Abstract
The E6 and E7 oncoproteins of the high-risk HPV type16 represent ideal targets for HPV vaccine development, they being consistently expressed in cervical cancer lesions. Since HPV-16 is primarily transmitted through genital mucosal route, mucosal immune responses constitute an essential feature for vaccination strategies against HPV-associated lesions. We present here evidence showing that mucosal immunization of mice by the intranasal route with a mixture of peptides E744–62 and E643–57 from the E7 and E6 oncoproteins of HPV-16, respectively, using a mutant cholera toxin adjuvant (CT-2*), primed strong antigen-specific cellular immune responses in systemic and mucosal tissues. Significant levels of IFN-γ production by both CD4 and CD8 cells were observed along with CTL responses that were effective against both peptide-pulsed targets as well as syngeneic tumor cells (TC-1) expressing the cognate E6 and E7 proteins. Furthermore, mice immunized with the peptide mixture and CT-2* effectively resisted TC-1 tumor challenge. These results together with our earlier observations that T cell responses to these peptides correlate with recurrence-free survival in women after ablative treatment for HPV-associated cervical intraepithelial neoplasia, support the potential of these E6 and E7 peptides for inclusion in vaccine formulations.
Keywords: HPV16 E7 and E6 peptides, mucosal immunization, mutant cholera toxin
INTRODUCTION
Papillomaviruses are small DNA viruses that infect vertebrate hosts, including humans and cause the formation of hyperproliferative epithelial lesions [1]. The Oncogenic high-risk types of human papillomaviruses (HPV) such as HPV-16 are the main causative factors in the pathogenesis of cervical carcinomas [2]. Several strategies for HPV16 vaccines have been developed and evaluated in animal models. These included the use of recombinant E7 protein [3], DNA vaccine encoding E7 [4], and bacterial/viral vectors expressing E7 or E7 epitopes [5–7] as well as CTL epitope peptides of E7 [8]. All of these strategies represent parenteral routes of immunization, but HPV is a sexually transmitted mucosal pathogen and therefore, mucosal vaccination may be necessary for protection. Both the oral and intranasal routes constitute attractive strategies for priming systemic as well as mucosal immunity against HPV.
Delivery of virus like particles (VLPs), corresponding to HPV16 and 18, by the oral route was shown to be effective in generating antigen-specific immune responses in mice and these could be significantly enhanced by the use of mucosal adjuvants like, LT192G, the non-toxic mutant form of Escherishia coli heat-labile enterotoxin (LT), or GpG-containing oligodeoxynucleotides [9]. However, intranasal immunization has emerged as the optimal vaccination strategy in rodents for induction of antibody responses in genital tissues [10–12] and was also shown to be effective in human studies [11, 13, 14]. Intranasal immunization of mice with HPV16 L1 protein or the HPV16 L1 gene in combination with the adjuvant cholera toxin was shown to elicit systemic and mucosal humoral and cellular immune responses [15]. Recently, it was reported that intranasal immunization of mice with live lactococci expressing the E7 antigen and IL-12 induced systemic and mucosal immune responses and also protected mice against challenge with an E7-expressing murine tumor cell line TC-1 [16].
Vaccines based on peptide antigens have been proposed and pursued by several groups for a variety of pathogens because the advantages related to safety and ease of production, but their weak immunogenic properties must be overcome through the use of adjuvants, fusion proteins, or anchor-modified peptide epitopes. The adjuvant properties of cholera toxin (CT), an enterotoxin produced by Vibrio cholerae have been described extensively [17, 18]. Since the native CT is unsuitable for human use because of its toxic effects, many mutants lacking toxicity but retaining their adjuvanticity have been developed and characterized [19, 20]. The mucosal adjuvanticity of CT-2*, a cholera toxin mutant derived by introducing two-codon substitutions (Arg7-Lys and Glu112-Gln) into the CT-A subunit [21] was reported earlier [22] where intranasal (i.n.) administration of a CTL epitope peptide from HIV-1 with CT-2* generated strong mucosal and systemic immune responses. Here, we obtained data demonstrating that i.n. administration of a combination of HPV-16 E7 44–62 and E6 43–57 peptides along with CT-2* as the adjuvant induced antigen-specific cellular immune responses at various mucosal and systemic compartments and protected mice against challenge with TC-1 tumor cells expressing the E6 and E7 oncoproteins of HPV-16.
2. MATERIALS AND METHODS
2.1. Animals
Female C57BL/6 mice of 6–8 weeks age were purchased from NCI. All the procedures for handling the animals were carried out in accordance with institutionally approved protocols. The animals were housed in microisolator cages and provided with sterile food and water. The animal facility is fully accredited by the Association for Assessment and Accreditation of Laboratory Animals Care International. The studies were conducted according to National Institute of Health Guidelines on the care and use of Laboratory Animals.
2.2. Cell Lines and cell cultures
The cell lines EL-4 (C57BL/6, H-2b, Thymoma) and YAC-1 were maintained in RPMI complete media (CM) supplemented with 10% heat-inactivated FBS, 50 U/ml of penicillin-streptomycin and 50μg/ml gentamycin. The TC-1 tumor cells are primary lung epithelial cells of C57BL/6 mice origin that were transfected to express the E6 and E7 oncogenes of HPV-16 as described earlier [23] and were a kind gift from Dr. T-C. Wu, Johns Hopkins Medical Institution, Baltimore, MD. The TC-1 cells were grown in complete RPMI supplemented with 400 μg/ml of G418. The E.G7-Ova cells were EL-4 thymoma cells transfected with chicken ovalbumin [24] and were a kind gift from Dr. Chen, Dept of Immunology, UTMD Anderson Cancer Center. They were maintained in DMEM supplemented with 10 % heat–inactivated FBS, 2 mM glutamine, 100 U/ml penicillin, 100 μg streptomycin and 400 μg/ml G418.
2.3. Peptides
The E744–62 peptide, Q19D, (QAEPDRAHVYNIVTFCCKCD) and the E643–57 peptide, Q15L, (QLLRREVYDFAFRDL) were used for immunization of mice. In addition to these peptides, the E749–57 peptide, R9F, (RAHVYNIVTF) and the E649–58 peptide, V10C, (VYDFAFRDLC), that represent murine H2b-restricted CTL epitopes, were used in the CTL assays. All the peptides were prepared in the institutional antigen-core facility utilizing FMOC solid phase chemistry on a PTI Symphony Peptide Synthesizer (Protein Technologies Inc., Tucson, Arizona). Peptide purity was determined to be >95% by high-pressure liquid chromatography (HPLC) and was validated by mass spectrometry.
