Abstract
Cables is a cyclin-dependent kinase-binding nuclear protein that maps to chromosome 18q11-12. Here, we assessed Cables expression in 160 colorectal cancers (CRCs), its role in colon cancer cell growth, and the potential mechanisms of Cables inactivation. Expression levels, promoter methylation, and mutational status of Cables were investigated in colon cancer cell lines and primary colon tumors. Chromosome 18q loss of heterozygosity (LOH) was evaluated with multiple polymorphic markers. Cables inhibited cellular proliferation and colony formation in colon cancer cell lines. Cables expression was reduced in 65% of primary CRCs. No mutations were detected in 10 exons of Cables in 20 primary colon tumors. Cables promoter was methylated in cell lines with decreased Cables expression and vice versa. 5-Aza-2′-deoxycytidine resulted in increased Cables expression in methylated cell lines. There was a significant correlation between promoter methylation and Cables gene expression in primary colon tumors. Sixty-five percent of primary colon tumors demonstrated chromosome 18q LOH. LOH involving the Cables region was observed in 35% of cases, including those in which more distal portions of chromosome 18q were retained, and Cables expression was decreased in all such cases. Loss of Cables expression in 65% of CRCs suggests that it is a common event in colonic carcinogenesis, with promoter methylation and LOH appearing to be important mechanisms of Cables gene inactivation.
Colorectal cancer (CRC) is characterized by several unique features that make it well suited for the study of the molecular genetics of tumor progression. A stepwise model of colorectal tumorigenesis has been well defined and validated.1 The inactivation of the adenomatous polyposis coli tumor suppressor gene is an early event that leads to the development of polyps, followed by oncogenic KRAS mutations in the adenomatous stage. Later events include deletions on chromosome 18q and inactivation of the tumor suppressor gene TP53 on chromosome 17p with the transition to malignancy.
Chromosome 18q is lost in a high proportion (approximately 70%) of CRCs.2 There are many candidate tumor suppressor genes on chromosome 18q, including deleted in colon cancer (DCC), SMAD4 (DPC4), and SMAD2.3,4,5 DCC was recently shown to be the netrin-1 receptor.6 DCC is expressed in normal colonic mucosa and in both primary and metastatic colonic cancer.7 However, mice lacking the functional DCC gene do not develop colon tumors.8 The SMAD proteins mediate transforming growth factor-β effects and regulate genes involved in cell cycle control. SMAD4 is biallelically inactivated in approximately 60% of pancreatic cancers.9 However, the number of mutations identified in the SMAD genes has been relatively small in colorectal cancer. Missense mutations of SMAD4 were found in 16% of primary colorectal cancers.10 SMAD2 mutations have been found in less than 10% of cases.11,12 Thus the observed frequency of alterations in the DCC and SMAD genes does not explain the high proportion of chromosome 18q loss in colorectal cancer. This suggests that other tumor suppressor genes, in addition to the DCC and SMAD genes, may be targets for the 18q loss.
Cables is a nuclear protein that plays a role in proliferation and/or differentiation and maps to human chromosome 18q11-12.13,14 Cables interacts with multiple cyclin-dependent kinases (cdks) and regulates cdk phosphorylation and activity. It functionally connects the Cdks and nonreceptor tyrosine kinases and modulates cdk activity by cdk-tyrosine 15 phosphorylation.13,14,15 Furthermore, ectopic expression of Cables inhibits cell proliferation and prevents formation of tumors in nude mice.13 Previous studies found that Cables gene deletion leads to abnormal endometrial epithelial growth in mice.16 Likewise, in response to a carcinogen (1,2-dimethylhydrazine), Cables−/− mice have an increased incidence of colorectal tumors and reduced survival rates compared with Cables+/+ mice.17 Furthermore, primary mouse embryonic fibroblasts from the Cables−/− mice show an increased rate of cell proliferation, delayed onset of senescence, and increased growth in low serum concentration.18 Thus, Cables seems to exhibit characteristics of a tumor suppressor gene. Our objectives in this study were to assess Cables expression in a large number of primary CRC samples, to define the function of Cables in colon cancer cell growth, and to identify potential mechanisms of Cables gene inactivation in these tumors.
Materials and Methods
Cell Lines and Tissues
We retrospectively collected discarded malignant human colonic tissue in accordance with the Massachusetts General Hospital Institutional Review Board Guidelines. In total, 160 colorectal adenocarcinomas, including 20 fresh frozen tissues, were collected. The colon cancer cell lines HT-29, WiDr, LoVo, DLD1, and HCT116 were maintained in Dulbecco’s modified Eagle’s medium, whereas the SNU81 line was maintained in RPMI 1640 medium (Mediatech, Herdon, VA), all supplemented with 10% fetal bovine serum, penicillin (100 μg/ml), and streptomycin (100 μg/ml) in humidified 5% CO2 atmosphere at 37°C.
