Abstract
Decorin, a proteoglycan that inhibits active transforming growth factor-β, is increased in diabetic nephropathy; however, its functional significance is unclear. In this study, we used low-dose streptozotocin to induce type 1 diabetes in wild-type (C57BL/6J Dcn+/+), Dcn−/−, and Dcn+/− mice and studied the mice for up to 1 year of diabetes. Decorin gene dose had no effect on severity of diabetes; however, the Dcn−/− diabetic mice died significantly earlier than nondiabetic controls (57 versus 7.3% mortality). In contrast to wild-type diabetic mice, which failed to develop significant nephropathy, the Dcn−/− diabetic mice developed a significant increase in albuminuria and plasma creatinine and a concurrent decrease in circulating adiponectin levels. Interestingly, adiponectin levels at 6 months of diabetes were predictive of mortality in diabetic mice. Dcn−/− diabetic mice exhibited advanced glomerular lesions, including diffuse mesangial matrix accumulation and fibrin cap formation. By immunohistochemistry, Dcn−/− diabetic mice exhibited significant increases in glomerular transforming growth factor-β, type I collagen, macrophage infiltration, and Nox4. We conclude that decorin is a natural protective factor against diabetic nephropathy and that the Dcn−/− diabetic mouse is a useful new model of progressive diabetic nephropathy.
Nephropathy is a major contributor to morbidity and mortality in patients with diabetes mellitus. Diabetic nephropathy is characterized by progressive albuminuria, glomerular matrix expansion, and a slow deterioration of renal function.1,2 Diabetes is now the leading cause of end-stage renal disease in the developed world.3 Furthermore, patients with renal insufficiency have substantially shortened life expectancy, even before they reach end-stage renal disease.4,5 Despite the importance of diabetic nephropathy, the molecular participants in this progressive disease have not been fully characterized.
The small leucine-rich proteoglycan decorin has been implicated in the regulation of collagen fibril assembly, cell adhesion, and growth factor activity.6,7,8 For several reasons, we sought to examine a role for decorin in diabetic nephropathy. First, decorin is an endogenous inhibitor of transforming growth factor-β (TGF-β),9 a profibrotic cytokine implicated in the pathogenesis of diabetic renal disease.10,11,12,13 In particular, the human diabetic kidney shows excess production of TGF-β,14 and administration of inhibitory anti-TGF-β antibodies to diabetic mice prevents the development of glomerular matrix expansion and renal insufficiency.13 Second, the expression of decorin is increased during the development of diabetic kidney disease,15,16 suggesting a role in the pathobiology of the disease, possibly as a compensatory response to antagonize local TGF-β activity. Third, decorin has been implicated as a protective factor in atherosclerotic vascular disease,17,18,19 and therefore decorin deficiency may promote a systemic vasculopathy found in diabetic kidney disease. Fourth, most of the available animal models of diabetic nephropathy show limited phenotypes that lack key features of the advanced renal pathology that occurs in humans,20 and thus models are needed that recapitulate the more advanced features of human diabetic nephropathy.
In the current study, we hypothesized that a genetic deficiency of decorin would accelerate nephropathy in mice with diabetes, thereby implicating decorin in the pathogenesis of diabetic nephropathy. For this work, decorin knockout mice21 were backcrossed onto the C57BL6 background,17 and diabetes was induced with a chronic low-dose streptozotocin protocol. Only the decorin- deficient diabetic mice showed significantly more advanced renal lesions and renal dysfunction.
Materials and Methods
Animals
Mice were housed in a barrier facility and cared for in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines. We introduced the decorin knockout allele21 onto the C57BL/6J background by seven backcrosses.17,18 Genotypes were determined by PCR of tail-clip DNA as described.18,21 After genotyping, male littermates were randomly assigned at 8 to 9 weeks of age to receive low-dose streptozotocin (50 mg/kg per day i.p. for 5 consecutive days) using an Animal Models of Diabetic Complications Consortium-established protocol for induction of diabetes22 or vehicle alone (nondiabetic mice). Diabetic and nondiabetic mice of each of wild-type Dcn+/+ and homozygous knockout Dcn−/− decorin genotypes were extensively studied.
Serial Monitoring
Body weights and blood glucose concentrations were monitored at 2 weeks and at 2, 4, 6, 8, and 10 months after induction of diabetes. At 6 and 10 months after induction of diabetes, each mouse was placed into a metabolic cage for a 24-hour collection of urine. Five hundred microliters of each urine sample was kept at 4°C for a murine albumin enzyme-linked immunosorbent assay (Albuwell M Kit; Exocell, Philadelphia, PA). The remainder was stored at −20°C for assays of creatinine (Creatinine Companion Kit; Exocell). Plasma was analyzed for creatinine concentration by high-performance liquid chromatography,23 which is the preferred method because of the presence in mouse plasma of substances that interfere with the chromagen-based creatinine colorimetric assay.23 A portion of plasma was analyzed for mouse adiponectin levels by enzyme-linked immunosorbent assay (Linco Research, Inc., St. Charles, MO) at 6 and 10 months of diabetes.
