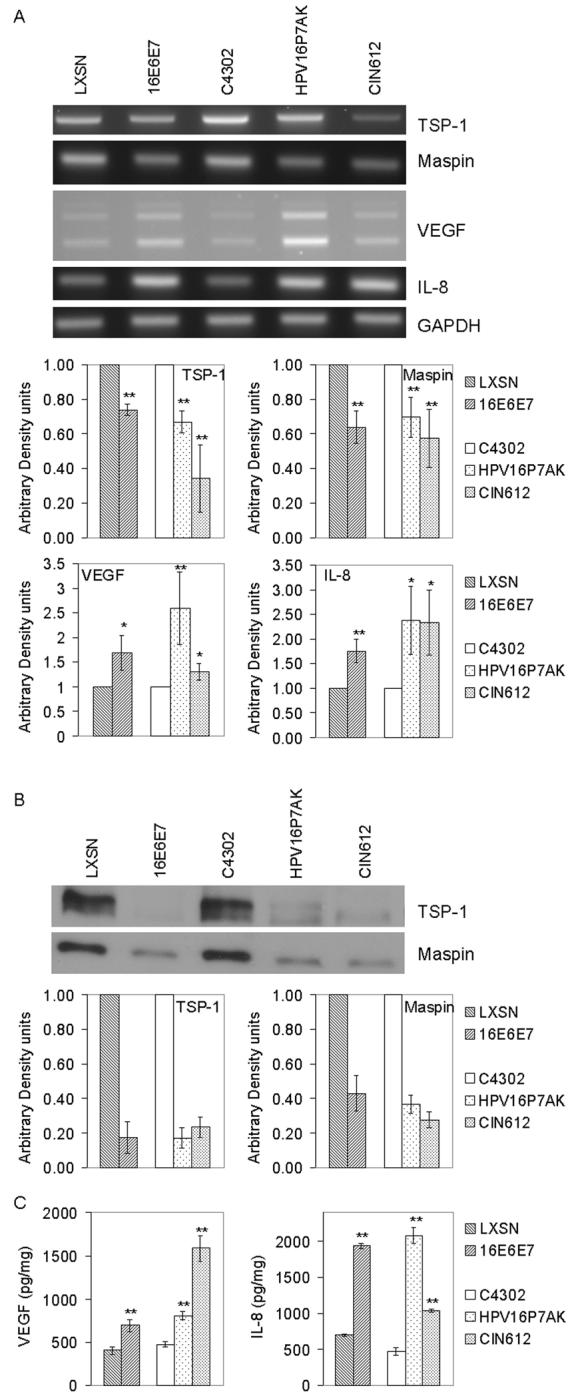
(A) Semi-quantitative RT-PCR analysis of thrombospondin-1, maspin, VEGF, and IL-8. RNA extracts from HFKs transduced with empty vector (LXSN) or HPV 16 E6E7 or from control human cervical keratinocytes (C4302 cells) or cervical keratinocytes containing the intact HPV 16 (HPV16P7AK) or HPV 31 (CIN612) were subjected to RT-PCR and analyzed on 2% agarose gels. GAPDH amplification was used as an internal control. The multiple bands seen in the VEGF panel represent different isoforms (Kodama et al., 1999). (B) Western analysis of thrombospondin-1 and maspin in conditioned media from cells transduced with LXSN or L(16E6E7)SN or from HPV16P7AK or CIN612 cells. Proteins were separated on 10% SDS-PAGE, transferred to nitrocellulose membrane and probed with antibodies to the indicated proteins. (C) ELISA for VEGF and IL-8 in conditioned media. The quantity of VEGF and IL-8 was determined and normalized to total protein in the conditioned media. The results shown are representative of three independent experiments conducted in triplicate (*p<0.05; **p<0.01).
The effect of HPV gene expression on the relative amounts of these angiogenic factors in the conditioned media was determined using immunoblots to quantitate thrombospondin-1 and maspin (Figure 3B) and ELISAs for IL-8 and VEGF (Figure 3C). Levels of thrombospondin-1 were decreased 5-fold in conditioned media from cells expressing HPV proteins and maspin levels were decreased by at least two-fold, compared to controls. Levels of VEGF in conditioned media from cells expressing retrovirally encoded HPV 16 E6E7 or the intact HPV 16 genome were increased approximately two fold compared to controls, or 4-fold in the case of CIN612 cells (p<.001). The levels of IL-8 were increased approximately 3-fold in the retrovirally transduced HFKs and the HPV16P7AK cells and 2-fold in the CIN612 cells, compared to controls (p<0.01).