Immunohistochemistry
All tissues had been conventionally fixed in 10% formaldehyde and paraffin embedded. Confirmation of the diagnosis was made on review of standard hematoxylin and eosin sections. Immunohistochemical staining was performed using a specific affinity-purified Cables antisera that was produced and purified as previously described.13 Sections of normal and pathological tissues under study were stained with the purified rabbit anti-Cables antisera at a 1:200 dilution, using a microwave for antigen retrieval in citrate buffer (pH 6.0) and using the avidin-biotin staining method (Vector Labs, Burlingame, CA). Negative control sections were immunostained under the same conditions substituting preabsorbed antisera and preimmune rabbit antisera for primary antibodies. Strong nuclear staining in more than 10% of tumor cells was considered to be positive immunolabeling for Cables.
Stable HT29 cell lines expressing a small-interfering RNA (siRNA) against Cables were generated to verify the specificity of immunohistochemistry of Cables. DNA-vector based siRNA construct was generated by GenScript using the 71-bp sequence (5′-GGATCCCGCAGTGATTGACTACGTGAATTCAAGAGATTCACGTAGTCAATCACTGTTTTTTCC-3′) and the pRNA-U6.1/Neovector. Stable HT29 cell lines were generated with the plasmid by transfection using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Stably transfected cells were selected by growth in G418 and Cables protein levels analyzed by Western blot. Controls were transfected with vector alone. Stable cell lines were also formalin-fixed, paraffin-embedded, and immunostained with Cables antisera as described above.
Loss of Heterozygosity Assay
Loss of heterozygosity (LOH) assay in the region of the Cables gene on chromosome 18q11-12 was examined using D18S44 and D18S1107. More distal 18q LOH was evaluated using microsatellite markers D18S55 (18q22.1), D18S56 (18q12.3), D18S67 (18q12.3), and D18S487 (18q21.1) as previously described.19 Archival DNA was extracted from formalin-fixed specimens by using standard methods. Sections 8-μm thick were mounted and microdissected into normal and tumor component by superimposing the unstained section with the corresponding stained section. We duplicated PCR reaction and electrophoresis in each sample to exclude allele dropouts of one of two alleles.
Cell Proliferation Assay in Colon Cancer Cell Lines
Stable colon cancer cell lines overexpressing Cables were generated by techniques described previously.13 Briefly, a plasmid containing full-length Cables in a pCIN4 vector was transfected into colon cancer cells using Lipofectamine 2000. Stably transfected cells were selected by growth in G418, and excess Cables protein was confirmed by Western blot analysis. Controls were transfected with vector alone. The proliferation rate of the Cables and control cell lines was determined by seeding 1 × 104 cell/well in 24-well tissue culture plates and counting the cells in triplicate for up to 10 days.
Colon cancer cells (8 × 105) were transfected as described above, divided into three 100-mm dishes, and selected after incubation for 19 days in appropriate media containing G418 selection solution that allows the killing of nontransfected cells. The cells were stained with Giemsa, and number of colonies was counted.
Luciferase Promoter Reporter Assay
To evaluate the potential promoter activity of the 5′ flanking region of Cables gene, different length fragments (2000, 1500, 1000, and 355 bp) upstream of the Cables gene translating initiating ATG codon were PCR-amplified from human genomic DNA. To facilitate directional cloning, a KpnI site was added to the 5′ ends of the forward primers, and a BgIII site was added to the 5′ end of the reverse primer. After digestion with KpnI and BgIII, the PCR products were cloned into pGL3 basic vector. For transient transfection, HT29 and SNU81 cells were seeded at 1 × 104 in 24-well tissue culture plate in Opti-MEM I reduced serum media the day before transfection. Cells were cotransfected using 0.6 μl for Lipofectin 2000 reagent with 400 ng of reporter plasmid and 40 ng of control plasmid phRL-null, which encodes Renilla luciferase control vector for each well. Luciferase assays were performed using the dual-luciferase reporter assay system and a microplate luminometer. Reporter activity was expressed as the ratio of luciferase activity of various sized constructs with activity in cells transfected with the empty vector.