Organ Harvesting and Histopathology
All surviving mice were sacrificed at 12 months after streptozotocin or vehicle injection. To obtain optimal light microscopic histology, the left kidney was perfusion-fixed before harvest, as described,24 and then paraffin-embedded. The fixed, embedded kidneys were cut into 3-μm sections and stained with periodic acid-Schiff reagent. Slides were read by a pathologist (P.M.) blinded to the code of the experimental groups. To assess glomerular disease, 50 glomeruli per mouse were rated using a semiquantitative scoring system for mesangial matrix expansion: minimal (grade 1, 0 to 25% of glomerular volume occupied by matrix), mild (grade 2, 25 to 50%), moderate (grade 3, 51 to 75%), and severe (grade 4, 75 to 100%).24 For electron microscopic analysis, selected right kidneys were removed, and a portion of the cortex fixed in 2.5% glutaraldehyde in Millonig solution, cut into 1-mm cubes, and embedded in PolyBed 812 (Polysciences, Inc., Warrington, PA), as previously described.25
NADPH Oxidase mRNA Analysis
Another portion of the right kidney cortex was immediately snap frozen. Total RNA was extracted by TRI Reagent (MRC, Inc., Cincinnati, OH). NADPH oxidase subunit mRNA levels were measured by quantitative real-time PCR using primers and a probe specific for mouse Nox1, Nox2, Nox4, p22phox, and p67phox (sequences included as supplement). Values were normalized to β-actin mRNA levels, as described previously.26
Immunohistochemistry and Quantification
Immunohistochemistry was performed as previously described.27 In brief, 3-μm paraffin sections prepared from the left kidneys were dewaxed, antigen retrieval was performed by 15 to 25 minutes of microwave exposure in antigen retrieval buffer (Citra Plus; BioGenex, San Ramon, CA), and then the sections were blocked by incubation in nonimmune goat serum. Each of these sections received one primary antibody, which was against TGF-β1/2/3 (rabbit polyclonal antibody, applied at 1:50 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), type I collagen (Southern Biotechnology, Birmingham, AL), the macrophage marker Mac-3 (rat monoclonal antibody, applied at 1:50 dilution; Santa Cruz Biotechnology), or Nox4 (rabbit polyclonal antibody kindly provided by Dr. Barry Goldstein (Division of Endocrinology, Thomas Jefferson University, Philadelphia, PA) and used at 1:300 dilution). Biotin-labeled anti-rabbit (Invitrogen, Carlsbad, CA) or anti-rat (Jackson ImmunoResearch Laboratories, West Grove, PA) secondary antibodies were then applied, followed by blockage of endogenous peroxidase with 3% H2O2, rinsing, and then the addition of avidin-linked peroxidase (Vectastain Elite Kit; Vector Laboratories, Burlingame, CA). Color development was achieved with the peroxidase substrate 3,3-diaminobenzidine (DAB Substrate Kit; Vector Laboratories). Sections were counterstained with Hematoxylin (Gill’s Hematoxylin Stain; Fisher Scientific).
Quantification of TGF-β immunohistochemical positive areas in glomeruli was performed by color-subtractive, computer-assisted image analysis, as described,27 using Photoshop CS2 (Adobe Systems, Mountain View, CA) and NIH Image J 1.36 (Bethesda, MD). Quantification of type I collagen was performed by scoring the presence or absence of glomerular type I collagen staining. The average number of cells per glomerulus that stained for Mac-3 or for Nox4 was determined by counting immunopositive cells. All immunohistochemical analyses were performed on 50 glomeruli from at least four separate mice in each group by a pathologist (P.M. or H.K.U.) blinded to the code of the experimental groups.
Measurement of Active TGF-β in Isolated Glomeruli
Glomeruli were isolated using the Dynabead infusion protocol as described by Takemoto.28 Three Dcn+/+ and three Dcn−/− mice were used as the source of glomeruli for each experiment. Equal numbers of glomeruli were plated onto 24-well plates and cultured in Dulbecco’s modified Eagle’s medium (DMEM)/10% serum overnight. The medium was then gently changed to DMEM normal glucose (NG; 100 mg/dl d-glucose) or DMEM high glucose (HG; 450 mg/dl d-glucose), and glomeruli were cultured for an additional 48 hours. TGF-β activity was measured with mink lung epithelial cells stably transfected with the plasminogen activator inhibitor-1 (PAI-1) promoter-luciferase construct as previously described.29 In brief, the mink lung epithelial cells were plated onto a 96-well plate (1.6 × 104 cells per well in DMEM G450/10% serum overnight) concurrently during the glomerular culture. On the day of harvesting conditioned media from glomeruli, the growth media of the mink lung epithelial cells were replaced with DMEM G100/0.1% bovine serum albumin. Conditioned media from glomeruli culture medium in NG or high glucose were added (1:5 dilution) in triplicate and incubated for an additional 18 hours. To subtract non-TGF-β-induced PAI-1 promoter activity, cultured media from glomeruli were preincubated with pan-isoform-neutralizing anti-TGF-β antibody13 (2G7; 50 μg/ml) for 40 minutes before the addition to mink lung epithelial cells in parallel wells. A standard curve was established with known concentrations of TGF-β1 (1 pg/ml to 1 ng/ml). After incubation, the mink lung epithelial cells were lysed, and luciferase measurements were performed with the Promega Luciferase Assay System (Madison, WI). Experiments were repeated three times.
Statistical Analyses
Kaplan-Meyer survival analysis through 12 months after induction of diabetes was performed using the log-rank statistic to test for a significant difference among the six survival curves. Because they were significantly different, pairwise comparisons between curves were then performed by the Holm-Sidak method. All other data are presented as means ± standard errors with statistical analysis performed using one-way analysis of variance from GraphPad Prism 4.03 (GraphPad Software, Inc., San Diego, CA) or Sigma Stat version 3 (SPSS Inc., Chicago, IL) and posthoc testing using the Newman-Keuls method.
Results
Decorin Deficiency Does Not Alter Severity of Diabetes
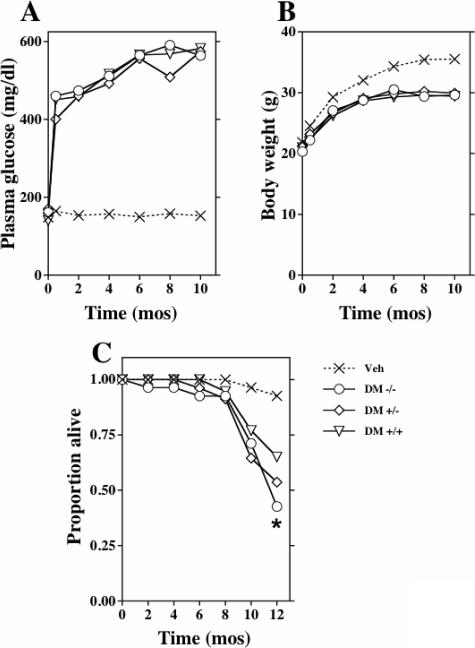
Type 1 diabetes was induced with a multiple low-dose streptozotocin protocol in male mice at 8 to 9 weeks of age. Blood glucose levels averaged 400 mg/dl at 2 weeks, gradually increased to 500 to 600 mg/dl during the first 6 months of diabetes, and subsequently remained unchanged (Figure 1, A). The diabetic mice required no exogenous insulin and did not exhibit ketonuria. The diabetic mice continued to gain weight, although less than the nondiabetic, vehicle-treated controls (Figure 1B). Blood glucose levels and body weights were indistinguishable among the diabetic groups, regardless of decorin gene dose. Thus, wild-type C57BL/6J male mice made diabetic using the low-dose streptozotocin protocol developed and maintained stable hyperglycemia for up to 1 year.