2.4. Mutant cholera toxin (CT-2*)
The CT-2* protein, a mutant form of the cholera toxin, produced by a vaccine strain of V. cholerae, was purified to homogeneity by sodium hexametaphosphate precipitation, affinity purification on a galactose column and Sephadex G75 gel filtration chromatography as described earlier [25–28]. The purified toxin was dissolved in pyrogen-free water, and the lipopolysaccharide (LPS) contamination was determined by the Limulus amebocyte lysate assay (QCL-1000kit, BioWhitaker, Walkerville, MD). The amount of LPS detected in 1 μg of CT-2* (amount used as adjuvant in mice) was 0.5 pg, which did not stimulate production of any cytokine in the mouse ligated ileal loops [25–28].
2.5. Immunizations
Mice (n=5) were immunized by the intranasal route twice at 5-day intervals with a mixture of the Q19D and Q15L peptides (100 μg of each/mouse) along with CT-2* (1 μg/mouse). The mice were anaesthetized by the intraperitoneal injection of ketamine-xylaxine mixture and were immunized by introducing a small volume (10–15 μl) consisting of the peptide mixture and the CT2* adjuvant in PBS into each nostril. Five days after the last immunization, mice were sacrificed and cell suspensions were prepared from the spleen, cervical lymph nodes (CLN), mesentric lymph nodes (MLN), Peyer’s patches (PP), and the vaginal-associated lymphoid tissue (VALT) by homogenization or enzymatic dissociation using collagenase type IV (Sigma).
2.6. CTL assay
The CTL assay was carried out as described previously [29]. Briefly, cells isolated from different tissues were restimulated for 5 days with the cognate peptide mixture used for immunizing the mice. The cytolytic activity of the restimulated cells was assayed against syngeneic 51Cr-labeled EL-4 cells that were incubated with either medium or the individual cognate peptides. Additional targets included the 51Cr-labeled TC-1 cells expressing the E6 and E7 oncogenes of HPV-16. Unlabeled Yac-1 cells were mixed with the labeled TC-1 cells to eliminate contribution from the NK cell lysis for calculating the antigen-specific CTL activity. The percentage (%) of specific lysis was calculated using the following formula: % specific lysis = (experimental release−spontaneous release)/(maximum release−spontaneous release) × 100, where the spontaneous release represents the radioactivity obtained when the target cells were incubated in culture medium without effectors and maximum release represents the radioactivity obtained when the target cells were lysed with 1 % Triton X-100.
2.7. Intracellular cytokine staining and flow cytometry analysis
Cells isolated from the various tissues were stimulated with the individual E6 and E7 peptides used for immunizing mice, and the percentages of CD4+ and CD8+ T cells producing IFN-γ were determined by the intracellular cytokine flow cytometry [30]. The peptides for stimulating the various cells were used at a concentration of 2μg/ml for 4 h at 37°C, with the Golgistop reagent (1 μl/ml, Pharmingen, San Diego, CA) added for an additional 8h before harvesting the cells from the culture. Cells were then washed once with FACS buffer (PBS + 1% FBS + 0.1 % sodium azide) and stained with the following florochrome-conjugated monoclonal rat anti-mouse antibodies (Pharmingen, San Diego, CA): CD3-APC, CD8-FITC and CD4-PerCP. Cells were then subjected to intracellular cytokine staining using the Cytofix/Cytoperm kit according to the manufacturer’s instructions (Pharmingen) and PE-conjugated anti-IFN-γ antibody or the immunoglobulin isotype control antibody (rat IgG1), both purchased from Pharmingen. Sample acquisition was done on a FACScalibur and analyzed using the Flowjo 6.4.2 software (Becton Dickinson, CA).
2.8. Measurement of IFN-γ production by ELISPOT assay
Cells isolated from spleen, CLN, and MLN of immunized mice were subjected to ELISPOT assay for antigen specific IFN-γ-producing cells as described earlier [22] using the kit from Pharmingen, San Diego, CA. The spots, representing individual IFN-γ-producing cell as spot forming cells (SFC), on the membrane were enumerated by Zellnet Consulting Inc., New York, NY using the KS-ELISPOT automatic system (Carl Zeisis Inc., Thornwood, NY). Responses were considered positive when they were above 50 SFC/well and at least double the number obtained in cells cultured with medium alone. A non-specific negative peptide was included as another control. Peptide specific and negative control responses were compared with the responses of medium and p values ≤ 0.05 (*) were considered significant.
2.9. In Vivo tumor protection experiments
Two groups of mice (n=5) were immunized by the i.n. route twice at 5d intervals with the mixture of the HPV-16 peptides E7 44–62 and E6 43–57 (100 μg each) along with the mutant cholera toxin, CT-2* (1 μg). Five days after the last immunization, one group of mice were injected in the right flank by the subcutaneous route with 2 × 105 TC-1 tumor cells, and the other group of mice, that served as a negative control group, were injected with 5 × 106 EG7.Ova tumor cells. A separate group of naïve unvaccinated mice (n=5) injected with the TC-1 tumor cells (2 × 105) served as another negative control group. Mice in all the groups were monitored twice a week for tumor growth, and the tumor size was measured using a caliper and was recorded as mean diameter: longest surface length (a) and width (b), and the tumor size calculated using the formula, (a+b)/2. Mice were euthanized when the tumor size reached 20 mm in mean diameter.
3. RESULTS
3.1 Intranasal immunization with a combination of the E7 peptide Q19D and the E6 peptide Q15L of HPV-16 along with the mutant cholera toxin CT-2* adjuvant primes antigen-specific CTL that lyse syngeneic TC-1 tumor cells expressing the cognate proteins
A group of five C57BL/6 mice were intranasally immunized twice at five-day intervals with a mixture of HPV-16 peptides Q19D and Q15L (E744–62 and E643–57, respectively) admixed with CT-2*, a two-codon mutant of cholera toxin, observed in our earlier studies to be effective as a mucosal adjuvant [22]. Analyses of cells from the spleens and draining cervical lymph nodes (CLN) showed strong CTL activity against target cells pulsed with either Q19D or Q15L peptides (Fig. 1). The specific lysis values obtained at an effector to target cell (E:T) ratio of 100:1 were 75% in spleen and 50% in CLN for Q19D peptide and 58% in spleen and 40% in CLN for Q15L peptide. These responses were found to be highly significant when compared to EL-4 targets (p ≤ 0.00005). These effectors also lysed EL-4 target cells pulsed with peptides R9F (E749–57) and V10C (E649–58), (p ≤ 0.05), the CTL epitopes within the Q19D and Q15L sequences, respectively. Furthermore, significant lysis of the TC-1 tumor cells that express the E7 and E6 oncoproteins of HPV-16 was also observed by the spleen and CLN cells from these immunized mice. The specific lysis values at an E:T ratio of 100:1 were 38% and 20% for the spleen and the CLN cells, respectively (Fig. 1). The values were compared to those obtained with EG7.Ova targets and were found to be highly significant (p ≤ 0.00005). No CTL activity was observed both in the spleen and CLN against either the Yac-1 cells (NK targets) or the EG7.Ova cells (syngeneic tumor cells expressing the non-specific ovalbumin antigen) used as targets (Fig. 1) indicating the antigen-specificity of the CTL responses induced.