Real-Time RT-PCR
Total RNA was isolated using TRIzol reagent (Sigma, St. Louis, MO) according to the manufacturer’s instructions. All RNA samples were DNase-treated using the DNA-free kit (Ambion, Austin, TX) and reverse transcribed to cDNA using the SuperScript III First Strand Synthesis cDNA kit (Invitrogen). Real-time PCR was performed by Cepheid SmartCycler II using Lightcycler Taqman Master kit (Roche, Mannheim, Germany). TaqMan probes were synthesized for both amplicons to be quantified (Biosearch Technologies, Novato, CA): Cables probe, 5′-6-FAMd[CTGATGGGAAGACTGTTTCCTATACCCAA]BHQ-1-3′; and β-actin probe, 5′–6-FAMd[ATCCACGAAACTACCTTCAACTCCATCA]BHQ-1-3′. The following master mix of reaction components was prepared to the indicated end concentration; sense primer (0.4 μmol/L), antisense primer (0.4 μmol/L), probe (0.2 μmol/L), water, and 4 μl of Master Mix. One microliter of cDNA (sample or standard) was added to the respective reaction tubes. Specific primers used for Cables are as follows: Cables sense, 5′-GGACGGAGGAAGACAATCAA-3′; Cables antisense, 5′-CAGGTTACGGAACTGGGAGA-3′; β-actin sense, 5′-CTTCCAGCCTTCCTTCCTG-3′; and β-actin antisense, 5′-TTGGCGTACAGGTCTTGC-3′. Real-time PCR cycling conditions were as follows: for Cables, 1 cycle of 95°C for 10 minutes, 40 cycles of 95°C for 10 seconds, 60°C for 45 seconds, and 72°C for 35 seconds; and for β-actin, 1 cycle of 95°C for 10 minutes, 40 cycles of 95°C for 10 seconds, 65°C for 45 seconds, and 72°C for 35 seconds. Relative expression levels were calculated automatically using the cycle threshold value from each individual samples. Standard curves containing a known number cDNA copies were generated in 10-fold increments for Cables and β-actin. cDNA copy numbers generated for Cables were then normalized to β-actin numbers.
Mutation Assay for Cables
The Cables coding exons and their flanking intronic regions were amplified by PCR from genomic DNA extracted from colonic adenocarcinoma tissue. Five microliters of genomic DNA was PCR amplified in a final volume of 50 μl containing buffer D (Epicenter Technologies, Madison, WI). PCR primer sets for each of 10 exons, including intron-exon boundary, are provided in detail in Table 1. PCR was performed using the following conditions: DNA denaturing at 95°C for 30 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds, for a total of 45 cycles. Amplified products were purified by QIAquick PCR purification kit (Qiagen, Valencia, CA) and sequenced by a BigDye terminator cycle sequencing kit (Applied Biosystems, Foster City, CA) with an ABI PRISM 3100 sequencer according to the manufacturer’s guidelines (Applied Biosystems).
Table 1.
Primer Sequences for Mutation Analysis of 10 Exons in Cables
| Primer name | Primer sequence | Product size (bp) |
|---|---|---|
| Mutation assay | ||
| Exon 1A | F-5′-CTCTCGCGTCCTCCTCTTC-3′ | 477 |
| R-5′-GCCATTGTGTCCGTCTGC-3′ | ||
| Exon 1B | F-5′-GAGCTAGGTAGCGAGGCGT-3′ | 556 |
| R-5′-AGCAAGCTGAACCGAGTCCT-3′ | ||
| Exon 1C | F-5′-CCTCTCCTTCCTCACCAACA-3′ | 750 |
| R-5′-ATGGGAGCAACCCGTGTC-3′ | ||
| Exon 2 | F-5′-GGAACATGGCAGCCTCTTAG-3′ | 354 |
| R-5′-CCCATAGTCCCTGGAGTTCA-3′ | ||
| Exon 3 | F-5′-GGGTGACTCAAGCCATTTGT-3′ | 362 |
| R-5′-ATGAGTGGGGTGGAAATTGT-3′ | ||
| Exon 4 | F-5′-GCTATGAATGCCATGAATGCT-3′ | 373 |
| R-5′-GCACACACACAACGAGGAAG-3′ | ||
| Exon 5 | F-5′-CAGGGCTCACCTGGTCATT-3′ | 350 |
| R-5′-CATCACATGCCTGGGAATC-3′ | ||
| Exon 6 | F-5′-TGAATGTGGGGTGACAACTG-3′ | 350 |
| R-5′-CCCTGTCTTCCAGTCTGCAT-3′ | ||
| Exon 7 | F-5′-CCAGCCTTTCTTCATTCCAG-3′ | 373 |
| R-5′-GGACAGGAGGCAATACTGGT-3′ | ||
| Exon 8 | F-5′-CCGAGCCCTTGTTGTAGCTC-3′ | 354 |
| R-5′-TCAATCACTGGAACCACAGG-3′ | ||
| Exon 9 | F-5′-ATGGGGGAGTTTTTCTGTCC-3′ | 357 |
| R-5′-TGACCGAATCCTTCTGAGAGA-3′ | ||
| Exon 10A | F-5′-TGGCTGGTGGATAGAGAAGC-3′ | 622 |
| R-5′-ACTCTCTTCCCCCGTATGCT-3′ | ||
| Exon 10B | F-5′-TCTGTCCACTAGCGAGAATCC-3′ | 612 |
| R-5′-TGGAGAAAGTGCGAGGGTTA-3′ | ||
| Exon 10C | F-5′-AGGCGAGTTCTGAGTGTTGG-3′ | 625 |
| F-5′-TAGAATCAGTGTGCGCAAGG-3′ |
F, forward; R, reverse.