Figure 1.
Decorin deficiency does not affect severity of diabetes but significantly accelerates mortality. At age 7 to 8 weeks (t = 0), animals were treated with saline or low-dose streptozotocin. Displayed are mean plasma glucose concentrations (A), body weights (B), and survival (C) over time for diabetic Dcn−/− (DM −/−), Dcn+/− (DM +/−), and wild-type (DM +/+) mice, as well as for saline-treated animals of all three genotypes (Veh). Because they were statistically indistinguishable, the nondiabetic groups were pooled for data display. There was a statistically significant difference in all diabetic groups with respect to blood glucose beyond 2 weeks and body weights beyond 2 months versus nondiabetic vehicle groups (P < 0.05 versus nondiabetic vehicle groups). The three diabetic groups were indistinguishable with respect to blood glucose and body weights. Kaplan-Meyer survival curves are depicted in C. By the log-rank test, survival curves for the six groups were significantly different (P < 0.001). Pairwise comparisons by the Holm-Sidak method indicated that the diabetic Dcn−/− mice, but no other group, showed significantly worse survival than wild-type nondiabetic controls (*P < 0.05; unadjusted P = 0.0019) (n/group 10 to 20 at induction of diabetes and n/group 8 to 18 at 10 months of diabetes).
The Dcn−/− diabetic mice exhibited an increase in mortality (Figure 1C). By 12 months of diabetes, mortality was 57.3% in Dcn−/− mice (P < 0.002 versus nondiabetic Dcn+/+ controls) and 34.9% in wild-type diabetic mice. Vehicle-treated mice showed an overall mortality of 7.3%. Of note, the increased mortality in Dcn−/− diabetic mice occurred primarily after 8 months of diabetes (Figure 1C).
Functional Parameters of Nephropathy in Dcn-Deficient Diabetic Mice Demonstrate Accelerated Renal Deterioration
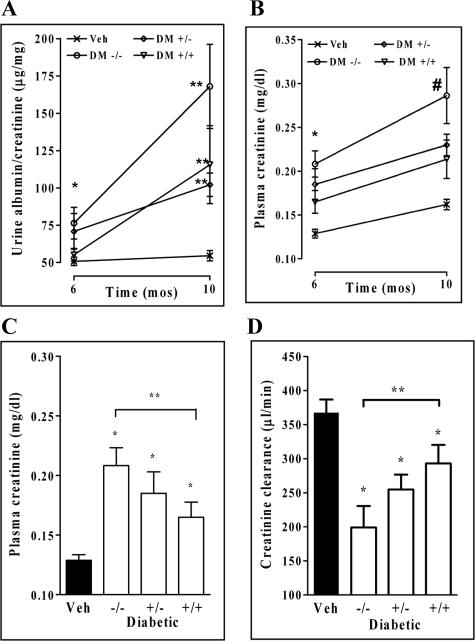
The degree of albuminuria was not increased before 6 months of diabetes but was increased in the Dcn−/− diabetic mice by 6 and 10 months of diabetes as compared with nondiabetic controls (P < 0.001). Albuminuria was significantly increased in the diabetic Dcn+/+ group only at 10 months of diabetes (Figure 2A).
Figure 2.
Decorin deficiency accelerates diabetic nephropathy. Displayed are urinary albumin/creatinine ratios (A), plasma creatinine concentrations (B and C), and creatinine clearance (D). Statistically significant differences are indicated by asterisks. For urinary albumin/creatinine ratios at 6 months, the diabetic Dcn−/− group was the only one to differ from the nondiabetic controls (*P < 0.01). At 10 months, urine albumin/creatinine ratios were increased in all diabetic groups versus nondiabetic controls (**P < 0.01) (A). The plasma creatinine was elevated in all of the diabetic groups at 6 and 10 months compared with nondiabetic controls (B, *P < 0.01 versus Veh at 6 months, #P < 0.01 versus Veh at 10 months). In addition, the diabetic Dcn−/− was significantly different from the diabetic Dcn+/+ mice at 6 months (**P < 0.05 versus Dcn+/+ diabetic group) (C). There was a corresponding reduction in creatinine clearance in all of the diabetic groups with the diabetic Dcn−/− also significantly different from the diabetic Dcn+/+ mice at 6 months of diabetes (D, *P < 0.01 versus Veh at 6 months, **P < 0.05 versus Dcn+/+ diabetic group) (n/group 10 to 20 at 6 months of diabetes and n/group 8 to 18 at 10 months of diabetes).
Renal function was assessed by measuring plasma creatinine concentrations by high-performance liquid chromatography. There was a significant decline in renal function by 6 months of diabetes as the Dcn−/− diabetic groups showed plasma creatinine concentrations significantly above the normal level and the diabetic Dcn+/+ group (P < 0.05) (Figure 2, B and C). At 10 months of diabetes, there was an even greater increase in plasma creatinine in the Dcn−/− diabetic group, indicating that renal disease was progressive (Figure 2B). The creatinine clearance showed a similar pattern in the Dcn−/− diabetic mice (Figure 2D). Thus, decorin deficiency accelerates the decline in renal function in experimentally induced diabetic nephropathy.
Markers of Mortality in Diabetic Mice
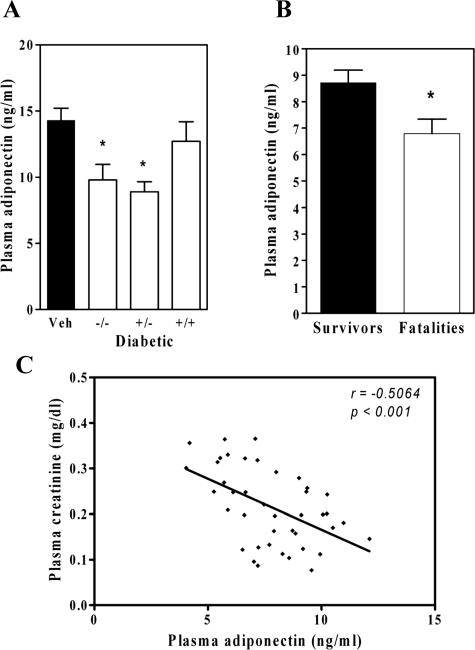
In human patients with impaired renal function, low plasma levels of adiponectin correlate with cardiovascular morbidity and mortality.30,31 Although there is no previously known relationship between decorin and adiponectin, we found that adiponectin levels at 10 months of diabetes were significantly decreased in the decorin-deficient Dcn−/− diabetic groups (Figure 3A). Adiponectin levels in the nondiabetic groups were not different based on gentoype. Interestingly, the diabetic mice that died by 12 months were characterized at month 6 by significantly lower adiponectin levels than was observed in the diabetic survivors (Figure 3B). Numerous other parameters did not differ at 6 months between diabetic mice that would die versus survive (body weight, blood glucose, streptozotocin dose), indicating a similar severity of diabetes.