Fig. 1.
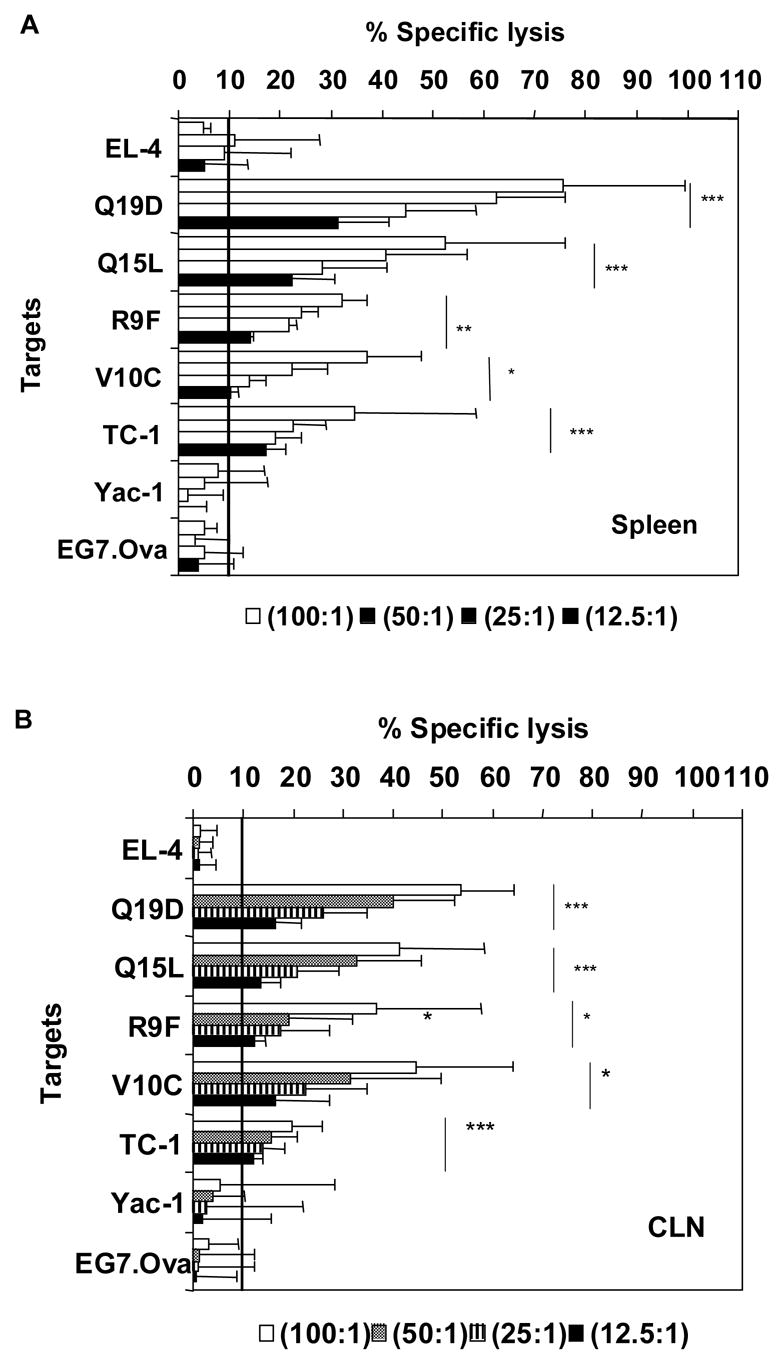
Intranasal immunization with the mixture of E744–62 (Q19D) and E643–57 (Q15L) peptides from HPV-16 using the mutant cholera toxin (CT-2*) adjuvant induces CTL responses. C57Bl/6 mice were immunized by the intranasal route with peptides Q19D and Q15L (100 μg each) and CT-2* (1 μg) a total of 2 times at 5 day intervals. Cells isolated from the spleen and cervical lymph nodes (CLN) were restimulated for 5 days in vitro with Q19D and Q15L peptides before assaying for CTL activity. (A) The restimulated spleen cells (panel A) and CLN (panel B) were tested for CTL activity against MHC-matched EL-4 target cells pulsed with Q19D, R9F, Q15L or V10C peptides or TC-1 tumor cells. The 51Cr-labelled TC-1 cells were mixed with unlabelled YAC-1 cells that serve as targets for NK activity, to determine the tumor-cell-specific lysis. The EG7.Ova tumor targets expressing Ova protein served as negative control target cells for ascertaining the antigen specific lysis of the TC-1 cells. The horizontal line in each panel indicates the cut-off value for positive response (10% antigen-specific lysis). Data shown is an average of at least three separate experiments. Student t-test analysis was done by comparing with the EL-4 empty targets to determine the p values. Values ≤ 0.00005 have been assigned = ***, p ≤ 0.0005 = ** and p ≤ 0.005 = *.
3.2 Immunization with the mixture of the Q19D and Q15L peptides induces IFN-γ production by CD4+ and CD8+ T cells from the systemic and mucosal compartments
We also determined whether the mucosal immunization protocol adopted was effective in priming antigen-specific cellular immune responses in different mucosal and systemic compartments. For this, cells isolated from different tissues of mice immunized with the mixture of Q19D and Q15L peptides along with the CT2* adjuvant were assayed for antigen-specific IFN-γ production by CD8+ and CD4+ T cells. As shown in figure 2A we observed significant percentages of CD8+ T cells producing IFN-γ in response to stimulation with the Q19D peptide in the spleen (7.88%), the draining cervical lymph nodes (4.58%), as well as multiple mucosal tissues like the MLN (6.26%), Peyer’s patches (9.41%), and the VALT (14.5%). Similar responses were observed in the cells from the various tissues in response to stimulation with the Q15L peptide (7.92% in spleen, 5.95% in CLN, 5.96% in MLN, 11.7% in PP, and 11.3% in VALT). We also observed strong IFN-γ production by CD4+ T cells in response to both Q19D and Q15L (Fig. 2B). No positive CD8 and CD4 responses were observed for all the tissues with the negative control peptide used for stimulation (Fig. 2A & 2B). Overall, the amounts of IFN-γ producing CD8+ T cells were comparable to that of CD4+ T cells in response to Q19D as well as Q15L peptides (Fig 2C).
Fig. 2.