Bisulfite PCR and Sequencing
Sodium bisulfite treatment was performed by EZ DNA methylation kit (Zymo Research, Orange, CA) per the manufacturer’s instruction. DNA was amplified in a final volume of 50 μl containing buffer F (Epicenter Technologies), 1.25 U of TaqDNA polymerase (Roche) and 0.2 μmol/L each specific sense and antisense primer. The PCR primers were as follows: CabMet1, 5′-agtttatgagggagaagagat-3′ (sense) and 5′-atccttttcataataacac-3′ (antisense) (−762 to −378 bp from TSS site); CabMet2, 5′-F-5-gtgttattatgaaaaggat-3′ (sense) and 5-ccctctttgcaataacac-3′ (antisense) (−396 to 54 from TSS site); and CabMet3, 5′-F-5-gtgttattgcaaagaggG-3′ (sense) and 5-ctcctcgccaccgccccact-3′ (antisense) (37 to 663 from TSS site). PCR reaction was performed using the following conditions: DNA denaturing at 95°C for 30 seconds, annealing at 50°C for 30 seconds, and extension at 68°C for 30 seconds for total of 45 cycles. PCR products were cloned into pCR2.1 (Invitrogen) using TOPO-TA Cloning kit (Invitrogen). At least 10 clones per reaction were sequenced by a BigDye terminator cycle sequencing kit (Applied Biosystems) with an ABI PRISM 3100 sequencer according to the manufacturer’s guidelines (Applied Biosystems). For the fresh colon tumor samples, amplified bisulfite PCR products were purified by QIAquick PCR purification kit (Qiagen) and directly sequenced by a BigDye terminator cycle sequencing kit (Applied Biosystems) with an ABI PRISM 3100 sequencer according to the manufacturer’s guidelines (Applied Biosystems) and quantified by percentage of ratio of the peak heights of the cytosine to cytosine plus thymine signals as previously described.20 The direct sequencing method was verified by comparison with the bisulfite cloning sequencing method in selected colon cancer cell lines and all colon tumor samples (Figure 1).
Figure 1.
Comparison of direct bisulfite DNA sequencing method (A) and cloning method (B) in selected colon cancer cell lines and colon tumor tissues. The bisulfite-sequencing results by cloning generally matched with those of direct sequencing in clinical samples and also in selected colon cancer cell lines. Number of column represents ordinal number for the 16 CpG sites between −628 to −307 bp from transcription start of Cables (A). Ten clones were evaluated from each selected cell line and tumor samples for 16 CpG sites between −628 and −307 bp from transcription start site. Each column and row represents an individual CpG site and clone, respectively. B: ▪, methylated CpG sites; □, unmethylated CpG sites.
5-Aza-2′-Deoxycytidine and Trichostatin A Treatment
Colon cancer cell lines were seeded in 100-mm tissue culture dishes and treated with the demethylating reagent 5-Aza-2′-deoxycytidine (5-aza-dC; Sigma) at a final concentration of 8 μmol/L for 4 days or with a histone deacetylase inhibitor trichostatin A (Calbiochem, San Diego, CA) at final concentration of 300 nmol/L for 2 days. PBS was used as a control for nonspecific solvent effects on cells. Methylation status of 5-aza-dC-treated cell lines was confirmed by cloning and sequencing individual clones as described above.