Figure 3.
Adiponectin levels are suppressed in decorin-deficient diabetic mice and in diabetic fatalities. Displayed are plasma adiponectin levels at 10 months (A) (means ± SEM, n ≥ 8 per diabetic group). The diabetic Dcn−/− and diabetic Dcn+/− diabetic groups had significantly lower adiponectin levels than nondiabetic controls (*P < 0.05). B: Plasma adiponectin concentrations at 6 months in all diabetic survivors versus all diabetic fatalities (means ± SEM, n = 24 survivors, n = 16 fatalities; *P < 0.001). C: An inverse correlation between plasma adiponectin levels and plasma creatinine levels at 6 months of diabetes (r = −0.506, P = 0.0007, n = 40 pairs).
Interestingly, there was a negative correlation between plasma creatinine levels and adiponectin levels (r = −0.506, P < 0.001) (Figure 3C) and between urine albumin/creatinine and adiponectin (r = −0.327, P < 0.05) in all diabetic mice at 6 months of diabetes, indicating that lower adiponectin levels are associated with an early decline in renal function. This correlation was not observed at 10 months of diabetes, at which time there was further deterioration of renal function and greater albuminuria.
Advanced Diabetic Renal Pathology in Dcn−/− Mice
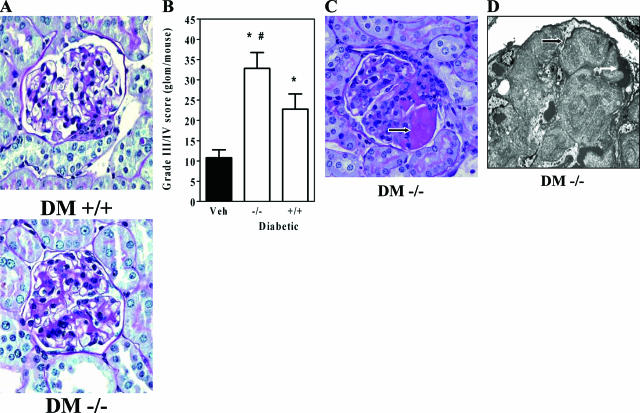
Renal pathology was assessed by light and electron microscopy. Periodic acid-Schiff-stained kidney sections revealed diffuse mesangial matrix accumulation in the Dcn−/− diabetic mice (Figure 4A). There was a statistically significant increase in grades 3 and 4 mesangial matrix lesions in the Dcn−/− diabetic mice (Figure 4B). In addition, only the Dcn−/− mice exhibited advanced glomerular pathology (Figure 4C), which was identified as fibrin caps in 38% of diabetic Dcn−/− mice as determined by electron microscopy (Figure 4D). Further ultrastructural and special stain analysis did not demonstrate tubulointerstitial fibrosis, although there was an overall thickening of both glomerular and tubular basement membranes in all diabetic mice (data not shown). Overall, our results indicate a substantially advanced renal pathology in the Dcn−/− diabetic mice, although the data probably underestimate the true severity of their lesions, given that many mice in that group (57%) died before pathological assessment of renal structure.
Figure 4.
Decorin deficiency worsens diabetic glomerulopathy. A: Representative periodic acid-Schiff-stained glomeruli from 12-month diabetic kidneys of diabetic Dcn+/+ (DM +/+) and Dcn−/− (DM −/−) mice (magnification, ×40). B: Counts of glomeruli with advanced matrix scores from individual mice (means ± SEM, n ≥ 8 per group; the diabetic mice were significantly different from controls, *P < 0.05; the diabetic Dcn−/− mice were significantly different from diabetic Dcn+/+ mice, #P < 0.05). C: Glomerular fibrin cap lesions, seen only in diabetic Dcn−/− glomeruli (arrow) (original magnification, ×40). D: An electron micrograph of a glomerulus fibrin cap (black arrow), which was identified as a luminal fibrin cap because of its proximity to erythrocytes (white arrow) (magnification, ×4000).
Increased Glomerular TGF-β, Type I Collagen, Macrophage Infiltration, and Nox4 in Dcn-Deficient Mice
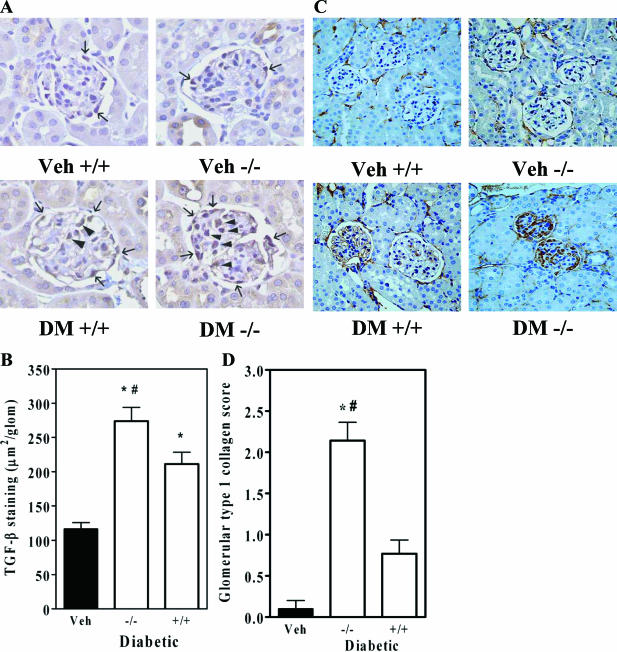
To identify mechanisms by which decorin deficiency enhances diabetic renal pathology, we performed immunostaining for potential pathways in diabetic wild-type and Dcn−/− mice. Since decorin deficiency would be expected to result in increased TGF-β activity, and TGF-β itself is positively regulated by active TGF-β, we examined tissues for TGF-β with immunostaining. There was a substantial up-regulation of TGF-β in the glomeruli of Dcn+/+ diabetic mice over nondiabetic and a further increase in Dcn−/− diabetic mice (Figure 5, A and B). Increased staining for TGF-β was found in the location of mesangial cells and podocytes in diabetic glomeruli (Figure 5A). Another major target of TGF-β is type I collagen. Glomeruli from Dcn−/− diabetic mice showed a marked increase in type I collagen that was not evident in either decorin deficiency alone or diabetes alone (Figure 5C). Quantitative grading revealed a significant increase in type I collagen staining in Dcn−/− diabetics (Figure 5D, P < 0.05).
