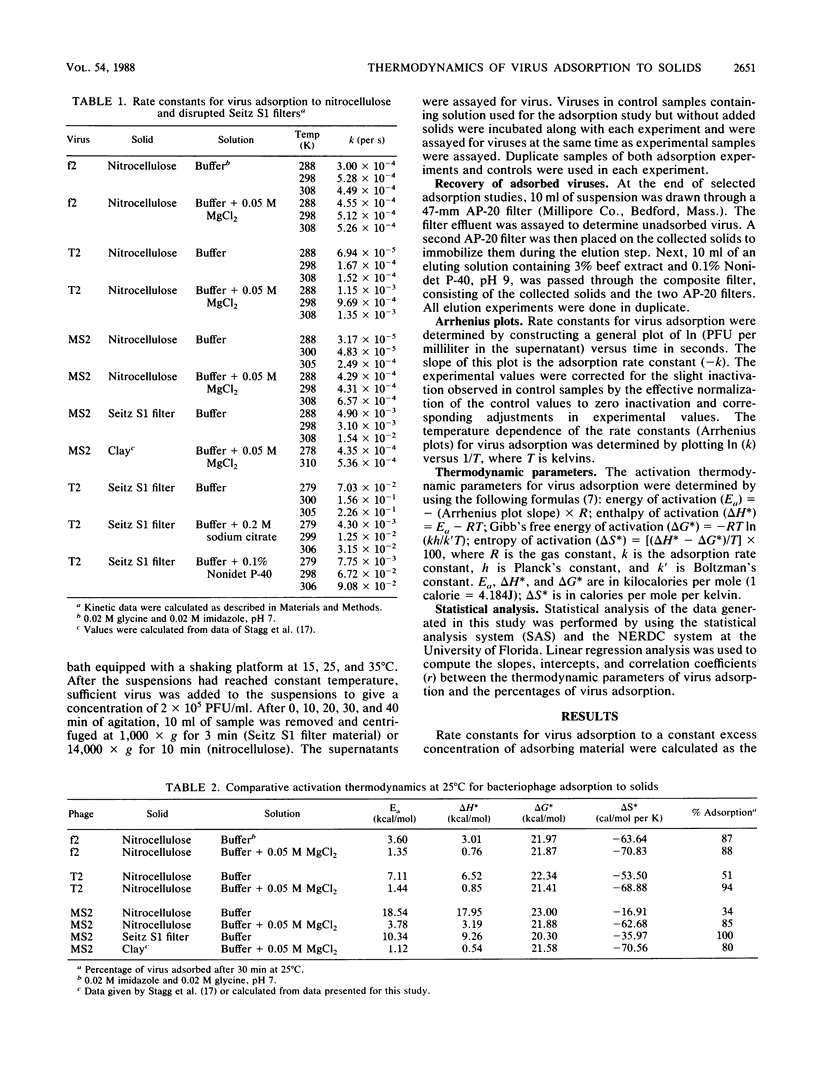
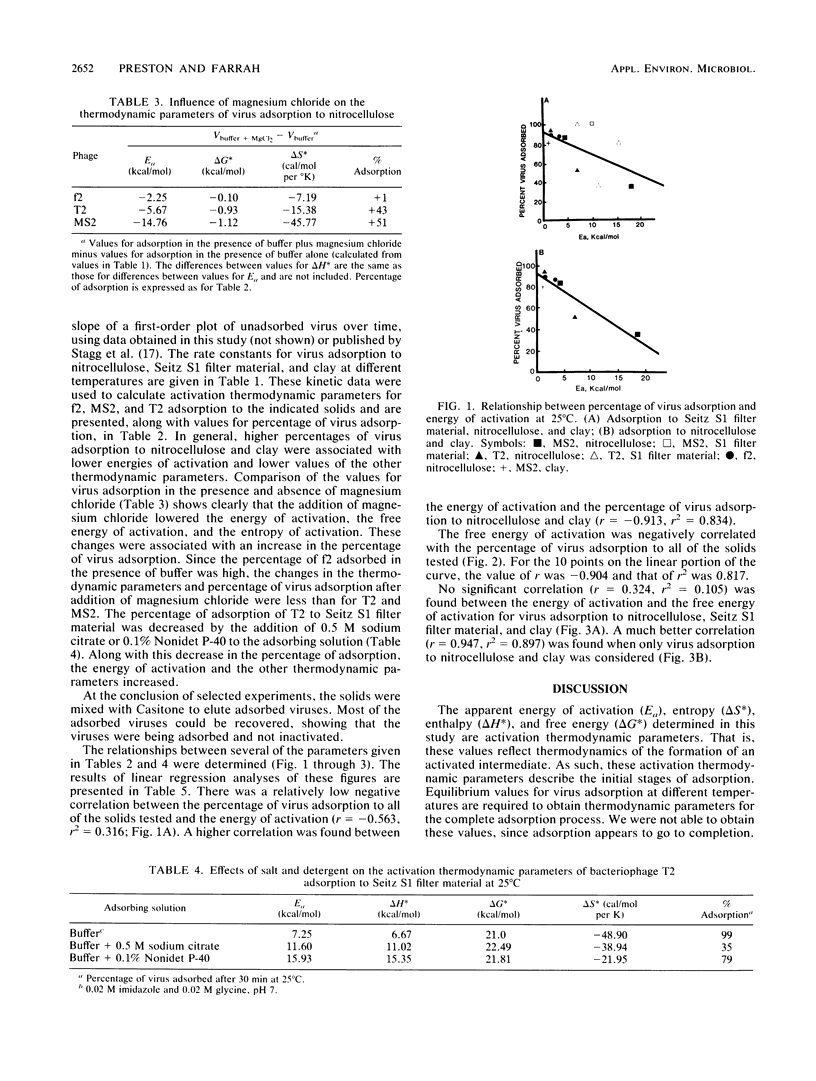
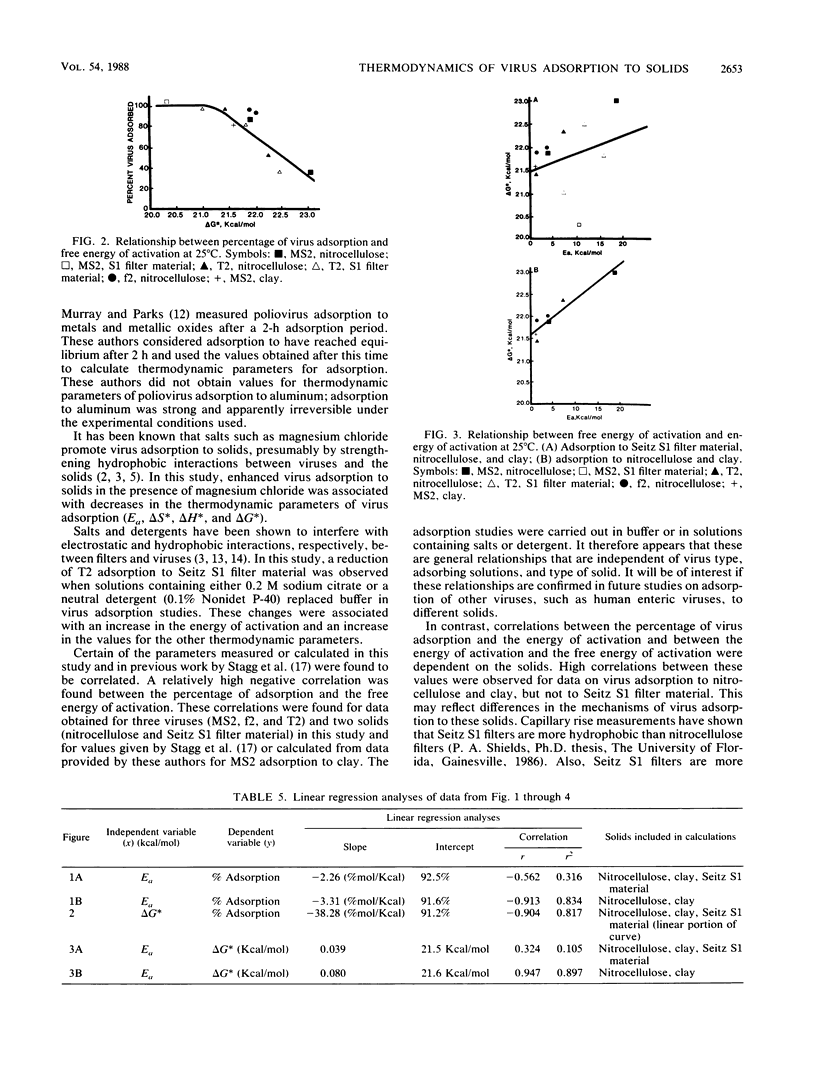
Abstract
The kinetics of bacteriophage MS2, T2, and f2 adsorption to powdered nitrocellulose and disrupted Seitz S1 filters at pH 7 were determined as a function of temperature. Data from these studies were combined with data produced in a previous study on MS2 adsorption to clay by Stagg et al. (Appl. Environ. Microbiol. 33:385-391, 1977). These workers studied the adsorption of MS2 to bentonite clay as a function of temperature. Data from both this previous study and the current one were used to calculate the thermodynamic parameters of virus adsorption. The results show that adsorption of bacteriophages to the solids tested is a physical process (energy of activation, less than 40 kcal [168 J]/mol) rather than a chemical process (energy of activation, greater than 40 kcal/mol). The free energy of activation showed a high negative correlation (r = -0.904, r2 = 0.817) with the percentage of virus adsorption to the solids tested. The energy of activation was highly negatively correlated with the percentage of virus adsorption to nitrocellulose and clay (r = -0.913, r2 = 0.834) but poorly correlated with the percentage of virus adsorption to disrupted Seitz S1 filters (r = -0.348, r2 = 0.121). In general, under conditions in which the percentage of virus adsorption was low, the energy of activation, the free energy of activation, and the entropy of activation were high. Increasing the percentage of virus adsorbed by changing the