Discussion
The experiments described here were undertaken to determine whether HPV proteins might contribute to the angiogenic response seen in the early stages of HPV-mediated disease. To do so, HPV-expressing HFKs were analyzed early after transduction, when the control cells were still proliferating. Similarly, the stably transfected cervical keratinocytes were analyzed at early passage, prior to the induction of telomerase. Angiogenesis is a multistep process, beginning at the pre-malignant stage and involving the progressive enhancement of the angiogenic switch as a lesion progresses from hyperplasia or low grade dysplasia to high grade dysplasia and carcinoma (Dobbs et al., 1997;Guidi et al., 1995; Hanahan and Folkman, 1996; Smith-McCune et al., 1997). In the case of cervical carcinomas and in the HPV 16 transgenic mouse model, a correlation has been made between the upregulation of HPV E6 and E7 gene expression and the upregulation of VEGF expression, increases in microvascular density, and close apposition of neovasculature to the affected tissue (Smith-McCune et al., 1997). Because high risk HPV E6 and E7 affect a number of factors implicated in the regulation of the angiogenic switch, most notably the tumor suppressors, p53 and pRb, we tested the hypothesis that HPV 16 E6 and E7 together would contribute to the angiogenesis required to sustain tumor growth. We showed that the expression of HPV 16 E6 and E7 in human foreskin keratinocytes caused a change in the expression of angiogenic factors (Toussaint-Smith et al., 2004). Here we have shown that the conditioned media from these retroviral transduced human foreskin keratinocytes alter EC behavior in vitro and in vivo. In addition, we have shown that conditioned media from cervical keratinocytes stably transfected with the intact HPV 16 genome also alter EC behavior in vitro and in vivo. These results establish that HPV proteins, expressed at physiological levels, cause a sufficient alteration in the expression of angiogenic factors in keratinocytes to affect EC behavior.
Different cell lineages within the tumor microenvironment, not just the tumor-initiating cell itself, secrete growth factors, which combine to recruit ECs from the local vasculature as well as from the bone marrow to the tumor (Orimo et al., 2005). Thus stromal fibroblasts and mast cells have also been documented to be contributors to the angiogenic process (Bhowmick et al., 2004; Coussens et al., 1999). Data described here are consistent with an effect of the microenvironment on amplification of the HPV-initiated signal. There is a greater angiogenic response to the conditioned media from HPV-expressing keratinocytes in the Matrigel plug assay in vivo (at least a 10-fold stimulation, Figure 2) than in either the proliferation or migration assay in vitro (approximately 2-fold stimulation, Figure 1). In contrast to the in vivo assay, only endothelial cells are exposed to the media in the in vitro assays.
The angiogenic response requires that the levels of angiogenic inducers increase and/or the level of angiogenic inhibitors decrease (Bouck et al., 1996). Four angiogenic factors were analyzed in this study. They were chosen because VEGF, IL-8, and thrombospondin-1 levels had been reported to be altered in cervical carcinomas and cervical carcinoma cell lines (Bequet-Romero and Lopez-Ocejo, 2000; Dobbs et al., 1997; Guidi et al., 1995; Smith-McCune et al., 1997); a limited microarray analysis showed altered levels of expression of VEGF, maspin, and thrombospondin-1 RNA in HPV 16 E6E7 transduced HFKs (Toussaint-Smith et al., 2004); and IL-8 was additionally found to be upregulated in oral squamous cell carcinomas (Lingen et al., 1996) and affected by HPV gene expression (Huang and McCance, 2002; Nees et al., 2001; Toussaint-Smith et al., 2004). Further experiments will be needed to determine whether alterations in one or more of these factors, or yet to be defined factors, is responsible for the HPV-mediated angiogenic switch seen in keratinocytes which subsequently affects ECs.