Figure 5.
Decorin deficiency increases glomerular TGF-β staining and type I collagen in diabetes. A: Representative photomicrographs of TGF-β immunostaining in glomeruli from the indicated experimental groups (magnification, ×40). Arrowheads indicate immunostaining in the mesangium; small arrows indicate immunostaining in the region of podocytes. B: Quantification of glomerular TGF-β staining areas (means ± SEM, n = 4; P < 0.001 by the one-way analysis of variance; *P < 0.01 versus nondiabetic controls; #P < 0.01 versus Dcn+/+ diabetic mice). C: Representative immunostains of type I collagen in glomeruli (magnification, ×20). Brown indicates type I collagen; blue is the counterstain. D: Quantification of glomerular type I collagen staining in glomeruli (means ± SEM, n = 4; P < 0.001 by the one-way analysis of variance; *P < 0.01 versus nondiabetic controls; #P < 0.01 versus Dcn+/+ diabetic mice).
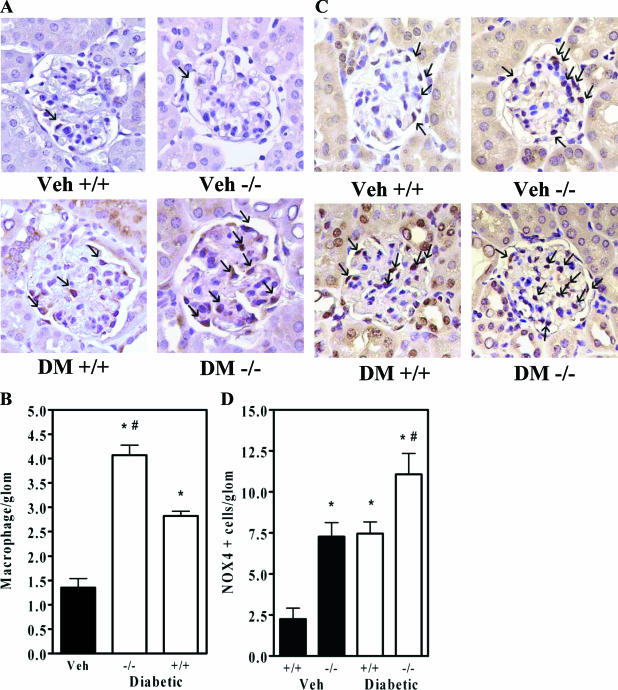
Recently, macrophage infiltration in diabetic glomeruli has been considered to contribute to progressive disease.27 Immunostaining with an antibody against Mac-3 revealed an increase in macrophage infiltration in diabetic Dcn+/+ glomeruli over nondiabetic and an even greater increase in Dcn−/− glomeruli (Figure 6, A and B).
Figure 6.
Decorin deficiency worsens glomerular macrophage infiltration and increases Nox4 in diabetes. A: Representative immunostains of the macrophage marker Mac-3 (magnification, ×40). Small arrows indicate macrophages. B: Macrophage counts per glomerulus (means ± SEM, n = 4; P < 0.001 by the one-way analysis of variance; *P < 0.01 versus nondiabetic controls; #P < 0.01 versus Dcn+/+ diabetic mice). C: Representative immunostains of glomerular Nox4 (magnification, ×40). Small arrows indicate Nox4-positive cells. D: Counts of Nox4-positive cells per glomerulus (means ± SEM, n = 4). There was a significant increase in glomerular Nox4 in nondiabetic Dcn−/− versus Dcn+/+ controls (*P < 0.001 versus Dcn+/+ nondiabetic control). The diabetic Dcn−/− had a significantly greater degree of glomerular Nox4 than the diabetic Dcn+/+ group (#P < 0.001).
Finally, the proinflammatory enzyme NADPH oxidase has been recently considered to play important roles in podocyte dysfunction32 and mesangial matrix accumulation33,34 in diabetic kidney disease. Our initial screening of kidneys by real-time PCR for mRNAs for components of the NADPH oxidase system (Nox1, Nox2, p22, p47, and p67) did not demonstrate up-regulation in Dcn−/− kidneys (data not shown), except for a preferential increase in renal Nox4 mRNA levels in Dcn−/− mice (to 174 ± 18% of Dcn+/+; P < 0.001). Based on these results, we examined renal Nox4 protein by immunostaining. Decorin deficiency alone and diabetes alone each significantly increased glomerular Nox4, and the combination caused the largest increase (Figure 6, C and D). Nox4 immunoreactivity was located primarily in mesangial cells and podocytes.
TGF-β Bioactivity Regulated by Decorin and High Glucose in Isolated Glomeruli
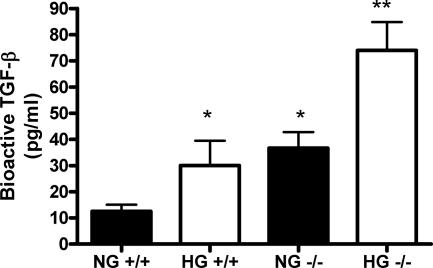
To clarify the role of decorin in regulating TGF-β activity, glomeruli were isolated from Dcn+/+ and Dcn−/− mice and cultured in normal (100 mg/dl) or high glucose (450 mg/dl). The secreted TGF-β activity from wild-type glomeruli exhibited increased TGF-β activity with high glucose, as expected (Figure 7). Deficiency of decorin in isolated glomeruli was sufficient to increase TGF-β activity in normal glucose and was increased further in high glucose (Figure 7).
Figure 7.
