Abstract
Background and purpose:
Since the vasorelaxant potency of the endocannabinoid anandamide is enhanced in perfused mesenteric vascular beds from rats made hypertensive by chronic inhibition of NO synthase (L-NAME in drinking water), we hypothesized that in vivo, anandamide-induced vasodilatation would be similarly enhanced in L-NAME-treated animals.
Experimental approach:
Male Sprague-Dawley rats were given L-NAME in drinking water (7.5 mg kg−1 day−1) for 4 weeks. Relaxant effects of anandamide were measured in perfused mesenteric vascular beds and in isolated small mesenteric arteries. Renal, mesenteric and hindquarters haemodynamic responses to anandamide, methanandamide, the synthetic cannabinoid agonist WIN-55212-2 and the cannabinoid receptor antagonist AM251 were assessed in conscious, chronically-instrumented rats.
Key results:
Vasorelaxant responses to anandamide were enhanced in the perfused mesentery but not in isolated mesenteric resistance vessels. In vivo, anandamide caused vasodilatation only in the hindquarters vascular bed and only in control rats. Methanandamide caused a late-onset (40 min after administration) tachycardia, mesenteric and hindquarters vasoconstriction, and renal vasodilatation, which did not differ between control and L-NAME-treated rats. AM251 had no effect on resting blood pressure in control or L-NAME-treated rats and WIN55212-2 caused pressor and renal and mesenteric vasoconstrictor responses, with hindquarters vasodilatation in both groups of animals.
Conclusions and Implications:
The results provide no in vivo evidence for enhanced vasodilator responses to cannabinoids, or up-regulation of endocannabinoids or their receptor activity, following chronic NO synthase inhibition.
Keywords: anandamide, cannabinoids, hypertension, nitric oxide synthase, regional haemodynamics
Introduction
Currently, much interest surrounds the physiological and pathophysiological cardiovascular actions of the endogenous cannabinoid (CB) system (see Randall et al., 2004 for a review). In vitro, anandamide-induced vasorelaxation of mesenteric resistance vessels is well documented and involves multiple mechanisms, including CB1 receptors, vanilloid (TRPV1) receptors, and in some cases, endothelium-derived hyperpolarizing factor (EDHF)-mediated relaxations and nitric oxide (NO) production (Randall et al., 1996; Deutsch et al., 1997; White and Hiley, 1997; White and Hiley, 1998; Wagner et al., 1999; Zygmunt et al., 1999; O'Sullivan et al., 2004). Other CB1 receptor-independent actions include inhibition of L-type calcium channels (Johnson et al., 1993; Jarrahian and Hillard, 1997), and effects on gap junction function (Chaytor et al., 1999; Howlett and Mukhopadhyay, 2000). Anandamide may also act via an as yet unidentified, non-CB1/CB2, endothelial ‘anandamide receptor' (Járai et al., 1999; Offertaler et al., 2003; O'Sullivan et al., 2004). However, vasodilator effects of anandamide in vivo are difficult to demonstrate, and indeed, the more recent literature indicates that in normotensive animals, a major component of the hypotensive effect of anandamide is due to decreased cardiac output rather than reduced peripheral vascular resistance (Pacher et al., 2004).
There is in vitro evidence to suggest that the vascular effects of anandamide may be augmented by inhibition of nitric oxide synthase (NOS). Specifically, in isolated, perfused mesenteric vascular beds, Mendizábal et al. (2001) demonstrated that the anandamide-induced inhibition of noradrenaline-evoked vasoconstriction was enhanced in vessels from rats given the NOS inhibitor, NG-nitro-L-arginine methyl ester (L-NAME), in the drinking water for 4 weeks. On the basis of the results from a series of experiments, Mendizábal et al. (2001) concluded that anandamide caused mesenteric vasorelaxation via activation of potassium channels and TRPV1 receptors, an effect that was augmented by chronic NOS inhibition, most likely via a downregulation of anandamide metabolism and uptake, as methanandamide was more potent than anandamide in controls, yet equipotent in vessels from L-NAME-treated rats. However, to our knowledge these findings have not been followed up in vivo.
Therefore, the first aim of the present study was to evaluate the regional haemodynamic effects of anandamide in conscious rats after a 4-week treatment with L-NAME in drinking water compared to tap water-drinking, control rats, to test the hypothesis that the vascular effects of anandamide would be enhanced in L-NAME-treated rats. In addition, in separate groups of L-NAME and control rats, responses to methanandamide, the metabolically stable analogue of anandamide, were compared.
Chronic administration of L-NAME causes hypertension, and although the findings of Mendizábal et al. (2001) were shown to be not related to the hypertensive state and not CB receptor-mediated, others have shown enhanced CB1-receptor-mediated cardiodepressor and vasodilator effects of endocannabinoids in other models of hypertension (Bátkai et al., 2004). Therefore, a second aim was to evaluate the effects of the cannabinoid (CB1) receptor antagonist, AM251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide), on baseline cardiovascular variables in L-NAME-treated and control rats, to test the hypothesis that there is a compensatory role for endocannabinoids in controlling vascular tone in hypertension induced by NOS inhibition, which would be manifest as an exaggerated response to antagonism of the endogenous ligand. In addition, the regional haemodynamic effects of the synthetic CB-receptor agonist, WIN55212-2, were compared in control and L-NAME-treated rats.
The dose of L-NAME administered (∼10 mg kg−1 day−1 for 4 weeks) was chosen on the basis of the effects on blood pressure measured by telemetry (Tep-Areenan et al., 2002) and was less than that used by Mendizábal et al. (2001) (70 mg kg−1 day−1 for 4 weeks). Therefore, before the in vivo experiments began, in vitro experiments were performed in perfused mesenteric vascular beds taken from rats treated with L-NAME, to ascertain that augmented vasorelaxant responses to anandamide were evident under those conditions. In addition, some experiments were performed in isolated third-order mesenteric arteries taken from control and L-NAME-treated rats, to determine the extent to which the results obtained in the isolated perfused vascular bed were reflected in this preparation. Overall, this study has provided no in vivo evidence for enhanced vasodilator responses to cannabinoids, or upregulation of endocannabinoids or their receptor activity, following chronic NOS inhibition, despite the in vitro evidence presented.
Methods
Animals
Male, Sprague–Dawley rats (Charles River, UK) were housed in a temperature-controlled environment (20–22°C) with a 12 h light/dark cycle (lights on at 0600). The rats were held within the Biomedical Services Unit at the University of Nottingham for at least a week before commencement of any procedures. All procedures were approved by the University of Nottingham Ethical Review Committee, and were performed under UK Home Office Project and Personal Licence Authority.