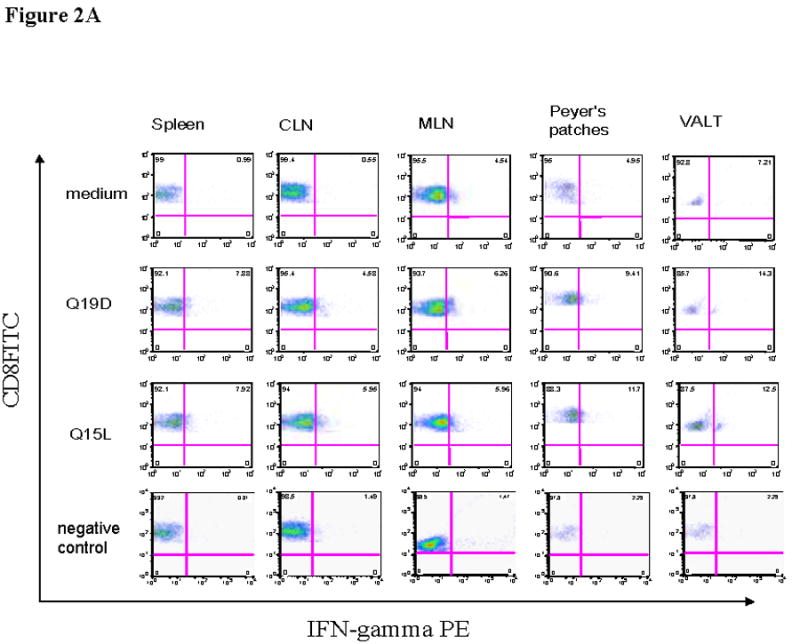
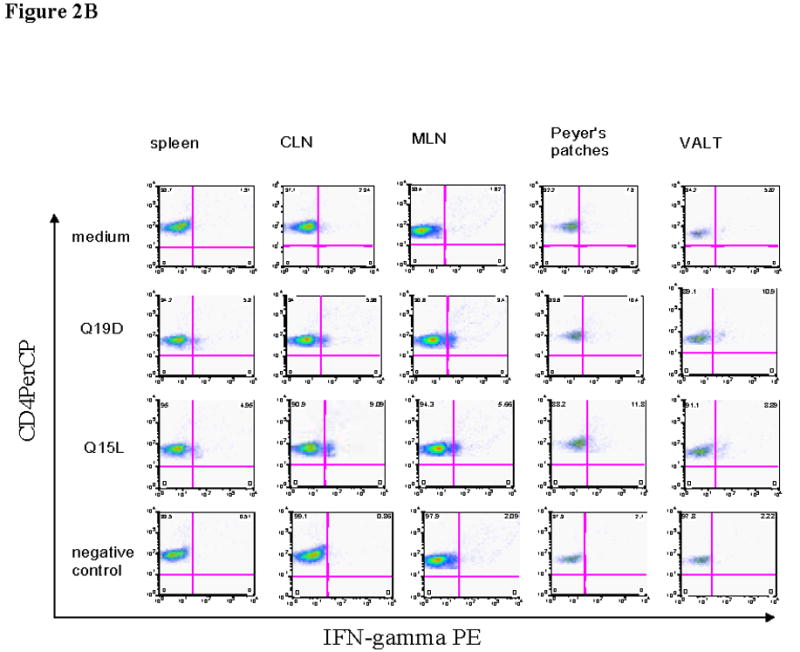
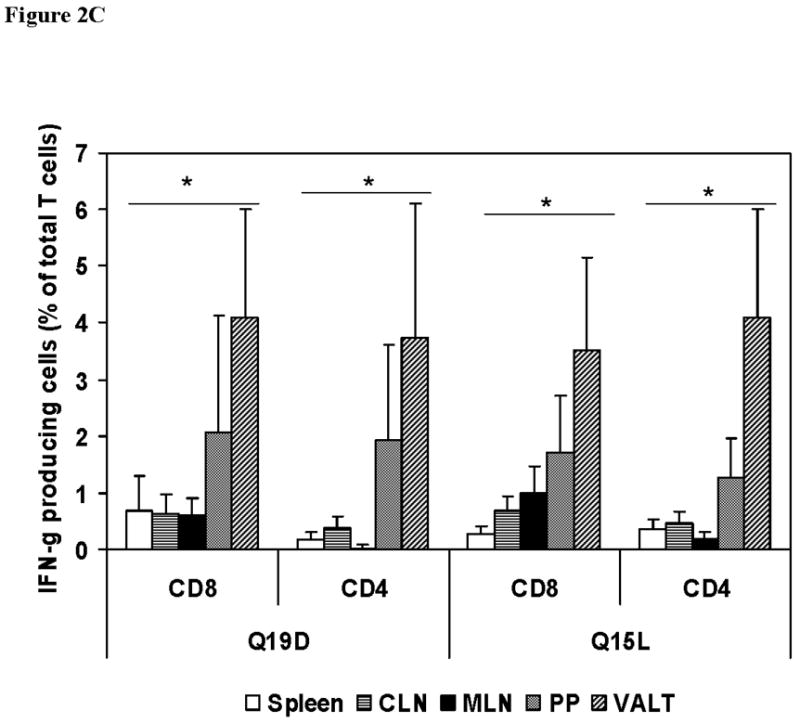
Intranasal immunization with the mixture of E744–62 (Q19D) and E643–57 (Q15L) peptides from HPV-16 using the mutant cholera toxin (CT-2*) adjuvant primes antigen-specific IFN-γ production by CD4+ and CD8+ T cells. Cells isolated from various mucosal and systemic tissues of C57BL/6 mice immunized by the intranasal route with mixture of Q19D and Q15L peptides were stimulated in vitro using medium cognate peptides or a non-specific negative control peptide individually (2μg/ml) for determining the IFN-γ producing in (A) CD8+ and (B) CD4+ T cells. Cells were stained for CD3, CD4 and CD8 surface markers and for intracellular IFN-γ as described in the methods section. The numbers of IFN-γ-producing T cells were analyzed by flow cytometry and the percentages of CD8+/IFN-γ+ CD4+/IFN-γ+ (double-positive) T cells are shown in the upper right corner of each dot plot. The data shown is one representative of 5 separate experiments performed. (C) the bar graph shows the average values along with standard errors of percentages of CD8+/IFN-γ+ CD4+/IFN-γ+ (double-positive) T cells in 1 ×106 cells specific to peptides Q19D and Q15L in spleen, CLN, MLN, peyer’s patches and VALT. The p values were determined by comparing with medium alone and values ≤ 0.05 (*) are considered significant.