Decorin deficiency enhances TGF-β bioactivity in isolated glomeruli. Equal numbers of glomeruli were isolated from Dcn+/+ and Dcn−/− mice and plated onto 24-well plates. After 48 hours of culture in normal glucose (100 mg/dl, NG) or high glucose (450 mg/dl, HG), aliquots of the conditioned media were added to proliferating mink lung epithelial cells stably transfected with a PAI-1 promoter-luciferase construct. After 24 hours, the mink lung cells were harvested, and luciferase activity was measured. Bioactive TGF-β was measured based on a standard curve and after subtraction of nonspecific stimulation. The experiment contained three wells for each condition and each experiment was repeated three times. (*P < 0.05 versus NG+/+, **P < 0.05 versus NG−/−).
Discussion
In the current study, we found that decorin deficiency substantially worsens the progression of diabetic kidney disease in mice, with features that closely mimic advanced human nephropathy. There were progressive increases in albuminuria, plasma creatinine, and mesangial matrix expansion with fibrin caps and macrophage infiltration. These results conclusively identify decorin as a protective agent in this murine model of diabetic nephropathy.
Despite the clinical importance of progressive diabetic nephropathy, mechanistic research has been limited by the lack of murine models with advanced phenotypes. Experimental type 1 diabetes with low-dose streptozotocin does not produce diabetic nephropathy in many strains, especially C57BL6 mice.24 Although high-dose streptozotocin (>75 to 100 mg/kg/day) has been associated with reduced renal function and proteinuria with short durations of hyperglycemia, it is difficult to separate out the potential toxic effect of streptozotocin from the effect of diabetes alone.35 Type 2 models, such as the db/db mouse, develop early albuminuria and mesangial matrix accumulation, but there is often no further progression of the renal disease.36 Recently, several additional models of spontaneous diabetic nephropathy with decline in renal function have been described in the endothelial nitric-oxide synthase-deficient mouse37,38 and in the OVE26 mouse on the FVB background.39 These models either induced hypertension or were described in different strains that may be more prone to renal disease from diabetes. To date, the C57BL6 mouse has been considered to be relatively resistant to advanced nephropathy and renal failure with diabetes alone.24 Our data in diabetic C57BL6 mice with 10 months of diabetes demonstrate that there are significant increases in albuminuria, plasma creatinine, and mesangial matrix expansion. In addition, deficiency of decorin is sufficient to result in a faster and more progressive diabetic nephropathy in the C57BL6 mouse based on clinical, histopathological, and ultrastructural characteristics.
Based on prior work13,15,16 and our current study, decorin seems to act as a counter-regulatory factor in the diabetic kidney, possibly to compensate for increased local TGF-β production. Decorin expression in cultured mesangial cells is stimulated by high glucose concentrations,40,41 and decorin has been reported to be up-regulated in the kidney of diabetic animal models16 and human diabetic patients.15 The pathology that was enhanced in our decorin-deficient diabetic mice was primarily mesangial matrix accumulation, consistent with a regulatory role for decorin in this location. In support of the role of decorin to regulate TGF-β and its downstream bioactivity, we found an increase in glomerular TGF-β and glomerular type I collagen, both major targets of TGF-β action, in decorin-deficient diabetic kidneys. It is of interest that decorin deficiency alone is sufficient to increase TGF-β bioactivity in isolated glomeruli (Figure 7), although increased TGF-β production was not observed in the absence of diabetes in the Dcn−/− kidney (Figure 5). These data suggest that decorin deficiency can contribute to TGF-β activity/production in vivo only when superimposed with other stimuli of TGF-β in the diabetic milieu (ie, angiotensin II, glycated proteins, etc). Work in other animal models of kidney diseases, such as glomerulonephritis9 and obstructive uropathy,9,42 also suggests a protective role for decorin as an antifibrotic molecule.
Two unexpected findings related to in vivo pathophysiology emerged from our studies. First, we found an unexpected link between decorin deficiency and suppression of plasma adiponectin levels, suggesting a role for decorin in the regulation of this adipokine. Although low adiponectin levels have been clearly linked with insulin resistance and obesity, two independent studies have also found that low adiponectin levels in patients with type 1 diabetes are associated with increased cardiovascular disease.43,44 In our study, the diabetic mice that eventually died exhibited significantly lower adiponectin levels months earlier compared with diabetic survivors. The relationship of adiponectin levels with diabetic kidney disease is controversial. In humans with mild increases in albuminuria there is a negative correlation with adiponectin levels,45 similar to what is observed in the diabetic mice in our study. However, with progressive kidney disease, as evidenced by overt proteinuria and decline in renal function, adiponectin levels are increased, and there is a positive correlation with proteinuria and plasma creatinine.46,47 In the present study with a model of type 1 diabetes, there is a negative correlation with plasma creatinine levels and albuminuria at the onset of renal decline (at 6 months) but not with further decline in renal function (at 10 months). These associations suggest a close and complex relationship between kidney function and circulating adiponectin. Of significance and great relevance, a recent study in patients with type 1 diabetes found a close link between an adiponectin single nucleotide polymorphisms and diabetic nephropathy,48 suggesting that alteration of adiponectin may contribute to diabetic nephropathy. The cause of death in the decorin-deficient diabetic mice is presently unclear, although it is intriguing that decorin may play a protective role in cardiovascular disease,17,19 and adiponectin levels have been linked with cardiovascular disease in patients with type 1 diabetes43,44 and mortality with chronic renal disease.30,31
The second unexpected finding was that the decorin-deficient mice exhibited increased renal expression of Nox4, the NADPH oxidase isoform that has been closely linked to diabetic nephropathy in several animal models.33,49 Because Nox4 was increased in Dcn−/− kidneys even in the absence of diabetes, the data suggest that decorin may have a direct effect in regulating Nox4 expression. The effect on Nox4 may well be related to TGF-β, given our recent demonstrations that TGF-β-induced reactive oxygen species production in endothelial cells is mediated by Nox-450 and that TGF-β can stimulate Nox4 production.51 Nox4 was found to be expressed in both mesangial cells and podocytes, suggesting that increased oxidant tone in these cell types contribute to cell dysfunction. Thus, we consider the finding of increased Nox4 in the context of decorin deficiency and accelerated diabetic nephropathy to be a significant clue to pathogenesis and adds to the evidence favoring a deleterious role for Nox4 in diabetic nephropathy.
In summary, our results show that decorin plays a protective role against the progression of diabetic kidney disease. Diabetic mice with decorin deficiency have increased albuminuria, impaired renal function, and an increased degree of mesangial matrix expansion and macrophage infiltration. Type 1 collagen and Nox-4, both targets of TGF-β, were substantially increased by decorin deficiency. Diabetic mice also exhibit increased mortality in association with low adiponectin levels. This model of diabetic kidney disease establishes a protective role for decorin and provides a murine model of advanced diabetic kidney disease.