Animals used for in vitro studies (n=46) were housed in pairs, whereas those used in in vivo studies (n=37) were housed individually after surgery. In each study, half of the group received tap water (control group) and the rest received a 0.1 mg ml−1 solution of the NOS inhibitor, L-NAME, to drink ad libitum. Fluid intake was measured over 4 weeks, with fresh water or L-NAME solution given every 2–3 days. On average, the fluid intake was ∼75 ml kg−1 giving an L-NAME intake of ∼7.5 mg kg−1 day−1. In vitro and in vivo experiments were performed after rats had been drinking L-NAME for 4 weeks.
Isolated perfused mesenteric arterial beds
Male, Sprague–Dawley rats (340–495 g) were stunned by a blow to the back of the head and killed by cervical dislocation. As previously described by Harris et al. (2002), cannulation of the superior mesenteric artery was followed by removal of the whole mesenteric bed, and its perfusion at 5 ml min−1 with either normal Krebs–Henseleit buffer (composition (mM): NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, CaCl2 2, D-glucose 10) containing 3 μM indomethacin (n=6 controls), or with this buffer containing 3 μM indomethacin and 300 μM L-NAME (n=10 controls, n=9 L-NAME-treated rats).
Following a 30-min equilibration period at a basal tone of approximately 40 mm Hg, the beds were contracted with methoxamine (ca 5 μM) to increase tone by around 80 mm Hg. In the presence of L-NAME, preparations showed increased sensitivity to methoxamine; hence, a lower concentration of methoxamine was used (<1 μM). Once the tone had stabilized, a cumulative concentration–response curve to anandamide (1 nM–10 μM) was constructed.
Wire myography of isolated mesenteric vessels
Male, Sprague–Dawley rats (300–450 g) were stunned by a blow to the back of the head and killed by cervical dislocation. The mesenteric arterial bed was removed immediately and transferred into cold Krebs–Henseleit buffer containing 300 μM L-NAME as above. Segments of third-order branches off the superior mesenteric artery (G3), 2 mm in length, were dissected from the mesenteric bed and cleared of connective and adipose tissue. The vessels were mounted on tungsten wires (40 μM diameter) on a Mulvany–Halpern myograph (Myo-Interface Model 410A, Danish Myo Technology, Denmark) (Mulvany and Halpern, 1977), kept at 37°C in Krebs–Henseleit buffer, gassed with 5% CO2/95% O2 and allowed to equilibrate under a tension of 5 mN (O'Sullivan et al., 2004). Tension was measured and recorded on a MacLab 4e recording system (ADInstruments, Oxford, UK).
Vessels were considered viable if tension increased by at least 5 mN in the presence of 60 mM KCl. If acceptable, the vessels were washed and left to equilibrate at a tension of 5 mN for 1 h. Following this, the vessels were washed and contracted three times with 60 mM KCl, and washed again to regain a basal tension of 5 mN. All vessels were contracted using a combination of the alpha-adrenoceptor agonist, methoxamine (5–30 μM), and the thromboxane-A2 analogue, U46619 (10–100 mM), to raise the tone by at least 5 mN. Once the contraction had stabilized, a cumulative concentration-response curve to anandamide (10 nM–30 μM) was constructed.
In vivo cardiovascular measurements
All surgery was carried out under general anaesthesia (fentanyl and medetomidine, 300 μg kg−1 of each, i.p.), which was reversed by nalbuphine and atipamezole (1 mg kg−1 of each, s.c.), with nalbuphine also providing analgesia. For the experiments in animals that received methanandamide and AM251, buprenorphine (0.02 mg kg−1 s.c.) was used in place of nalbuphine as the latter was no longer available.
The first surgical procedure was the implantation of miniaturized Doppler flow probes. This took place after 2 weeks of water/L-NAME-drinking. Probes were sutured around the left renal and superior mesenteric arteries, and around the distal abdominal aorta below the level of the ileocaecal artery allowing hindquarters flow to be measured.
Following another 2 weeks of water/L-NAME-drinking, the second stage of surgery, that is, catheterization, was conducted, subject to the animals passing veterinarian checks. This procedure used the same general anaesthesia as for probe implantation. Under anaesthesia, the wires attached to the Doppler flow probes were soldered into a plug that was held in a harness worn by the rat. Catheters were inserted into the distal abdominal aorta via the caudal artery, allowing measurement of arterial blood pressure and heart rate, and in the right jugular vein for drug administration. Following surgery, anaesthesia was reversed and analgesia provided as before. The animals were then left to recover for 24 h to ensure they were fully conscious and freely moving before commencement of experimentation. The catheters were connected to fluid-filled, double-channel swivels to allow overnight intra-arterial infusion of heparinized (15 U ml−1, 0.4 ml h−1) saline to maintain catheter patency, and intravenous (i.v.) infusion of saline (control rats; 0.4 ml h−1) or L-NAME (7.5 mg kg−1 day−1). The latter approach was taken to ensure constancy of L-NAME administration in the first 24 h postsurgery, when fluid intake would be expected to be lower than normal. Thereafter, L-NAME or saline was infused i.v. for the rest of the experimental period and all animals were given tap water to drink. At the time of experimentation, animals weighed 400–500 g.
On the morning of each experimental day, the arterial catheters were connected to a fluid-filled (degassed water) pressure transducer (Gould, type 4-442, OH, USA) with a modified, low volume-displacement dome. The pressure signal was fed into a Gould transducer amplifier (model 13-4615-50, OH, USA) and thence to a customized data capture system (Haemodynamics Data Acquisition System (HDAS), University of Limburg, Maastricht, Netherlands). The leads from the Doppler flow probes were connected to a Doppler flow meter (Crystal Biotech VF-1 Mainframe fitted with high velocity (HVPD-20) modules, Holliston, USA). This information was also recorded using HDAS. The system sampled the data every 2 ms, averaged every cardiac cycle and then stored to disc at 5 s intervals (or on a beat-by-beat basis for the first minute of responses to anandamide and methanandamide), enabling recording of heart rate, blood pressure and processed Doppler shift signals.
In vivo cardiovascular responses to anandamide and WIN55212-2
In one group of animals (n=9 controls, n=9 L-NAME treated), a 0.12 ml i.v. bolus dose of vehicle (Tocrisolve) was administered 1 h before four i.v. bolus doses of anandamide (0.1, 0.3, 1 and 3 mg kg−1), which were given in ascending order, separated by 60 min.
In the same animals, on the second experimental day, a 0.12 ml i.v. bolus dose of vehicle was administered 1 h before i.v. bolus doses of WIN55212-2 (50 and 150 μg kg−1), which were given in ascending order, separated by 60 min.