adsorbing conditions or changing the adsorbing solid decreased the energy of activation, the free energy of activation, and the entropy of activation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Farrah S. R. Chemical factors influencing adsorption of bacteriophage MS2 to membrane filters. Appl Environ Microbiol. 1982 Mar;43(3):659–663. doi: 10.1128/aem.43.3.659-663.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Shah D. O., Ingram L. O. Effects of chaotropic and antichaotropic agents on elution of poliovirus adsorbed on membrane filters. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1229–1232. doi: 10.1073/pnas.78.2.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P. Applied and theoretical aspects of virus adsorption to surfaces. Adv Appl Microbiol. 1984;30:133–168. doi: 10.1016/s0065-2164(08)70054-6. [DOI] [PubMed] [Google Scholar]
- Shields P. A., Farrah S. R. Influence of salts on electrostatic interactions between poliovirus and membrane filters. Appl Environ Microbiol. 1983 Feb;45(2):526–531. doi: 10.1128/aem.45.2.526-531.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Jones B. L. Concentration of poliovirus from tap water using positively charged microporous filters. Appl Environ Microbiol. 1979 Mar;37(3):588–595. doi: 10.1128/aem.37.3.588-595.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C. H., Wallis C., Ward C. H. Inactivation of clay-associated bacteriophage MS-2 by chlorine. Appl Environ Microbiol. 1977 Feb;33(2):385–391. doi: 10.1128/aem.33.2.385-391.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