We also measured the number of antigen-specific IFN-γ producing cells generated by intranasal immunization with the HPV peptides and CT-2* on the day of sacrifice by ELISPOT assay. Figure 3A depicts the number of IFN-γ spot forming cells observed in the spleens of mice immunized with CT-2* and the combination of HPV16 HPV16E7 44–62 and E6 43–57 peptides. The number of spot forming cells in the spleen was significantly higher for the peptides (Q19D and Q15L) when compared to those in the medium alone control (p ≤ 0.05). Similar analyses were performed using cells isolated from the CLN and MLN. As shown in figure 3B positive peptide specific responses were significantly higher than the medium control (p ≤ 0.05) in mice immunized with CT-2* and the combination of HPV16 HPV16E7 44–62 and E6 43–57 peptides. Responses to the negative peptide were not positive according to the criteria described in the methods section in spleen (Fig 3A), CLN and MLN (Fig 3B).
Fig. 3.
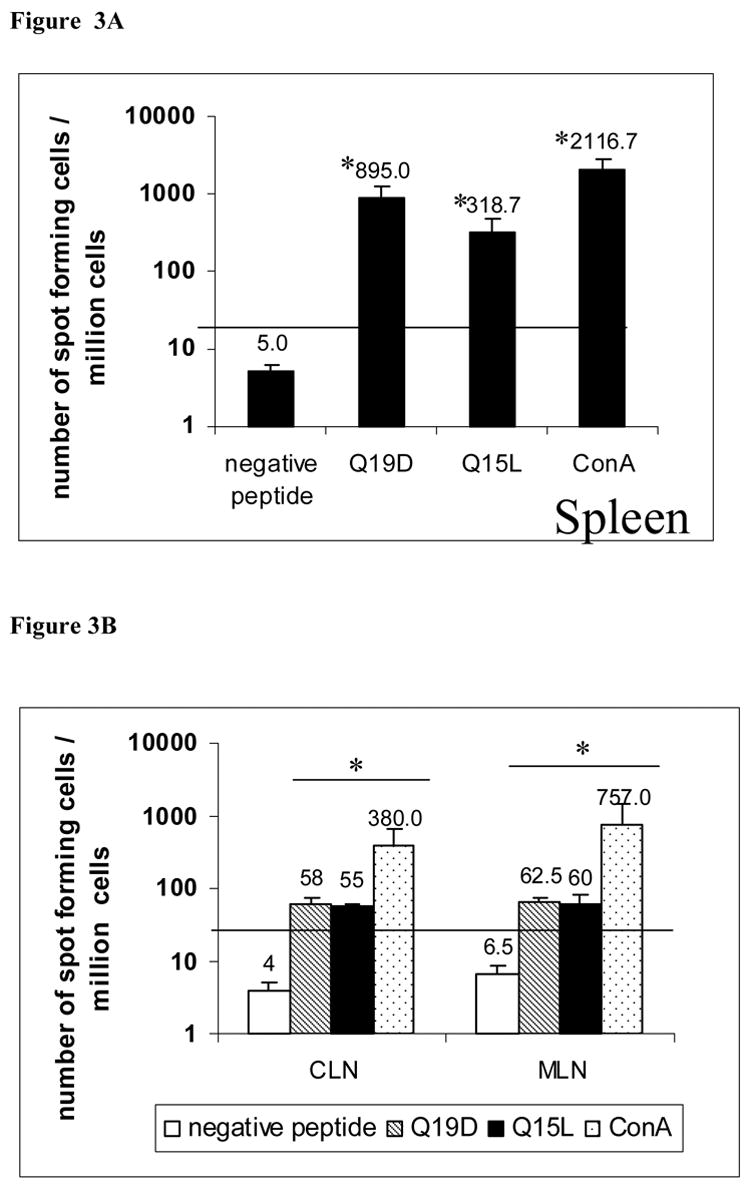
Induction of peptide specific IFN-γproducing cells by intranasal immunization with the mixture of E744–62 (Q19D) and E643–57 (Q15L) peptides from HPV-16 using the mutant cholera toxin (CT-2*) as adjuvant. Cells isolated from spleen, CLN and MLN of C57BL/6 mice immunized by the intranasal route with mixture of Q19D and Q15L peptides were stimulated in vitro using medium, cognate peptides or a non-specific negative control peptide individually (2μg/ml) in a 96 well plate ELISPOT. After 48h the plates were washed and developed and the number IFN-γproducing spot forming cells were enumerated in each well and the number of spot forming cells calculated per 1 ×106 cells is plotted. Average values and errors bars were calculated from 2 separate experiments consisting of 3 mice in each. Responses were considered positive when they were above 50 SFC/well and at least double the number obtained in cells cultured with medium alone (this cut-off value shown as horizontal line in the two panels). The p values were calculated comparing with the medium alone control and values ≤ 0.05 (*) are considered significant.
3.3 Protection against TC-1 tumor growth by intranasal immunization with the mixture of the Q19D and Q15L peptides
In order to determine the in vivo efficacy of the cellular immune responses induced after i.n. immunization of mice with the combination of the Q19D and Q15L peptides using the CT2* adjuvant, we adopted a tumor-challenge model. The TC-1 cells are syngeneic to the C57BL/6 mice and express the E6 and E7 oncoproteins of HPV-16 [23]. A group of naïve mice injected with the TC-1 tumor cells served as unvaccinated controls (group 1). At seven days after the second i.n. immunization, mice were injected with either the EG7.Ova tumor cells that are syngeneic but express the irrelevant ovalbumin antigen (group 2) or the TC-1 tumor cells (group 3). Mice in all the three groups were monitored for tumor development over 15 days. As shown in Fig. 4A, seven days after injection of TC-1 tumor cells mice in group 1 developed tumors with a mean size of 14.15 ± 1.15 mm. Similarly, mice in group 2 that received the EG7.Ova tumor cells, tumors started growing by day seven and reached a mean size of 11.15 ± 0.36 mm. However, mice in group 3 immunized with the HPV peptides (Q19D and Q15L along with the CT2* antigen) and injected with TC-1 tumors had no measurable tumors (p<0.05 as compared with EG7.Ova group and naïve group). By day 15, naïve mice injected with the TC-1 tumor cells (group 1) as well as the mice injected with the EG7.Ova tumor cells (group 2) showed tumors measuring and 17.0 ± 0.5 mm and 16.15 ± 0.23, respectively (Fig 4A). On the other hand, mice in group 3 immunized with the HPV peptides and injected with TC-1 tumor cells did not develop any measurable tumors by day 15. Analyses of the data for the percentages of mice in each group that were free of tumor over a period of 60 days follow-up showed 15% of the naïve unimmunized mice challenged with TC-1 tumors (group 1) and 20% of immunized mice challenged with the EG7.Ova tumors (group 2) were without tumors (Fig. 4B) while none in the group 3 mice showed tumors. Between days 15–18 days, all the mice in the groups 1 and 2 developed large tumors while 100% of the mice immunized with the mixture of E7 and E6 peptides (Q19D and Q15L, respectively) along with the CT-2* as adjuvant and challenged with the TC-1 tumors were tumor-free throughout the follow-up period of 60 days. These results clearly demonstrate the efficacy of intranasal immunization with the mixture of the HPV-16 peptides against the TC-1 tumor challenge.