In vivo cardiovascular responses to methanandamide and AM251
In a separate group of animals (n=10 controls, n=8 L-NAME-treated), a 0.12 ml i.v. bolus dose of vehicle (Tocrisolve) was administered followed by an i.v. bolus dose of methanandamide (3 mg kg−1) 3 h later.
On the second experimental day, the same animals were infused i.v. with the CB1 receptor antagonist AM251 (3 mg kg−1, infused 2 ml h−1 over 30 min).
Data analysis
In vitro studies
Vasorelaxation data were expressed as concentration–response curves fitted by GraphPad Prism (version 4.00 for Windows, GraphPad Software, San Diego, CA, USA) using a sigmoidal logistic equation. In perfused mesenteric beds, the negative log of the anandamide concentration required to give a 50% relaxation of tone (pEC50) was calculated. Statistical significance between groups was calculated using a one-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test. For myography experiments, sigmoidal curves fitted by GraphPad Prism were used to calculate the negative log of the anandamide concentration that reduced tone by 50% (pEC50%). This value was used because sigmoidal response curves did not produce a plateau. Statistical significance between these groups was calculated using two-tailed, unpaired t-tests. All data were expressed as mean±s.e.m. with n representing the number of animals in each group.
In vivo studies
All in vivo data were analysed offline using Datview software (University of Limburg, Maastricht, Netherlands), which interfaced with HDAS, allowing the analyst to select and average data at chosen intervals during the experiment. Values were then extracted into custom-designed software (Biomed, University of Nottingham) to enable statistical tests to be performed. The Friedman's test (non-parametric version of ANOVA allowing for multiple comparisons) was used for within-group analysis and Mann—Whitney U tests were used for between group comparisons, where P⩽0.05 was taken as significant.
Drugs
All drugs used in vitro were supplied by Sigma Chemical Co. (UK), with the exception of anandamide and AM251, which were from Tocris, UK. Anandamide was dissolved in ethanol to 10 mM. AM251 was dissolved in dimethyl sulfoxide (DMSO) to 10 mM. Stock solutions were further diluted with distilled water.
For in vivo experimentation, fentanyl citrate was purchased from Martindale; medetomidine hydrochloride (Domitor) and atipamezole hydrochloride (Antisedan) were obtained from Pfizer; Du Pont supplied nalbuphine hydrochloride (Nubain). Buprenorphine (Vetergesic) was supplied by Alstoe Animal Health (York, UK). Anandamide, Tocrisolve, methanandamide, WIN-55212-2, ((R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo [1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate) and AM251 were obtained from Tocris UK, with anandamide and methanandamide supplied dissolved in Tocrisolve. AM251 solutions were made in saline with 5% propylene glycol (Sigma, Poole, UK) and 2% Tween-80 (BDH, Poole, UK). AM251 was infused (2 ml h−1) over 30 min, and all other drugs were administered as bolus i.v. injections given in a volume of 0.12 ml.
Results
Vasorelaxant responses to anandamide in vitro
In isolated mesenteric beds, perfused at 5 ml min−1, the basal perfusion pressure was measured as 40.8±2.3 mm Hg (n=6) for controls, 41.6±3.5 mm Hg (n=10) for beds from control rats with L-NAME in the perfusate and 53.4±9.1 mm Hg (n=9) for preparations from L-NAME-treated rats. Following administration of the spasmogen methoxamine, the maximum perfusion pressures before adding anandamide were 147±10, 125±6 and 132±7 mm Hg, respectively. These values did not differ significantly.
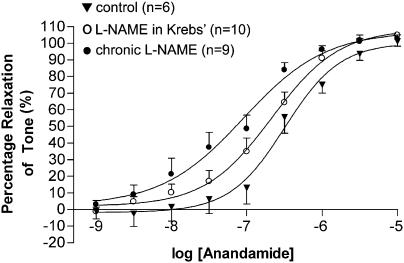
Anandamide (1 nM–10 μM) caused a concentration-dependent relaxation (Rmax 100±5% relaxation, pEC50=6.50±0.07, n=6) in control-isolated, perfused mesenteric vascular beds (Figure 1). The presence of L-NAME (300 μM) in the perfusate did not significantly affect the vasorelaxant response to anandamide (Rmax 109±3% relaxation, pEC50=6.69±0.04, n=10). However, the potency of anandamide was significantly (P<0.01) enhanced compared to controls (pEC50=7.05±0.11, n=9) in preparations taken from rats drinking L-NAME for 4 weeks, although reactivity was unaffected (Rmax 107±6% relaxation) (Figure 1).
Figure 1.
Concentration–response curves for the vasorelaxant responses to anandamide in perfused mesenteric beds of chronic L-NAME- and water-drinking rats, and control rats with L-NAME in the Krebs' solution. Values are mean and vertical bars show s.e.m.
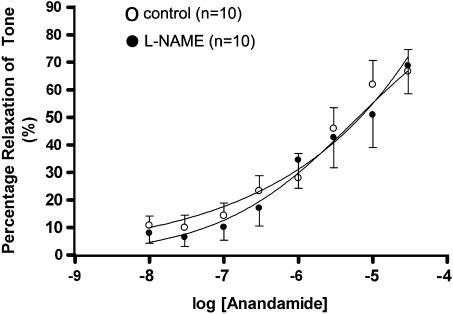
Anandamide (10 nM–30 μM) caused a concentration-dependent vasorelaxation in isolated third-order (G3) mesenteric arteries from control (pEC50%=5.73±0.25, n=10) and L-NAME-treated rats (pEC50%=5.60±0.26, n=10), with no difference in the effects of anandamide between preparations from control and L-NAME-treated rats (Figure 2).
Figure 2.
Concentration–response curves for the vasorelaxant effects of anandamide in third-order mesenteric vessels from chronic L-NAME- and water-drinking rats. Values are mean and vertical bars show s.e.m.
Cardiovascular responses to anandamide in conscious rats (Experiment 1)
Resting cardiovascular variables prior to administration of anandamide are shown in Table 1. In L-NAME-treated rats, resting mean arterial pressure was higher, and vascular conductances were lower (P⩽0.05, Mann–Whitney U-test) than the corresponding values in control rats. Heart rate was similar in controls and L-NAME-treated rats.
Table 1.