Materials and methods
Cell culture and collection of conditioned media
Pools of primary human foreskin keratinocytes were prepared and infected with parental vector control virus LXSN, or recombinant retrovirus L(16E6E7)SN (ATCC) as previously described (Toussaint-Smith et al., 2004). Following selection, the cells were expanded in complete E media on mitomycin-treated J2-3T3 feeder cells (Wu et al., 1982). For collection of conditioned media, 2.5×106 cells (at passage 3) were plated in E media in 10 cm plates with 2×105 feeder cells for 24 hrs. Cells were then starved for four hrs in 4 ml of basal E media (3 parts DMEM, 1 part Ham's F-12 with 100 U/ml of penicillin and 100 μg/ml of streptomycin) before switching to 8 ml of the same media and conditioned media were harvested 24 hrs later. In parallel, cells were lysed and RNA and protein were collected. HPV16P7AK cells (originally called C66 C1F P7) are early passage cervical keratinocytes stably transfected with and maintaining extrachomosomal HPV 16, from A. Klingelhutz (U. Iowa) (Sprague et al., 2002). The cells are not tumorigenic (Berger et al., 2006). C4302 are primary cervical keratinocytes. CIN612 cells contained episomal HPV 31. HPV16P7AK, C4302, and CIN612 cells were maintained in E media on feeder cells and conditioned media were harvested as described for the retrovirally transduced HFKs. Conditioned media were quick frozen in an alcohol/dry ice bath and stored at −80°C. HMVECs (Cambrex, Walkersville, MD) were maintained in complete EGM-2 supplemented with 10% FBS in tissue culture flasks precoated with type I rat tail collagen (BD Biosciences). Experiments were done at passage 5 to 8.
RNA isolation and semi-quantitative reverse transcription (RT)-PCR analysis
Total RNA was prepared using RNeasy Minikit (Qiagen, Valencia, CA, USA) and contaminating DNA was removed by digestion with the RNase-Free DNase Set (Qiagen). 0.5 μg of RNA was reverse-transcribed and amplified using Superscript Onestep RT-PCR kit (Invitrogen) and primers for thrombospondin-1, maspin and GAPDH (Toussaint-Smith et al., 2004), IL-8 (Seo et al., 2004); and VEGF (Kodama et al., 1999). Reactions were carried out with 52°C annealing temperature, and stopped at 22 cycles for GAPDH, 28 cycles for IL-8 and 26 cycles for all others. The RT-PCR products were visualized on a 2% agarose gel with ethidium bromide staining, pictures were analyzed with Quantity one software (Bio-Rad, Hercules, CA, USA). Arbitrary density units were adjusted based on GAPDH and calculated by setting each negative control to one.
ELISA analysis
Conditioned media were analyzed in triplicate using human CXCL-8 (IL-8) and VEGF Quantikine ELISA kits purchased from R&D systems (Minneapolis, MN, USA).
Western blot analysis
Proteins in conditioned media, concentrated using an Amicon ultra centrifugal devise filter (5000 MWCO), were separated by 10% SDS-PAGE, transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA), and probed with antibodies to GAPDH (MAB374, Chemicon, Temecula, CA, USA), maspin (554292, BD Biosciences, Pharmingen, San Diego, CA, USA), and thrombospondin (MS-1066, Lab Vision, Fremont, CA, USA). Quantity one software was used for densitometry analysis. Arbitrary density units were calculated as described for RT-PCR.
In vitro proliferation assay
Cell proliferation was determined by [3H] thymidine incorporation as previously described (Munchhof et al., 2006). HMVECs were plated in collagen coated six-well plates (BD Biosciences, Bedford, MA, USA) at 5×104 cells/well and were deprived of serum. Conditioned media were then added for 8 hr in a 37μC, 5% CO2, humidified incubator. Cells were pulse-labeled with 1 μCi/ml of [3H] thymidine (Perkin-Elmer Life Sciences Products, Boston, MA, USA) for 5 hr, washed, lysed with 0.1N NaOH and measured with a beta counter (Beckman Coulter Inc., Fullerton, CA, USA). Assays were performed in triplicate.
In vitro migration assay
Migration assays were performed as previously described (Munchhof et al., 2006) with minor modifications. Transwell cell culture inserts (BD Biosciences, Bedford, MA, USA) coated with collagen were placed into the lower chamber containing 600 μl conditioned media. 5×104 cells/well of serum deprived HMVECs were placed on top of the insert and incubated for 3 hr. Non-migrating cells were removed using a cotton swab, and migrating cells were fixed and stained with Diff-quick kit (Invitrogen). The average number of migrated cells was counted with an inverted microscope at 20x magnification. 25 ng/ml of VEGF with 0.1% BSA was also used a positive control (data not shown). Assays were performed in triplicate.