Fig. 4.
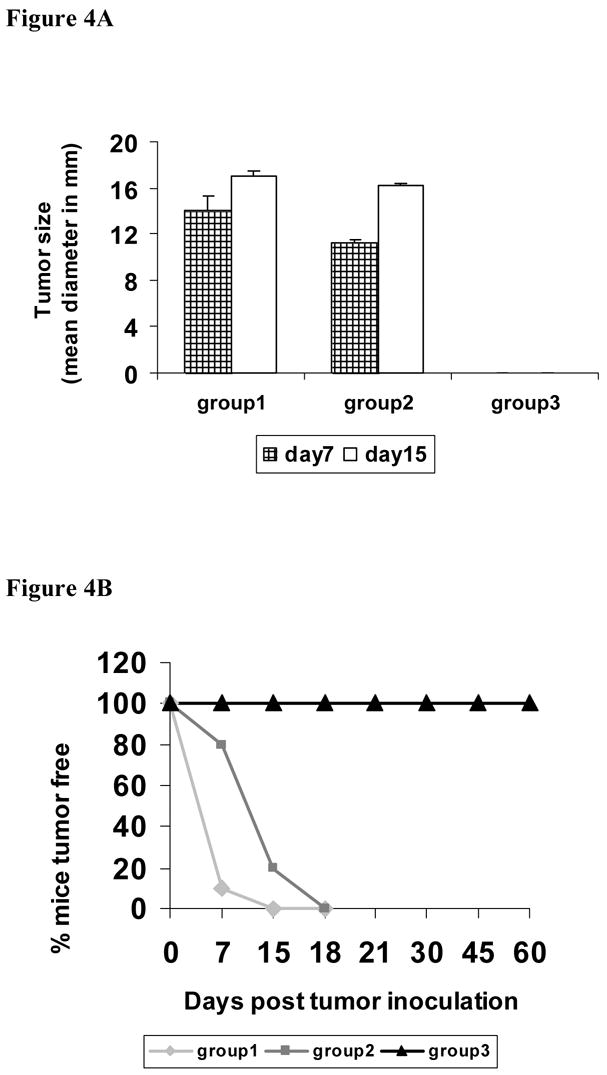
Intranasal immunization with the mixture of E744–62 (Q19D) and E643–57 (Q15L) peptides from HPV-16 using the mutant cholera toxin (CT-2*) adjuvant protects mice against challenge with the TC-1 tumor cells. Three experimental groups of C57BL/6 mice were included: naïve unimmunized mice were injected subcutaneously on the right flank with 1 × 105 TC-1 tumor cells per mouse (group 1, n=6); mice immunized intranasally with the mixture of Q19D and Q15L peptides (100 μg each) along with CT-2* adjuvant (1 μg) twice at 5 day intervals and one week after the last immunization injected subcutaneously on the right flank with either 1 × 106 EG7.Ova tumor cells per mouse (group 2, n=6) or 2 × 105 TC-1 tumor cells per mouse (group 3, n=10). The size of the tumors (length+width/2) on days 7 and 15 was measured in all three groups were measured and the average values plotted (panel A). Panel B shows the percentages of mice tumor-free over a period of 60 days follow-up in the three different groups: Data for group 1 is shown as light gray line, as dark gray line for group 2, and as black line for group 3. Statistical analysis between the 3 groups was done using the students t-test.
4. DISCUSSION
Both the E7 and E6 oncoproteins of HPV are expressed in pre-neoplastic as well as cancerous lesions of the cervix and represent potential targets for prophylaxis and immunotherapy approaches. Most studies to date focused on the development of vaccines employing either the whole E7 or E6 proteins in separate formulations or individual immunogenic peptides corresponding to these proteins [30–33]. Peptide-based vaccines have gained much importance over the last decade as they are relatively easy to construct and produce, chemically stable, and unlike the E6 and E7 proteins, lack oncogenic potential. Many investigators have developed powerful paradigms to choose peptides from the E6 and E7 oncoproteins of HPV-16 for immunization and established the pre-clinical evaluation to develop peptide-based tumor vaccines [8, 34, 35].
In a previous study [36] we demonstrated the immunogenic potential of the Q19D and Q15L peptides from the E7 and E6 oncoprotein of HPV-16 (E744–62 and E643–57, respectively), delivered individually by the parenteral route using the Freund’s complete adjuvant, for inducing antigen-specific CTL responses in mice. A 35-amino acid E7 peptide overlapping with the Q19D peptide, but also encompassing an E7-derived Th epitope, potentiated antigen specific CTL responses in mice [32]. The Q15L peptide from the E6 oncoprotein used in our study overlaps with a murine CTL epitope peptide (E6 48–57, presented by H-2Kb) reported by Peng et al [37]. Results from the present investigation demonstrated that intranasal immunization with a mixture of the E7 and E6 peptides, Q19D and Q15L, using the mucosal adjuvant CT-2*, induced strong antigen-specific T cell responses that afforded protection against challenge with tumor cells expressing the E6 and E7 oncoproteins of HPV-16. Importantly, antigen-specific T cell responses, in terms of IFN-γ producing CD4+ and CD8+ T cells in response to the peptides used for immunization of mice were observed in the VALT. No positive peptide specific antibody responses (IgG and IgA) were observed in the serum, saliva and vaginal washes (data not shown) tested at different time points by the immunization procedure followed (2 doses at 5 day intervals). The significance of this data lies in the fact that HPV is a sexually transmitted pathogen, and the development of an HPV vaccine/adjuvant formulation capable of eliciting mucosal immune responses, including at the genital mucosal surfaces, may be important for protection against HPV-associated cervical lesions. Furthermore, in a previous cross-sectional study we observed a significant association of cellular immune responses specific to these E7 and E6 peptides, Q19D and Q15L, with recurrence-free survival of women that underwent ablative treatment for HPV-associated cervical intraepithelial neoplasia.
Even though literature reports described DNA vaccines based on the E7 protein or the immunodominant CTL epitopes to be effective in generating HPV-specific systemic immunity [30], prevalence of antigen specific T cell responses at distant mucosal sites has not been investigated in detail. Similarly, the efficacy of delivering the E7 protein along with CpG-oligodeoxynucleotide (ODN), a strong immunomodulatory agent [38–41], for priming systemic but not mucosal immune responses was reported by Tae-Yoon Kim et al., [31]. One recent report described intranasal immunizations with an adeno-associated virus (AAV2) vector construct expressing the codon-optimized major capsid gene L1 (L1H) from HPV-16 to induce long-lasting humoral and cellular immune responses [42]. In our study, we show for the first time that co-delivery of peptides from the E7 and E6 proteins of HPV-16 along with the non-toxic mucosal adjuvant CT-2 * by the intranasal route elicited strong systemic and mucosal cellular immune responses along with anti-tumor efficacy. We believe that an ideal vaccine for the prophylaxis and therapy of HPV-associated cancers should prime strong immunity at multiple mucosal sites and exhibit protective efficacy, and results from the present investigation provide strong support for the utility of these E6 and E7 peptides and the intranasal delivery strategy for potential clinical testing.