Basal cardiovascular variables
|
Experiment 1 |
Experiment 2 |
Experiment 3 |
||||
|---|---|---|---|---|---|---|
| Control | L-NAME | Control | L-NAME | Control | L-NAME | |
| Heart rate (beats min−1) | 390±13 | 365±17 | 352±8 | 359±11 | 351±7 | 346±12 |
| Systolic blood pressure (mm Hg) | 158±3 | 199±6* | 138±3 | 173±8* | 151±3 | 176±3* |
| Mean arterial pressure (mm Hg) | 121±3 | 153±6* | 107±2 | 137±7* | 113±3 | 133±3* |
| Diastolic blood pressure (mm Hg) | 99.3±3.6 | 126±5* | 88.2±1.8 | 114±6* | 91.6±3.2 | 107±3* |
| Renal VC ((kHz mm Hg−1) × 103) | 93.5±13.3 | 66.3±5.9* | 77.8±7.6 | 67.2±9.9 | 89.4±10.3 | 71.5±4.2 |
| Mesenteric VC ((kHz mm Hg−1) × 103) | 72.8±6.1 | 43.9±4.2* | 62.0±6.0 | 56.6±5.7 | 83.2±4.5 | 57.7±6.0* |
| Hindquarters VC ((kHz mm Hg−1) × 103) | 36.9±1.7 | 26.6±1.6* | 41.8±6.2 | 26.0±2.2* | 39.2±1.6 | 29.8±1.9* |
Abbreviations: L-NAME, NG-nitro-L-arginine methyl ester; VC=vascular conductance.
Basal cardiovascular variables (mean±s.e.m.) before administration of: anandamide (Experiment 1), methanandamide (Experiment 2) and WIN55212-2 (Experiment 3) in control and L-NAME-treated Sprague–Dawley rats.
Significant difference (P<0.05) to corresponding control rats (Mann–Whitney U-test).
Tocrisolve, the vehicle in which anandamide was dissolved, did not cause any consistent cardiovascular changes in either group, and at the lower doses (0.1 and 0.3 mg kg−1) the cardiovascular effects of anandamide were inconsistent in both groups of rats (data not shown).
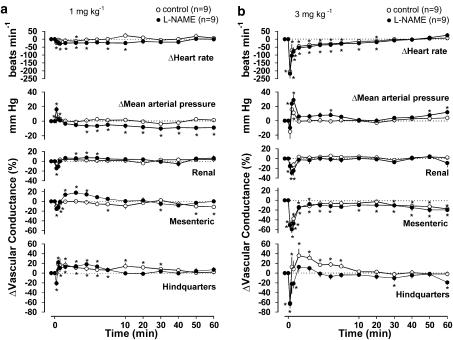
In control rats, anandamide (1 mg kg−1) caused an initial increase in blood pressure (10 s postdose), accompanied by short-lived mesenteric vasoconstriction and hindquarters vasodilatation without any consistent changes in the renal vasculature (Figure 3a). In L-NAME-treated rats, anandamide (1 mg kg−1) caused modest bradycardia and an initial rise in blood pressure, which was followed by a small, but sustained, fall in blood pressure (Figure 3a). There was an initial constriction and subsequent vasodilatation in all three vascular beds, although from 10 min after administration of anandamide, when blood pressure remained low, there was no significant vasodilatation in any vascular bed (Figure 3a).
Figure 3.
Cardiovascular changes elicited by anandamide (a, 1 mg kg−1; b, 3 mg kg−1) in the same conscious, control and L-NAME-treated, Sprague–Dawley rats. Values are mean and vertical bars show s.e.m. *Significant change versus baseline, P⩽0.05 (Friedman's test).
In control rats, anandamide (3 mg kg−1) caused a biphasic cardiovascular response. Initially (Phase 1, 10 s postdose), there was marked, transient bradycardia (−205±42 beats min−1), which rapidly reversed, although the heart rate generally remained below baseline for up to 20 min. The initial, marked bradycardia was accompanied by a transient depressor response (−15±10 mm Hg) and reduced conductances in the mesenteric and hindquarters vascular beds (Figure 3b). Following the acute depressor phase, there was a short-lived pressor response (Phase 2, 1 min postdose), accompanied by vasoconstriction in the renal and mesenteric vascular beds, but vasodilatation in the hindquarters. Thereafter, the mesenteric vascular bed remained constricted while the other cardiovascular variables returned to baseline values (Figure 3b). In rats treated with L-NAME, anandamide (3 mg kg−1) also caused a biphasic cardiovascular response, similar to that observed in controls, with Phase 1 bradycardia (−217±30 beats min−1) accompanied by a small depressor response (−6±3 mm Hg, not significant) and reduced conductances in the mesenteric and hindquarters vascular beds (Figure 3b). During Phase 2, the changes in heart rate, blood pressure and renal and mesenteric vascular conductances were not different from those in control rats, but there was no hindquarters vasodilatation in L-NAME-treated rats (Figure 3b).
Cardiovascular responses to methanandamide in conscious rats (Experiment 2)
In L-NAME-treated rats, before methanandamide (3 mg kg−1) administration, resting mean arterial pressure was higher, and hindquarters vascular conductance was lower than in control rats (P⩽0.05, Mann–Whitney U-test), but there were no differences between the heart rates, renal vascular conductances or mesenteric vascular conductances (Table 1).
In the control animals, Tocrisolve caused a slight initial increase in heart rate (+28.2±8.8 beats min−1, at 20 s) and a small fall in mesenteric vascular conductance (−8.4±2.6 (kHz mm Hg−1) × 103), but the changes were not sustained.
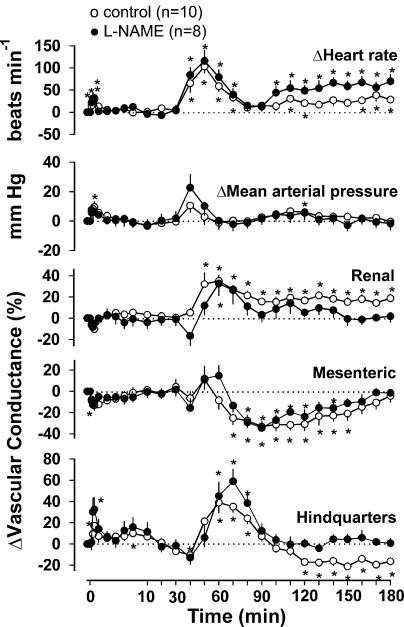
In control rats, methanandamide (3 mg kg−1) caused a small initial (at 30 s) tachycardia (+28.1±10.2 beats min−1), which was not different from the vehicle effect although there was also a small rise in blood pressure (+9.60±3.60 mm Hg), together with an increased hindquarters vascular conductance (+6.9±2.5 (kHz mm Hg−1) × 103) (Figure 4). Thereafter, starting at around 40 min after administration of methanandamide, more marked changes in the cardiovascular variables occurred, comprising tachycardia (maximum at 50 min, +103±15 beats min−1), an increase in renal vascular conductance (+35.1±5.1% at 60 min), a decrease in mesenteric vascular conductance (−25.1±6.2% at 70 min) and an increase (+39.0±9.8% at 60 min) followed by a decrease (−17.0±2.1% at 120 min) in hindquarters vascular conductance.
Figure 4.