Footnotes
Address reprint requests to Kumar Sharma, M.D., Director, Translational Research for Kidney Disease, 9500 Gilman Dr., Stein Building, Room 406, University of California at San Diego, La Jolla, CA 92093-0711. E-mail: KumarSharma@ucsd.edu.
Portions of this manuscript were presented in abstract form at the ASN meeting 2004. Funding for this study was provided by grant U01 DK60995 (E.B., K.J.W., and K.S.). Additional funding for portions of this work was provided by National Institutes of Health grants DK053867 (K.S.), HL56984 (K.J.W.), and CA 39481 (R.V.I.).
References
- Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74:1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan T, McCue P, Sharma K. Diabetic nephropathy. Clin Lab Med. 2001;21:111–146. [PubMed] [Google Scholar]
- US Renal Data System: USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Division of Kidney, Urologic, and Hematologic Diseases 2005. Bethesda, MD, National Institutes of Health, National Institute of Diabetic and Digestive Kidney Disease, 2005 [Google Scholar]
- Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau J-L, Drury PL, Esmatjes E, Hricik D, Pohl M, Raz I, Vanhille P, Wiegmann TB, Wolfe BM, Locatelli F, Goldhaber SZ, Lewis EJ, for the Collaborative Study Group Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan diabetic nephropathy trial. J Am Soc Nephrol. 2005;16:2170–2179. doi: 10.1681/ASN.2004090763. [DOI] [PubMed] [Google Scholar]
- Go A, Chertow G, Fan D, McCulloch C, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Yamaguchi Y, Hildebrand A, Border WA. Extracellular matrix/growth factor interactions. Cold Spring Harbor Symp Quant Biol. 1992;57:309–315. doi: 10.1101/sqb.1992.057.01.035. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–174. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem. 1999;274:18843–18846. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Rouslahti E. Natural inhibitor of transforming growth factor-β protects against scarring in experimental kidney disease. Nature. 1992;360:361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- Wolf G, Sharma K, Chen Y, Ericksen M, Ziyadeh FN. High glucose-induced proliferation in mesangial cells is reversed by autocrine TGF-β. Kidney Int. 1992;42:647–656. doi: 10.1038/ki.1992.330. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA. 1993;90:1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Ziyadeh FN. Hyperglycemia and diabetic kidney disease: the case for transforming growth factor-β as a key mediator. Diabetes. 1995;44:1139–1146. doi: 10.2337/diab.44.10.1139. [DOI] [PubMed] [Google Scholar]
- Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency excess matrix gene expression and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA. 2000;97:8015–8020. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Ziyadeh FN, Alzahabi B, McGowan TA, Kapoor S, Kurnik BRC, Kurnik PB, Weisberg LS. Increased renal production of transforming growth factor-β1 in patients with type II diabetes. Diabetes. 1997;46:854–859. doi: 10.2337/diab.46.5.854. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Raslik I, Grone H-J, Schonherr E, Macakova K, Ugorcakova J, Budny S, Schaefer R, Kresse H. Small proteoglycans in human diabetic nephropathy: discrepancy between glomerular expression and protein accumulation of decorin, biglycan, lumican, and fibromodulin. FASEB J. 2001;15:559–561. doi: 10.1096/fj.00-0493fje. [DOI] [PubMed] [Google Scholar]
- Mogyorosi A, Ziyadeh FN. Increased decorin mRNA in diabetic mouse kidney and in mesangial and tubular cells cultured in high glucose. Am J Physiol. 1998;275:F827–F832. doi: 10.1152/ajprenal.1998.275.5.F827. [DOI] [PubMed] [Google Scholar]
- Mazany KD, Kuriakose G, Eichstetter I, Iozzo RV, Tabas I, Williams KJ. Genetic modification of arterial matrix in vivo substantially alters the development of early atherosclerosis: the decorin null mouse. Circulation. 2000;102(Suppl):II-38. [Google Scholar]
- Williams KJ. Arterial wall chondroitin sulfate proteoglycans: diverse molecules with distinct roles in lipoprotein retention and atherogenesis. Curr Opin Lipidol. 2001;12:477–487. doi: 10.1097/00041433-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Al Haj Zen A, Caligiuri G, Sainz J, Lemitre M, Demerens C, Lafont A. Decorin overexpression reduces atherosclerosis development in apolipoprotein E-deficient mice. Atherosclerosis. 2006;187:31. doi: 10.1016/j.atherosclerosis.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Breyer M, Bottinger E, Brosius FC, 3rd, Coffman T, Harris R, Heilig C, Sharma KA. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16:27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- Danielson K, Baribault H, Holmes D, Graham H, Kadler K, Iozzo R. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susztak K, Bottinger E, Novetsky A, Liang D, Zhu Y, Ciccone E, Wu D, Dunn S, McCue P, Sharma K. Molecular profiling of diabetic mouse kidney reveals novel genes linked to glomerular disease. Diabetes. 2004;53:784–794. doi: 10.2337/diabetes.53.3.784. [DOI] [PubMed] [Google Scholar]
- Dunn S, Qi Z, Bottinger E, Breyer M, Sharma K. Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int. 2004;65:1959–1967. doi: 10.1111/j.1523-1755.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes. 2005;54:2628–2637. doi: 10.2337/diabetes.54.9.2628. [DOI] [PubMed] [Google Scholar]
- Steffes MW, Schmidt D, McCrery R, Basgen JM. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int. 2001;59:2104–2113. doi: 10.1046/j.1523-1755.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- Oliverio M, Best C, Kim H-S, Arendshorst W, Smithies O, Coffman T. Angiotensin II responses in AT1A receptor-deficient mice: a role for AT1B receptors in blood pressure regulation. Am J Physiol. 1997;272:F515–F520. doi: 10.1152/ajprenal.1997.272.4.F515. [DOI] [PubMed] [Google Scholar]