Acknowledgments
All the culture media were produced by the central media lab, and all the synthetic peptides were prepared in the Synthetic Antigen Core Facility, both supported by funds from NIH grant CA 16672.
Studies in this manuscript are supported in part by funds from the NCI grant CA077378, NIAID AI42694, institutional grant MRP from MD Anderson Cancer Center.
Footnotes
None of the authors have a commercial association that might pose conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shah KV, Howley PM. Papillomaviruses. Virology. 1996;3 (2):2077–2110. [Google Scholar]
- 2.Zur Hausen H. Papillomavirus infections—a major cause of human cancers. Biochim Biophys Acta. 1996;1288:F55–F78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]
- 3.Fernando GJ, Murray B, Zhou J, Frazer IH. Expression, purification and immunological characterization of the transforming protein E7, from cervical cancer-associated human papillomavirus type 16. Clin Exp Immunol. 1999;115(3):397–403. doi: 10.1046/j.1365-2249.1999.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hung CF, Cheng WF, Hsu KF, Chai CY, He L, Ling M, Wu T-C. Cancer immunotherapy using a DNA vaccine encoding the translocation domain of a bacterial toxin linked to a tumor antigen. Cancer Res. 2001;9(61):3698–703. [PubMed] [Google Scholar]
- 5.Londono LP, Chatfield S, Tindle RW, Herd K, Gao XM, Frazer I, Dougan G. Immunisation of mice using Salmonella typhimurium expressing human papillomavirus type 16 E7 epitopes inserted into hepatitis B virus core antigen. Vaccine. 1996;6(14):545–52. doi: 10.1016/0264-410x(95)00216-n. [DOI] [PubMed] [Google Scholar]
- 6.Liu DW, Tsao YP, Kung JT, Ding YA, Sytwu HK, Xiao X, Chen SL. Recombinant adeno-associated virus expressing human papillomavirus type 16 E7 peptide DNA fused with heat shock protein DNA as a potential vaccine for cervical cancer. J Virol. 2000;6(74):2888–94. doi: 10.1128/jvi.74.6.2888-2894.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng WF, Hung CF, Hsu KF, Chai CY, He L, Polo JM, Slater LA, Ling M, Wu T-C. Cancer immunotherapy using Sindbis virus replicon particles encoding a VP22-antigen fusion. Hum Gene Ther. 2002;4(13):553–568. doi: 10.1089/10430340252809847. [DOI] [PubMed] [Google Scholar]
- 8.Feltkamp MC, Smits HL, Vierboom MP, Minnaar RP, De Jongh BM, Drijfhout JW, ter Schegget J, Melief CJ, Kast WM. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1996;1993;23:2242–2249. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 9.Gerber S, Lane DM, Brown E, Lord M, Dilorenzo M, Clements JD, Rybicki E, Williamson A-L, Rose RC. Human Papillomavirus Virus-Like Particles are efficient oral immunogens when coadministered with Escherichia coli heat-labile enterotoxin mutant R192G or CpG DNA. J Virol. 2001;75(10):4752–4760. doi: 10.1128/JVI.75.10.4752-4760.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamura S, Shoji Y, Hasiguchi K, Aizawa C, Kurata T. Effects of cholera toxin adjuvant on IgE antibody response to orally or nasally administered ovalbumin. Vaccine. 1994;12(13):1328–40. doi: 10.1016/0264-410x(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 11.Johansson EL, Rask C, Fredriksson M, Eriksson M, Czerkinsky C, Holmgren J. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect Immun. 1998;66(2):514–20. doi: 10.1128/iai.66.2.514-520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nardelli-Haefliger D, Benyacoub J, Lemoine R, Hopkins-Donaldson S, Potts A, Hartman F, Kraehenbuhl JP, De Grandi P. Nasal vaccination with attenuated Salmonella typhimurium strains expressing the Hepatitis B nucleocapsid: dose response analysis. Vaccine. 2001;19(20–22):2854–61. doi: 10.1016/s0264-410x(01)00009-3. [DOI] [PubMed] [Google Scholar]
- 13.Russell MW, Moldoveanu Z, White PL, Sibert GJ, Mestecky J, Michalek SM. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the Cholera toxin B subunit. Infect Immun. 1996;64(4):1272–83. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergquist C, Johansson EL, Lagergard T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun. 1997;65(7):2676–84. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupuy C, Buzoni-Gatel D, Touze A, Daniel Bout, Coursaget P. Nasal immunization with human papillomavirus type 16 (HPV-16) virus-like particles or with the HPV-16 L1 gene elicits specific cytotoxic T lymphocytes in vaginal draining lymp nodes. J Virol. 1999;73(11):9063–9071. doi: 10.1128/jvi.73.11.9063-9071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bermudez-Humaran LG, Cortes-Perez NG, Lefevre F, Guimaraes V, Rabot S, Alcocer-Gonzalez JM, Jean-Jacques G, Rodriguez-Padilla C, Tamez-Guerra RS, Corthier G, Gruss A, Langella P. A novel mucosal vaccine based on live Lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type16-induce tumors. J Immunol. 2005;175:7297–7302. doi: 10.4049/jimmunol.175.11.7297. [DOI] [PubMed] [Google Scholar]
- 17.Simmons CP, Mastroeni P, Fowler R, et al. MHC class I-restricted cytotoxic lymphocyte responses induced by enterotoxin-based mucosal adjuvants. J Immunol. 1999;163(12):6502–10. [PubMed] [Google Scholar]
- 18.Simmons CP, Hussell T, Sparer T, Walzl G, Openshaw P, Dougan G. Mucosal delivery of a respiratory syncitial virus CTL peptide with enterotoxin-based adjuvants elicits protective, immunopathogenic, and immunoregulatory antiviral CD8+ T cell response. J Immunol. 2001;166(2):1106–13. doi: 10.4049/jimmunol.166.2.1106. [DOI] [PubMed] [Google Scholar]
- 19.Hagiwara Y, Komas K, Chen Z, Matsuo K, Suzuki Y, Aizawa C, Kurata T, Tamura S-I. Mutants of cholera toxin as an effective and safe adjuvant for nasal influenza vaccine. Vaccine. 1999;17:2918–2926. doi: 10.1016/s0264-410x(99)00135-8. [DOI] [PubMed] [Google Scholar]
- 20.Pizza M, Guiliani MM, Fontana MR, Monaci E, Douce G, Dougan G, Mills KHG, Rappuoli R, Giudice GD. Mucosal vaccines: non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine. 2001;19:2534–2541. doi: 10.1016/s0264-410x(00)00553-3. [DOI] [PubMed] [Google Scholar]