Cardiovascular changes elicited by methanandamide (3 mg kg−1 i.v.) in conscious, control and L-NAME-treated, Sprague–Dawley rats. Values are mean and vertical bars show s.e.m. *Significant change versus baseline, P⩽0.05 (Friedman's test).
In L-NAME-treated rats, the initial small response to methanandamide was similar to that of the control rats, and the subsequent responses were generally comparable, except that the renal vasodilatation was more transient and no delayed hindquarters vasoconstriction was observed (Figure 4).
Cardiovascular responses to WIN55212-2 in conscious rats (Experiment 3)
Before administration of WIN55212-2 (50 μg kg−1), mean arterial pressure was higher, and mesenteric and hindquarters vascular conductances were lower (P⩽0.05, Mann–Whitney U-test) in L-NAME-treated rats than in control rats, but heart rates and renal vascular conductances were not significantly different (Table 1).
Following administration of WIN55212-2 (50 μg kg−1) to control and L-NAME-treated rats, there was a pressor response accompanied by a fall in mesenteric vascular conductance lasting up to 20 min (Figure 5a). Although the magnitude of the changes in heart rate were similar in the two groups of rats, the changes were only significant in the control rats (Figure 5a).
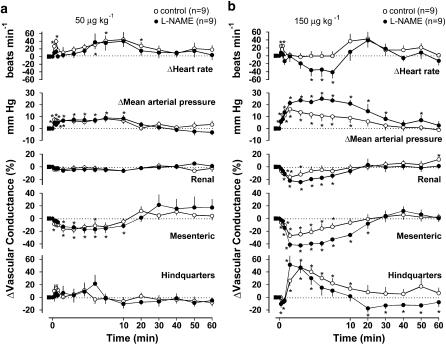
Figure 5.
Cardiovascular changes elicited by WIN55212-2 (a, 50 μg kg−1; b, 150 μg kg−1 i.v.) in the same conscious, control and L-NAME-treated, Sprague–Dawley rats. Values are mean and vertical bars show s.e.m. *Significant change versus baseline, P⩽0.05 (Friedman's test).
The high dose of WIN55212-2 (150 μg kg−1) caused an increase in blood pressure, a fall in mesenteric and renal vascular conductances, and an increase in hindquarters vascular conductance in both groups of rats (Figure 5b). The increase in blood pressure and decrease in mesenteric vascular conductance in response to WIN55212-2 in the L-NAME-treated rats were significantly greater (Mann–Whitney U-test on integrated (0–10 min) responses, P⩽0.05) than in the control group. In the control group, there was an initial transient tachycardia, whereas in the L-NAME-treated group, bradycardia developed (Figure 5b). In L-NAME-treated rats, there was a delayed hindquarters vasoconstriction starting 20 min after administration of WIN55212-2, which did not occur in the controls (Figure 5b).
Cardiovascular responses to AM251 in conscious animals (Experiment 4)
Cardiovascular variables before and 60 min following administration of AM251 in both groups of rats are shown in Table 2. In control rats there was a small, significant increase in mesenteric vascular conductance, and in L-NAME-treated rats there was a small, significant increase in hindquarters vascular conductance, but otherwise there were no significant effects of AM251.
Table 2.
Cardiovascular variables before and 60 min after administration of AM251 (3 mg kg−1) in control and L-NAME-treated Sprague–Dawley rats (Experiment 4)
|
Control |
L-NAME |
|||
|---|---|---|---|---|
| Basal | 60 min | Basal | 60 min | |
| Heart rate (beats min−1) | 363±11 | 360±14 | 373±14 | 396±7# |
| Mean arterial pressure (mm Hg) | 112±2 | 112±2 | 137±9# | 149±8# |
| Renal VC ((kHz mm Hg−1) × 103) | 81.5±6.8 | 84.3±6.5 | 56.4±8.3# | 59.6±9.6# |
| Mesenteric VC ((kHz mm Hg−1) × 103) | 62.4±7.8 | 68.7±8.2* | 54.9±6.8 | 51.8±5.4 |
| Hindquarters VC ((kHz mm Hg−1) × 103) | 47.7±5.1 | 47.2±3.2 | 28.0±2.3# | 31.2±2.9* # |
Abbreviations: L-NAME, NG-nitro-L-arginine methyl ester; VC, vascular conductance.
Values are mean±s.e.m.
P⩽0.05 versus basal within group (Friedman's test).
P<0.05 versus corresponding value in control group (Mann–Whitney U-test).
Discussion and conclusions
Against the background of evidence showing enhanced vasorelaxant responses to anandamide in isolated mesenteric vascular beds following chronic NOS inhibition (Mendizábal et al., 2001), together with reports of augmented endocannabinoid activity in hypertensive conditions (Bátkai et al., 2004), the present study was designed to test the hypotheses that: (1) in rats made hypertensive by chronic NOS inhibition, upregulation of the endocannabinoid system would appear as greater vasodilator responses to anandamide in vivo and (2) upregulation of CB1 receptor-mediated events would be evident as pressor and vasoconstrictor responses to the CB1-receptor antagonist, AM251, and augmented vasodilator responses to the synthetic CB receptor agonist, WIN55212-2. Our in vivo results showed no augmentation of any vasodilator effect of anandamide, no significant pressor and/or vasoconstrictor effect of AM251 and no enhancement of any vasodilator effect of WIN55212-2 in L-NAME-treated rats and thus our hypotheses cannot be accepted.
The dose of L-NAME used in this work was about 10-fold less than that used by Mendizábal et al. (2001), but it was clearly sufficient to substantiate their findings. Following our original description of the oral activity of L-NAME (Gardiner et al., 1990), two independent groups described long-term effects of L-NAME drinking in rats, one of which used a dose of ∼5 mg kg−1 day−1 (Baylis et al., 1992), and the other used a dose of ∼60 mg kg−1 day−1 (Ribeiro et al., 1992). As discussed elsewhere (Dunn and Gardiner, 1995; Zatz and Baylis, 1998; Gardiner et al., 1999), the mechanisms underlying the hypertension may become more complex with higher doses of L-NAME, including activation of the renin–angiotensin system. Therefore we reasoned that the dose we used (∼7.5 mg kg−1 day−1) was appropriate for studying a putative effect of NOS inhibition. We had shown in a pilot study, in which blood pressures were measured by radiotelemetry, that it caused an increase of 17±3 mm Hg after 28 days (Tep-Areenan et al., 2002), and in the present series of experiments, L-NAME-treated rats were consistently hypertensive and vascular conductances were generally reduced, although the differences in vascular conductances between the groups were not always significant. (It should be noted, however, that vascular conductances are calculated from the Doppler shift signal, and the latter depends not only on blood flow velocity, but also the angle of the crystal in the probes which varies to some extent. Hence, absolute values should be used cautiously). Although we used a lower dose of L-NAME than used by Mendizábal et al. (2001), and the degree of hypertension was not as extreme as in, for example, the SHRs used by Bátkai et al. (2004), we do not believe that the level of hypertension can explain our inability to measure enhanced vasorelaxant effects of the cannabinoids in vivo, since scrutinising the within-group data, it is clear that individual animals with the highest mean arterial pressures (∼155 mm Hg) did not show greater vasorelaxant responses. Furthermore, we are confident that there was a considerable degree of NOS inhibition in our L-NAME-treated animals, since elsewhere (Wakefield et al., 2003), we have shown that with the degree of L-NAME-induced hypertension seen here, there is almost complete inhibition of the vasodilator effect of acetylcholine in vivo.