In vivo Matrigel plug assay
C57BL/6.129 or C57BL/6 mice were used in accordance with a protocol approved by the Indiana University Animal Use and Care Committee. 10 mg/ml of Matrigel (BD Biosciences) were mixed with 60 units/ml heparin (Sigma) and 90 μg of conditioned media on ice before injection. Matrigel (300 μl) was injected subcutaneously into the murine groin. After 7 days, plugs were harvested, photographed and assayed for total hemoglobin using a hemoglobin assay kit (Pointe Scientific, Lincoln Park, MI, USA). 600 ng/ml bFGF (Peprotech, Rocky Hill, NJ, USA) was also used as a positive control (data not shown).
Statistical analysis
Student two tailed t-test was used for statistical analysis. p<0.05 was considered significant.
Acknowledgments
We thank Aloysius Klingelhutz for providing C4302 and HPV16AKP7 and Laimonis Laimins for providing CIN612 cells. This work was supported by NIH R01AI31494 and AHA Greater Midwest Affiliate 0550043Z (to AR), NIH T32 CA111198-02S1 (to JW), and NIH KO8 CA096579-01, P50 NS052606, NF043019 Department of Defense, Riley Children's Foundation, P30 CA82709 (to D.A.I.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arbeit JM, Olson DC, Hanahan D. Upregulation of fibroblast growth factors and their receptors during multi-stage epidermal carcinogenesis in K14-HPV16 transgenic mice. Oncogene. 1996;13:1847–1857. [PubMed] [Google Scholar]
- Bequet-Romero M, Lopez-Ocejo O. Angiogenesis modulators expression in culture cell lines positives for HPV-16 oncoproteins. Biochem.Biophys.Res.Commun. 2000;277:55–61. doi: 10.1006/bbrc.2000.3628. [DOI] [PubMed] [Google Scholar]
- Berger KL, Barriga F, Lace MJ, Turek LP, Zamba GJ, Domann FE, Lee JH, Klingelhutz AJ. Cervical keratinocytes containing stably replicating extrachromosomal HPV-16 are refractory to transformation by oncogenic H-Ras. Virology. 2006 doi: 10.1016/j.virol.2006.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J.Clin.Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck N, Stellmach V, Hsu SC. How tumors become angiogenic. Adv.Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. [DOI] [PubMed] [Google Scholar]
- Butz K, Ristriani T, Hengstermann A, Denk C, Scheffner M, Hoppe-Seyler F. siRNA targeting of the viral E6 oncogene efficiently kills human papillomavirus-positive cancer cells. Oncogene. 2003;22:5938–5945. doi: 10.1038/sj.onc.1206894. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilippis RA, Goodwin EC, Wu L, DiMaio D. Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J.Virol. 2003;77:1551–1563. doi: 10.1128/JVI.77.2.1551-1563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs SP, Hewett PW, Johnson IR, Carmichael J, Murray JC. Angiogenesis is associated with vascular endothelial growth factor expression in cervical intraepithelial neoplasia. Br.J.Cancer. 1997;76:1410–1415. doi: 10.1038/bjc.1997.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi AJ, Abu-Jawdeh G, Berse B, Jackman RW, Tognazzi K, Dvorak HF, Brown LF. Vascular permeability factor (vascular endothelial growth factor) expression and angiogenesis in cervical neoplasia. J.Natl.Cancer Inst. 1995;87:1237–1245. doi: 10.1093/jnci/87.16.1237. [DOI] [PubMed] [Google Scholar]
- Hall AH, Alexander KA. RNA interference of human papillomavirus type 18 E6 and E7 induces senescence in HeLa cells. J.Virol. 2003;77:6066–6069. doi: 10.1128/JVI.77.10.6066-6069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Herrero R, Castellsague X, Pawlita M, Lissowska J, Kee F, Balaram P, Rajkumar T, Sridhar H, Rose B, Pintos J, Fernandez L, Idris A, Sanchez MJ, Nieto A, Talamini R, Tavani A, Bosch FX, Reidel U, Snijders PJ, Meijer CJ, Viscidi R, Munoz N, Franceschi S. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J.Natl.Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- Huang SM, McCance DJ. Down regulation of the interleukin-8 promoter by human papillomavirus type 16 E6 and E7 through effects on CREB binding protein/p300 and P/CAF. J.Virol. 2002;76:8710–8721. doi: 10.1128/JVI.76.17.8710-8721.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Milner J. Selective silencing of viral gene expression in HPV-positive human cervical carcinoma cells treated with siRNA, a primer of RNA interference. Oncogene. 2002;21:6041–6048. doi: 10.1038/sj.onc.1205878. [DOI] [PubMed] [Google Scholar]