- Okada S, Shikata K, Matsuda M, Ogawa D, Usui H, Kido Y, Nagase R, Wada J, Shikata Y, Makino H. Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes. 2003;52:2586–2593. doi: 10.2337/diabetes.52.10.2586. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- Zoccali C, Mallamaci F, Tripepi G, Benedetto FA, Cutrupi S, Parlongo S, Malatino LS, Bonanno G, Seminara G, Rapisarda F, Fatuzzo P, Buemi M, Nicocia G, Tanaka S, Ouchi N, Kihara S, Funahashi T, Matsuzawa Y. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:134–141. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- Becker B, Kronenberg F, Kielstein JT, Haller H, Morath C, Ritz E, Fliser D, MMKD Study Group Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. J Am Soc Nephrol. 2005;16:1091–1098. doi: 10.1681/ASN.2004090742. [DOI] [PubMed] [Google Scholar]
- Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- Etoh T, Inoguchi T, Kakimoto M, Sonoda N, Kobayashi K, Kuroda J, Sumimoto H, Nawata H. Increased expression of NAD(P)H oxidase subunits. NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibility by intensive insulin treatment. Diabetologia. 2003;46:1428–1437. doi: 10.1007/s00125-003-1205-6. [DOI] [PubMed] [Google Scholar]
- Tay Y, Wang Y, Kairaitis L, Rangan GK, Zhang C, Harris DC. Can murine diabetic nephropathy be separated from superimposed acute renal failure? Kidney Int. 2005;68:391–398. doi: 10.1111/j.1523-1755.2005.00405.x. [DOI] [PubMed] [Google Scholar]
- Sharma K, McCue P, Dunn SR. Diabetic kidney disease in the db/db mouse. Am J Physiol. 2003;284:F1138–F1144. doi: 10.1152/ajprenal.00315.2002. [DOI] [PubMed] [Google Scholar]
- Zhao HJ, Wang S, Cheng H, Zhang M-Z, Takahashi T, Fogo AB, Breyer MD, Harris RC. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol. 2006;17:2664–2669. doi: 10.1681/ASN.2006070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Sato W, Glushakova O, Heinig M, Clarke T, Campbell-Thompson M, Yuzawa Y, Atkinson MA, Johnson RJ, Croker B. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol. 2007;18:539–550. doi: 10.1681/ASN.2006050459. [DOI] [PubMed] [Google Scholar]
- Zheng S, Noonan WT, Metreveli NS, Coventry S, Kralik PM, Carlson EC, Epstein PN. Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes. 2004;53:3248–3257. doi: 10.2337/diabetes.53.12.3248. [DOI] [PubMed] [Google Scholar]
- Wahab N, Parker S, Sraer J-D, Mason R. The decorin high glucose response element and mechanism of its activation in human mesangial cells. J Am Soc Nephrol. 2000;11:1607–1619. doi: 10.1681/ASN.V1191607. [DOI] [PubMed] [Google Scholar]
- Murphy M, Godson C, Cannon S, Kato S, Mackenzie HS, Martin F, Brady HR. Suppression subtractive hybridization identifies high glucose levels as a stimulus for expression of connective tissue growth factor and other genes in human mesangial cells. J Biol Chem. 1999;274:5830–5834. doi: 10.1074/jbc.274.9.5830. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Macakova K, Raslik I, Micegova M, Grone H-J, Schonherr E, Robenek H, Echtermeyer F, Grassel S, Bruckner P, Schaefer R, Iozzo R, Kresse H. Absence of decorin adversely influences tubulointerstitial fibrosis of the obstructed kidney by enhanced apoptosis and increased inflammatory reaction. Am J Pathol. 2002;160:1181–1191. doi: 10.1016/S0002-9440(10)64937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costacou T, Zgibor JC, Evans RW, Otvos J, Lopes-Virella MF, Tracy RP, Orchard TJ. The prospective association between adiponectin and coronary artery disease among individuals with type 1 diabetes. The Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2005;48:41–48. doi: 10.1007/s00125-004-1597-y. [DOI] [PubMed] [Google Scholar]
- Maahs DM, Ogden LG, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, Hokanson JE, Ehrlich J, Eckel RH, Rewers M. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111:747–753. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- Tsioufis C, Dimitriadis K, Chatzis D, Vasiliadou C, Tousoulis D, Papademetriou V, Toutouzas P, Stefanadis C, Kallikazaros I. Relation of microalbuminuria to adiponectin and augmented C-reactive protein levels in men with essential hypertension. Am J Cardiol. 2005;96:946. doi: 10.1016/j.amjcard.2005.05.052. [DOI] [PubMed] [Google Scholar]
- Schalkwijk CG, Chaturvedi N, Schram MT, Fuller JH, Stehouwer CD, EURODIAB Prospective Complications Study Group Adiponectin is inversely associated with renal function in type 1 diabetic patients. J Clin Endocrinol Metab. 2006;91:129–135. doi: 10.1210/jc.2005-1117. [DOI] [PubMed] [Google Scholar]
- Looker HC, Krakoff J, Funahashi T, Matsuzawa Y, Tanaka S, Nelson RG, Knowler WC, Lindsay RS, Hanson RL. Adiponectin concentrations are influenced by renal function and diabetes duration in Pima Indians with type 2 diabetes. J Clin Endocrinol Metab. 2004;89:4010–4017. doi: 10.1210/jc.2003-031916. [DOI] [PubMed] [Google Scholar]
- Vionnet N, Tregouet D, Kazeem G, Gut I, Groop P-H, Tarnow L, Parving H-H, Hadjadj S, Forsblom C, Farrall M, Gauguier D, Cox R, Matsuda F, Heath S, Thevard A, Rousseau R, Cambien F, Marre M, Lathrop M. Analysis of 14 candidate genes for diabetic nephropathy on chromosome 3q in European populations: strongest evidence for association with a variant in the promoter region of the adiponectin gene. Diabetes. 2006;55:3166–3174. doi: 10.2337/db06-0271. [DOI] [PubMed] [Google Scholar]
- Li J-M, Shah AM. ROS generation by nonphagocytic NADPH oxidase: potential relevance in diabetic nephropathy. J Am Soc Nephrol. 2003;14:S221–S226. doi: 10.1097/01.asn.0000077406.67663.e7. [DOI] [PubMed] [Google Scholar]
- Hu T, Ramachandra Rao SP, Siva S, Valancius C, Zhu Y, Mahadev K, Toh I, Goldstein BJ, Woolkalis M, Sharma K. Reactive oxygen species production via NADPH oxidase mediates TGF-β-induced cytoskeletal alterations in endothelial cells. Am J Physiol. 2005;289:F816–F825. doi: 10.1152/ajprenal.00024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor β1 induces Nox 4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol. 2005;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]