The pilot study (Tep-Areenan et al., 2002) also showed enhanced vasorelaxant responses to anandamide in perfused mesenteric vascular beds from rats given this dose of L-NAME for 28 days and this was confirmed here. Thus, the findings of Mendizábal et al. (2001) can be extended to conditions where the extent of NOS inhibition is likely to be less. However, the present results with in vitro myography and the in vivo observations indicate that the augmentation of the vasorelaxant potency of anandamide observed in perfused mesenteric vascular beds may not pertain to the resistance vasculature.
In contrast to the findings in whole vascular beds, there was no difference between the effects of anandamide in isolated G3 mesenteric blood vessels from control and L-NAME-treated rats. A reason for the clear differences between the whole mesenteric arterial bed and the G3 small mesenteric arteries could reflect the heterogeneity in the action of anandamide throughout the vascular tree. Specifically, O'Sullivan et al. (2004) reported that anandamide acted by a variety of mechanisms in G3 vessels (including vanilloid receptors, CB1 receptors and an endothelial receptor coupled EDHF release), while in the central mesenteric artery vanilloid receptors appeared to play a greater role. It is possible that L-NAME-induced hypertension may have influenced sensory nerve activity or function in the larger vessels, leading to enhanced relaxation, but in the smaller vessels where vanilloid receptors may have less of a role, the augmentation was not apparent.
The cardiovascular responses to anandamide in vivo are complex. A triphasic blood pressure response to i.v. anandamide has been described in vivo (Varga et al., 1995; Lake et al., 1997; Malinowska et al., 2001). Phase 1 occurs soon after administration and comprises a vagally-mediated bradycardia associated with hypotension, which may involve TRPV1 receptors (Malinowska et al., 2001; Smith and McQueen, 2001; Pacher et al., 2004), and has been shown to be accompanied by vasoconstriction (Gardiner et al., 2002a). Thus, the lack of any augmentation of this phase in L-NAME-treated rats is not surprising, although others have shown greater blood pressure and heart rate responses in another model of hypertension, that is, spontaneously hypertensive rats (Li et al., 2003).
Phase 2 follows shortly after with hypertension (Varga et al., 1995; Lake et al., 1997; Malinowska et al., 2001), which may be centrally or peripherally mediated (Kwolek et al., 2005), and we have previously shown this phase to be associated with renal and mesenteric vasoconstriction, and hindquarters vasodilatation that is β2-adrenoceptor mediated (Gardiner et al., 2002a). The present results show that the hindquarters vasodilatation is inhibited in L-NAME-treated rats, which is consistent with evidence that indicates that a large part of β2-adrenoceptor-mediated vasorelaxation may be NO-dependent (Gardiner et al., 1991; MacDonald et al., 1999; Ferro et al., 2004).
Phase 3 of the cardiovascular response to anandamide has been shown to occur in anaesthetized, but not conscious, normotensive rats (Varga et al., 1996) and in conscious, hypertensive animals (Lake et al., 1997). It occurs approximately 5–10 min after administration, is characterized by a delayed hypotension, and may be associated with vasodilatation in some vascular beds (Kunos et al., 2000). Anandamide-induced hypotension is absent in CB1 receptor-knockout mice (Járai et al., 1999; Ledent et al., 1999) and in the presence of SR141716A (Lake et al., 1997; Malinowska et al., 2001). Wang et al. (2005) also proposed the involvement of TRPV1 receptor-mediated CGRP release in the associated vasodilatation. We predicted that Phase 3 would occur in L-NAME-treated rats as in other hypertensive animals. At 1 mg kg−1, anandamide did cause a small delayed, prolonged fall in blood pressure in the L-NAME-treated rats, but this was not associated with long-lasting vasodilatation, and was not evident with the higher dose. Since doses were given sequentially, receptor downregulation could have occurred over time, but this is unlikely, since Phase 3 remained absent when the highest dose of anandamide (3 mg kg−1) was administered alone to some naïve rats (unpublished data).
Furthermore, to optimize the likelihood of eliciting Phase 3, the present study also used the stable anandamide analogue, methanandamide, in naïve animals, but as with anandamide, there was no evidence of enhanced vasodilator responses or hypotension in the L-NAME-treated animals. To our knowledge, this is the first description of the effects of methanandamide in conscious animals with prolonged cardiovascular monitoring postdosing. Malinowska et al. (2001) found that anandamide and methanandamide both resulted in a triphasic response under anaesthesia, but methanandamide was more potent. However, here the immediate effects of methanandamide were smaller than with the same dose of anandamide. Perhaps, this was because methanandamide was administered to naïve rats whereas anandamide was given in increasing doses, but this is unlikely since the effects of anandamide (3 mg kg−1) are not different in naïve rats compared to rats previously exposed to anandamide (see above). Another difference between the group of rats given anandamide and the group given methanandamide was that the former was given nalbuphine as an analgesic, whereas the latter was given buprenorphine (see Methods). However, it is most unlikely that this would have affected our results, as the analgesics were given at least 24 h before the cannabinoids, and they are both partial mixed opioid agonists. In the study of Malinowska et al. (2001), anandamide was given in vehicle used by us (Tocrisolve) whereas methanandamide was in ethanol. As the vascular effect of anandamide can vary depending on the vehicle used (Lopez-Miranda et al., 2004), the potency of methanandamide is lower in Tocrisolve than in ethanol in vitro (W-S V Ho, unpublished observations), and the effect of methanandamide at TRPV1 receptors is lower than anandamide (Ross et al., 2001), it is possible that in our experiments using methanandamide in Tocrisolve, we were below the threshold for activation of TRPV1 receptors.