- 21.Hasse CC, Thai LS, Boesman-Finkelstein M, et al. Construction and characterization of recombinant Vibrio cholerae strains producing inactive cholera toxin analogs. Infect Immun. 1994;62(8):3051–7. doi: 10.1128/iai.62.8.3051-3057.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lomada D, Gambhira R, Nehete PN, Guhad FA, Chopra AK, Peterson JW, Sastry KJ. A two-codon mutant of cholera toxin lacking ADP-ribosylating activity functions as an effective adjuvant for eliciting mucosal and systemic cellular immune responses to peptide antigens. Vaccine. 2004;23:555–565. doi: 10.1016/j.vaccine.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 23.Lin K-Y, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August T, Pardoll DM, Wu T-C. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 24.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing presentation. Cell. 1988;54:777. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 25.Peterson JW, Finkelstein RA, Cantu J, Gessell DL, Chopra AK. Cholera toxin B subunit activates arachidonic acid metabolism. Infect Immun. 1999;67(2):794–9. doi: 10.1128/iai.67.2.794-799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boesman-Frakelstein M, Peterson JW, Thai LS, Finkelstein RA. A nontoxic cholera enterotoxin (CT) analog is chimeric with regard to both epitypes of CT-B subunits, CT-B-1, CT-B-2. Infect Immun. 1996;64(1):346–8. doi: 10.1128/iai.64.1.346-348.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boesman Finkelstein M, Peterson JW, Thai LS, Finkelstein Ra. A nontoxic chimeric cholera toxin analog. Ann NY Acad Sci. 1996;797:266–8. doi: 10.1111/j.1749-6632.1996.tb52973.x. [DOI] [PubMed] [Google Scholar]
- 28.Fan JL, Peterson JW, Prabhakar BS. Adjuvant effects of cholera toxin B subunit on immune response to recombinant thyrotropin receptor in mice. J Autoimmun. 2000;14(1):43–52. doi: 10.1006/jaut.1999.0336. [DOI] [PubMed] [Google Scholar]
- 29.Sastry KJ, Nehete PN, Venkatnarayanan S, Morokowki J, Platsoucas CD, Arlinghaus RB. Rapid in vivo induction of HIV-specific CD8+ cytotoxic T lymphocytes by a 15-amino acid unmodified free peptide from the immunodominant V3-loop of GP120. Virology. 1992;88(2):502–9. doi: 10.1016/0042-6822(92)90504-i. [DOI] [PubMed] [Google Scholar]
- 30.Huang C-F, Peng S, He L, Tsai Y-C, Boyd DAK, Hansen TH, Wu T-C, Hung C-F. Cancer immunotherapy using DNA vaccine encoding a single –chain trimer of MHC class I linked to an HPV-16 E6 immunodominant CTL epitope. Gene Therapy. 2005:1–7. doi: 10.1038/sj.gt.3302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tae-Yoon K, Han-Jeong M, Ji-Hyun K, In-Sung M, Tai-Gyu K, Woong-Shick A, Jeong-Im S. Both E7 and CpG-Oligodeoxynucleotide are required for protective immunity against challenge with human papillomavirus 16 (E6/E7 immortalized tumor cells: involvement of CD4+ and CD8+ T cells in protection. Cancer Res. 2002;62:7234–7240. [PubMed] [Google Scholar]
- 32.Zwaveling S, Ferriera Mota SC, Nouta J, Johnson M, Lipford GB< Offringa R, Van der Burg SH, Melief CJM. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J Immunol. 2002;169:35–358. doi: 10.4049/jimmunol.169.1.350. [DOI] [PubMed] [Google Scholar]
- 33.Kim JW, Hung C-F, Juang J, He L, Kim TW, Armstrong DK, Pai SI, Chen P-J, Lin C-T, Boyd DA, Wu T-C. Comparison of HPV DNA vaccines employing intracellular targeting strategies. Gene Therapy. 2004:1–8. doi: 10.1038/sj.gt.3302252. [DOI] [PubMed] [Google Scholar]
- 34.Ressing ME, Stte A, Brandt RM, Ruppert J, Wentworth PA, Hartman M. Human CTL epitopes encoded by human papillomavirus type 16 E6 and E7 identified through in vivo and in vitro immunogenecity studies of HLA-A*0201-binding peptides. J Immunol. 1995;154:5934–43. [PubMed] [Google Scholar]
- 35.Van Driel WJ, Ressing ME, Kenter GG, Brandt RM, Krul EJ, van Rossum AB, et al. Vaccination with HPV16 peptides of patients with advanced cervical carcinoma: clinical evaluation of a phase I – II trial. Eur J Cancer. 1999;35:946–52. doi: 10.1016/s0959-8049(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 36.Sarkar AK, Tortolero-Luna G, Nehete PN, Arlinghaus RB, Mitchell MF, Sastry KJ. Studies on in vivo induction of cytotoxic T lymphocyte responses by synthetic peptides from E6 and E7 oncoproteins of human papillomavirus type 16. Viral Immunol. 1995;8(3):165–74. doi: 10.1089/vim.1995.8.165. [DOI] [PubMed] [Google Scholar]
- 37.Peng S, Ji H, Trimble C, He L, Tsai Y-C, Yeatermeyer J, Boyd DAK, Hung C-F, Wu T-C. Development of a DNA vaccine targeting human papillomavirus type 16 oncoprotein E6. J Virol. 2004;78(16):8468–8476. doi: 10.1128/JVI.78.16.8468-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis HL, McCluskie MJ, Gerin JL, Purcell RH. DNA vaccine for hepatitis B: evidence for immunogenecity in chimpanzees and comparison with other vaccines. Proc Natl Acad Sci USA. 1996;93:7213–7218. doi: 10.1073/pnas.93.14.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis HL, Weeranta R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol. 1998;160:870–876. [PubMed] [Google Scholar]
- 40.Weiner GJ, Liu H-M, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory oligodeoxynucleotides containing CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci USA. 1997;94:10833–10837. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott Gallichan W, Woolstencroft RN, Guarasci T, McCluskie MJ, Davis HL, Rosenthal KL. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J Immunol. 2001;166:3451–3457. doi: 10.4049/jimmunol.166.5.3451. [DOI] [PubMed] [Google Scholar]
- 42.Kuck D, Lau T, Leuchs B, Kern A, Muller M, Gissmann L, Kleinschmidt A. Intranasal vaccination with recombinant adeno-associated virus type 5 against papillomavirus type 16 L1. J Virol. 2006;80(6):2621–2630. doi: 10.1128/JVI.80.6.2621-2630.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