- Kodama J, Seki N, Tokumo K, Hongo A, Miyagi Y, Yoshinouchi M, Okuda H, Kudo T. Vascular endothelial growth factor is implicated in early invasion in cervical cancer. Eur.J.Cancer. 1999;35:485–489. doi: 10.1016/s0959-8049(98)00410-9. [DOI] [PubMed] [Google Scholar]
- Lingen MW, Polverini PJ, Bouck NP. Retinoic acid induces cells cultured from oral squamous cell carcinomas to become anti-angiogenic. Am.J.Pathol. 1996;149:247–258. [PMC free article] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat.Rev.Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- Munchhof AM, Li F, White HA, Mead LE, Krier TR, Fenoglio A, Li X, Yuan J, Yang FC, Ingram DA. Neurofibroma-associated growth factors activate a distinct signaling network to alter the function of neurofibromin-deficient endothelial cells. Hum.Mol.Genet. 2006;15:1858–1869. doi: 10.1093/hmg/ddl108. [DOI] [PubMed] [Google Scholar]
- Munoz N, Bosch FX, de SS, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N.Engl.J.Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- Nees M, Geoghegan JM, Hyman T, Frank S, Miller L, Woodworth CD. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-kappaB-responsive genes in cervical keratinocytes. J.Virol. 2001;75:4283–4296. doi: 10.1128/JVI.75.9.4283-4296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, renzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Seo KH, Lee HS, Jung B, Ko HM, Choi JH, Park SJ, Choi IH, Lee HK, Im SY. Estrogen enhances angiogenesis through a pathway involving platelet-activating factor-mediated nuclear factor-kappaB activation. Cancer Res. 2004;64:6482–6488. doi: 10.1158/0008-5472.CAN-03-2774. [DOI] [PubMed] [Google Scholar]
- Smith EM, Hoffman HT, Summersgill KS, Kirchner HL, Turek LP, Haugen TH. Human papillomavirus and risk of oral cancer. Laryngoscope. 1998;108:1098–1103. doi: 10.1097/00005537-199807000-00027. [DOI] [PubMed] [Google Scholar]
- Smith-McCune K, Zhu YH, Hanahan D, Arbeit J. Cross-species comparison of angiogenesis during the premalignant stages of squamous carcinogenesis in the human cervix and K14-HPV16 transgenic mice. Cancer Res. 1997;57:1294–1300. [PubMed] [Google Scholar]
- Sprague DL, Phillips SL, Mitchell CJ, Berger KL, Lace M, Turek LP, Klingelhutz AJ. Telomerase activation in cervical keratinocytes containing stably replicating human papillomavirus type 16 episomes. Virology. 2002;301:247–254. doi: 10.1006/viro.2002.1542. [DOI] [PubMed] [Google Scholar]
- Toussaint-Smith E, Donner DB, Roman A. Expression of human papillomavirus type 16 E6 and E7 oncoproteins in primary foreskin keratinocytes is sufficient to alter the expression of angiogenic factors. Oncogene. 2004;23:2988–2995. doi: 10.1038/sj.onc.1207442. [DOI] [PubMed] [Google Scholar]
- Walker JE. Angiogenic Protocols. In: in Methods in Molecular Medicine. Humana Press; Totowa, NJ: 2001. [Google Scholar]
- Wu YJ, Parker LM, Binder NE, Beckett MA, Sinard JH, Griffiths CT, Rheinwald JG. The mesothelial keratins: a new family of cytoskeletal proteins identified in cultured mesothelial cells and nonkeratinizing epithelia. Cell. 1982;31:693–703. doi: 10.1016/0092-8674(82)90324-5. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J.Natl.Cancer Inst. 2000;92:690–698. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]