Most unexpected in the present study was the delayed response to methanandamide, which developed after approximately 40 min, suggesting induction of some process and/or effects of metabolites. There is evidence to suggest that methanandamide induces expression of cyclo-oxygenase (COX)-2, independent of CB1 and TRPV1 receptors (Rösch et al., 2006), and that COX-derived eicosanoids mediate a vasoconstrictor effect of anandamide in cirrhosis (Yang et al., 2006). Thus, it is feasible that methanandamide-induced COX-2 activity resulted in delayed, complex haemodynamic effects via vasoconstrictor and vasodilator prostanoids. This was not seen with anandamide because it is quickly metabolized (Willoughby et al., 1997). The underlying mechanisms for this response will be the subject of further investigations but whatever the explanation, it was clear that the effects were not different in L-NAME-treated animals.
Administration of WIN55212-2 and AM251 allowed the study of CB receptor-mediated cardiovascular responses more directly. In contrast to findings by Bátkai et al. (2004), where CB1 receptor antagonism resulted in a pressor response in several models of hypertension, here, administration of AM251 had no significant effect on blood pressure in rats made hypertensive by L-NAME administration, suggesting that there was no upregulation of endocannabinoids after chronic NOS-inhibition. Furthermore, there was no enhancement of vasodilator effects of WIN55212-2 in L-NAME-treated rats.
The cardiovascular responses to WIN55212-2 described here are consistent with those reported previously by Gardiner et al. (2002b). In that study, the WIN55212-2-induced hindquarters vasodilatation was β2-adrenoceptor-mediated; therefore, it was anticipated that it would be inhibited in L-NAME-treated animals, as with anandamide (see above). However, this was not the case. Currently, we have no clear explanation for this, although it is notable that the pressor and mesenteric vasoconstrictor effects of WIN55212-2 were enhanced in the L-NAME-treated animals and were accompanied by bradycardia. The bradycardia was possibly a baroreceptor-mediated reflex, which, if it involved withdrawal of sympathetic tone, may have contributed to the hindquarters vasodilatation. As the pressor and vasoconstrictor effects of WIN55212-2 have been found to be sympathetically-mediated (Gardiner et al., 2002b), amplification of these responses in L-NAME-treated rats is consistent with evidence for augmented sympathetic activity in this model (Scrogin et al., 1998; Souza et al., 2001).
In summary, despite the in vitro evidence from perfused mesenteric vascular beds that anandamide is a more potent vasorelaxant after chronic NOS inhibition, our current myographic study showed that this did not pertain to the resistance vasculature. Furthermore, the in vivo results provide no evidence for upregulation of endogenous cannabinoids or their receptors in this hypertensive model.
Acknowledgments
Many thanks to JE March and PA Kemp for providing their technical assistance, and to the British Heart Foundation (PG 03/142) for funding this study.
Abbreviations
- AM251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- CB
cannabinoid
- EDHF
endothelium-derived hyperpolarizing factor
- G3
third-order branch of the superior mesenteric artery
- HDAS
Haemodynamics Data Acquisition System
- L-NAME
NG-nitro-L-arginine methyl ester
- TRPV1
transient receptor potential vanilloid 1 receptors or vanilloid receptors
- WIN55212-2
(R)-(+)-(2,3-dihydro-5-methyl-3-[(morphonolinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazin-yl)(1-napthalenyl)methanone mesylate
Conflict of Interest
The authors state no conflict of interest.
References
- Bátkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J, et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest. 1992;90:278–281. doi: 10.1172/JCI115849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor AT, Martin PE, Evans WH, Randall MD, Griffith TM. The endothelial component of cannabinoid-induced relaxation in rabbit mesenteric artery depends on gap junctional communication. J Physiol. 1999;520:539–550. doi: 10.1111/j.1469-7793.1999.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HH, Das SK, et al. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn WR, Gardiner SM. No evidence for vascular remodelling during hypertension induced by chronic inhibition of nitric oxide synthase in Brattleboro rats. J Hypertens. 1995;13:849–857. doi: 10.1097/00004872-199508000-00005. [DOI] [PubMed] [Google Scholar]
- Ferro A, Coash M, Yamamoto T, Rob J, Ji Y, Queen L. Nitric oxide-dependent beta2-adrenergic dilatation of rat aorta is mediated through activation of both protein kinase A and Akt. Br J Pharmacol. 2004;143:397–403. doi: 10.1038/sj.bjp.0705933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, Compton AM, Bennett T, Palmer RM, Moncada S. Regional haemodynamic changes during oral ingestion of NG-monomethyl-L-arginine or NG-nitro-L-arginine methyl ester in conscious Brattleboro rats. Br J Pharmacol. 1990;101:10–12. doi: 10.1111/j.1476-5381.1990.tb12079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, Dunn WR, Bennett T. Chronic nitric oxide inhibition model six years on. Hypertension. 1999;34:e4. doi: 10.1161/01.hyp.34.5.e4. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Kemp PA, Bennett T. Effects of NG-nitro-L-arginine methyl ester on vasodilator responses to acetylcholine, 5′-N-ethylcarboxamidoadenosine or salbutamol in conscious rats. Br J Pharmacol. 1991;103:1725–1732. doi: 10.1111/j.1476-5381.1991.tb09854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T. Complex regional haemodynamic effects of anandamide in conscious rats. Br J Pharmacol. 2002a;135:1889–1896. doi: 10.1038/sj.bjp.0704649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T. Influence of the CB1 receptor antagonist, AM 251, on the regional haemodynamic effects of WIN-55212-2 or HU 210 in conscious rats. Br J Pharmacol. 2002b;136:581–587. doi: 10.1038/sj.bjp.0704750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D, McCulloch AI, Kendall DA, Randall MD. Characterization of vasorelaxant responses to anandamide in the rat mesenteric arterial bed. J Physiol. 2002;539:893–902. doi: 10.1113/jphysiol.2001.013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Mukhopadhyay S. Cellular signal transduction by anandamide and 2-arachidonoylglycerol. Chem Phys Lipids. 2000;108:53–70. doi: 10.1016/s0009-3084(00)00187-0. [DOI] [PubMed] [Google Scholar]
- Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrahian A, Hillard CJ. Arachidonylethanolamide (anandamide) binds with low affinity to dihydropyridine binding sites in brain membranes. Prostaglandins Leukot Essent Fatty Acids. 1997;57:551–554. doi: 10.1016/s0952-3278(97)90559-7. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Heald SL, Dally RD, Janis RA. Isolation, identification and synthesis of an endogenous arachidonic amide that inhibits calcium channel antagonist 1,4-dihydropyridine binding. Prostaglandins Leukot Essent Fatty Acids. 1993;48:429–437. doi: 10.1016/0952-3278(93)90048-2. [DOI] [PubMed] [Google Scholar]
- Kunos G, Jarai Z, Batkai S, Goparaju SK, Ishac EJ, Liu J, et al. Endocannabinoids as cardiovascular modulators. Chem Phys Lipids. 2000;108:159–168. doi: 10.1016/s0009-3084(00)00194-8. [DOI] [PubMed] [Google Scholar]
- Kwolek G, Zakrzeska A, Schlicker E, Gothert M, Godlewski G, Malinowska B. Central and peripheral components of the pressor effect of anandamide in urethane-anaesthetized rats. Br J Pharmacol. 2005;145:567–575. doi: 10.1038/sj.bjp.0706195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake KD, Martin BR, Kunos G, Varga K. Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension. 1997;29:1204–1210. doi: 10.1161/01.hyp.29.5.1204. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Li J, Kaminski NE, Wang DH. Anandamide-induced depressor effect in spontaneously hypertensive rats: role of the vanilloid receptor. Hypertension. 2003;41:757–762. doi: 10.1161/01.HYP.0000051641.58674.F7. [DOI] [PubMed] [Google Scholar]
- Lopez-Miranda V, Herradon E, Dannert MT, Alsasua A, Martin MI. Anandamide vehicles: a comparative study. Eur J Pharmacol. 2004;505:151–161. doi: 10.1016/j.ejphar.2004.10.017. [DOI] [PubMed] [Google Scholar]
- MacDonald A, McLean M, MacAulay L, Shaw AM. Effects of propranolol and L-NAME on beta-adrenoceptor-mediated relaxation in rat carotid artery. J Auton Pharmacol. 1999;19:145–149. doi: 10.1046/j.1365-2680.1999.00128.x. [DOI] [PubMed] [Google Scholar]
- Malinowska B, Kwolek G, Gothert M. Anandamide and methanandamide induce both vanilloid VR1- and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:562–569. doi: 10.1007/s00210-001-0498-6. [DOI] [PubMed] [Google Scholar]
- Mendizábal VE, Orliac ML, Adler-Graschinsky E. Long-term inhibition of nitric oxide synthase potentiates effects of anandamide in the rat mesenteric bed. Eur J Pharmacol. 2001;427:251–262. doi: 10.1016/s0014-2999(01)01272-9. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA, Randall MD. Heterogeneity in the mechanisms of vasorelaxation to anandamide in resistance and conduit rat mesenteric arteries. Br J Pharmacol. 2004;142:435–442. doi: 10.1038/sj.bjp.0705810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offertaler L, Mo FM, Batkai S, Liu J, Begg M, Razdan RK, et al. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J Physiol. 2004;558:647–657. doi: 10.1113/jphysiol.2004.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall MD, Alexander SP, Bennett T, Boyd EA, Fry JR, Gardiner SM, et al. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem Biophys Res Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- Randall MD, Kendall DA, O'Sullivan S. The complexities of the cardiovascular actions of cannabinoids. Br J Pharmacol. 2004;142:20–26. doi: 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro MO, Antunes E, de Nucci G, Lovisolo SM, Zatz R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension. 1992;20:298–303. doi: 10.1161/01.hyp.20.3.298. [DOI] [PubMed] [Google Scholar]
- Rösch S, Ramer R, Brune K, Hinz B. R(+)-methanandamide and other cannabinoids induce the expression of cyclooxygenase-2 and matrix metalloproteinases in human nonpigmented ciliary epithelial cells. J Pharmacol Exp Ther. 2006;316:1219–1228. doi: 10.1124/jpet.105.092858. [DOI] [PubMed] [Google Scholar]
- Ross RA, Gibson TM, Brockie HC, Leslie M, Pashmi G, Craib SJ, et al. Structure–activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br J Pharmacol. 2001;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrogin KE, Hatton DC, Chi Y, Luft FC. Chronic nitric oxide inhibition with L-NAME: effects on autonomic control of the cardiovascular system. Am J Physiol. 1998;274:R367–R374. doi: 10.1152/ajpregu.1998.274.2.R367. [DOI] [PubMed] [Google Scholar]
- Smith PJ, McQueen DS. Anandamide induces cardiovascular and respiratory reflexes via vasosensory nerves in the anaesthetized rat. Br J Pharmacol. 2001;134:655–663. doi: 10.1038/sj.bjp.0704296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza HC, Ballejo G, Salgado MC, Da Silva VJ, Salgado HC. Cardiac sympathetic overactivity and decreased baroreflex sensitivity in L-NAME hypertensive rats. Am J Physiol Heart Circ Physiol. 2001;280:H844–H850. doi: 10.1152/ajpheart.2001.280.2.H844. [DOI] [PubMed] [Google Scholar]
- Tep-Areenan P, March JE, Kemp PA, Randall MD, Kendall DA, Bennett T, et al. Effects of chronic in vivo treatment with nitric oxide synthase inhibitor on vasorelaxant responses to anandamide in rat isolated arteries. Br J Pharmacol. 2002;137:55P. [Google Scholar]
- Varga K, Lake K, Martin BR, Kunos G. Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur J Pharmacol. 1995;278:279–283. doi: 10.1016/0014-2999(95)00181-j. [DOI] [PubMed] [Google Scholar]
- Varga K, Lake KD, Huangfu D, Guyenet PG, Kunos G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension. 1996;28:682–686. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Varga K, Jarai Z, Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- Wakefield ID, March JE, Kemp PA, Valentin JP, Bennett T, Gardiner SM. Comparative regional haemodynamic effects of the nitric oxide synthase inhibitors, S-methyl-L-thiocitrulline and L-NAME, in conscious rats. Br J Pharmacol. 2003;139:1235–1243. doi: 10.1038/sj.bjp.0705351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kaminski NE, Wang DH. VR1-mediated depressor effects during high-salt intake: role of anandamide. Hypertension. 2005;46:986–991. doi: 10.1161/01.HYP.0000174596.95607.fd. [DOI] [PubMed] [Google Scholar]
- White R, Hiley CR. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat mesenteric artery. Br J Pharmacol. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Hiley CR. The actions of the cannabinoid receptor antagonist, SR 141716A, in the rat isolated mesenteric artery. Br J Pharmacol. 1998;125:689–696. doi: 10.1038/sj.bjp.0702127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby KA, Moore SF, Martin BR, Ellis EF. The biodisposition and metabolism of anandamide in mice. J Pharmacol Exp Ther. 1997;282:243–247. [PubMed] [Google Scholar]
- Yang YY, Lin HC, Huang YT, Lee TY, Hou MC, Wang YW, et al. Roles of anandamide in the hepatic microcirculation in cirrhotic rats. Am J Physiol Gastrointest Liver Physiol. 2006;290:G328–G334. doi: 10.1152/ajpgi.00367.2005. [DOI] [PubMed] [Google Scholar]
- Zatz R, Baylis C. Chronic nitric oxide inhibition model six years on. Hypertension. 1998;32:958–964. doi: 10.1161/01.hyp.32.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]