Results
Loss of Cables Expression in Primary CRCs
Cables immunohistochemistry was performed on cultured cells and tissue sections using the affinity-purified Cables antisera as previously described.13 Preimmune and preabsorbed antisera were used as controls to demonstrate specificity. In addition, the immunohistochemical staining data were correlated to Western blots. Stable HT29 cell lines expressing siRNA against Cables were generated to verify the specificity of immunohistochemistry. Cables protein levels were reduced more than 75% in the siRNA cell line as detected by immunostaining and Western blot with the affinity-purified Cables antisera (Figure 2).
Figure 2.
Cables protein levels are reduced by Cables siRNA in HT 29 cell lines as detected by immunohistochemistry (A) and Western blotting (C) with the affinity-purified Cables antisera. There is strong expression of Cables by immunohistochemistry (B) and Western blotting (C) in the control cell line without siRNA.
In normal colonic epithelium, Cables staining was weak at the base of the crypts and increased with differentiation toward the luminal surface (Figure 3A). Cables expression was detected in 35% of the primary colorectal cancers analyzed, whereas the remaining 65% (105 of 160) showed decreased or absent nuclear staining (Figure 3, B and C). In each case, benign colonic epithelium or admixed inflammatory cells adjacent to negative tumor cells showed nuclear staining. One case that showed adenoma merging into carcinoma showed patchy positive cells in the residual adenoma, whereas no staining was observed in the adjacent colon cancer, consistent with the model that chromosome 18q loss occurs frequently with the transition to malignancy (Figure 3D). However, further studies with additional cases are needed.
Figure 3.
Normal colonic epithelium exhibits weak Cables expression by immunohistochemistry at the base of the crypts and stronger expression with differentiation toward the luminal surface (A). Cables expression in invasive adenocarcinomas was typically absent (C) but was retained in some cases (B). An adenomatous component of a tumor reveals preserved nuclear expression of Cables (arrow), but decreased expression was seen in the transition to the adenocarcinomatous component (D). Original magnification: ×100 (A); ×400 (B–D).
Overexpression of Cables Inhibits Growth of Colon Cancer Cells
We assessed Cables tumor suppressor activity using an in vitro colony formation assay and cell proliferation assay. Transfection of colon cancer cell lines with a Cables expression construct markedly reduced the number of G418-resistant colonies compared with transfection of empty vector. Cell lines with lower levels of Cables expression (DLD1, HCT116, and SNU81) showed a greater reduction in colony formation after Cables transfection compared with cell lines with higher levels of Cables (HT29 and WiDr) (Figure 4A). Growth characteristics of the colon cancer cell lines were compared between Cables stably transfected cell lines and control (vector only) cell lines. Decreased numbers of cells in Cables transfected colon cancer cell lines were present after 7 days and throughout the experiment (Figure 4B). There was a greater decrease in cell number in DLD1 (Cables methylated and down-regulated cell line) than HT-29 and LoVo (Cables unmethylated and retained cell lines). An insufficient number of colonies grew to perform a proliferation assay using SNU81 cells (Cables methylated and down-regulated cell line), probably because of greater cellular suppression by Cables. The doubling time in the exponential growth phase for the cell lines transfected with control/Cables vectors were as follows: HT-29, 22/37 hours; LoVo, 35/57 hours; and DLD1, 19/38 hours.
Figure 4.
Tumor suppressor activity of Cables. A: Colony formation assay showed marked reduction of G418-resistant colonies in Cables-transfected colon cancer cell lines compared with vector only transfected cells. Top: Representative example of colony formation assay for HCT116 cells. Bottom: Quantitative analysis of inhibition of colony formation by Cables. B: Proliferation rate of colon cancer cell lines stably overexpressing Cables or empty vector as control. The proliferation rate of the Cables and control cell lines was determined by seeding 1 × 104 cells/well and counting the cells in triplicate for up to 10 days. Three control and experimental cell lines were used for each cell line assay.
18q LOH with Loss of Cables Expression in Colon Tumors
To examine loss of chromosome 18q11-12, 20 cases of fresh colon tumors and paired normal tissue were studied for LOH using highly polymorphic markers that map to 18q11 (D18S44 and D18S1107). We also examined more distal 18q LOH microsatellite markers (D18S55, D18S56, D18S67, and D18S487), which included markers of DCC/Smad4 (D18S67 and D18S487). Eighteen cases were heterozygous at the D18S44 locus, whereas 15 cases were heterozygous at D18S1107. Of the 18 and 15 informative cases for D18S44 and D18S1107, six cases and two cases showed LOH, respectively (Figure 5). In addition, 19, 14, 14, and 14 cases were heterozygous for D18S55, D18S56, D18S67, and D18S487, and seven, five, five, and five cases showed LOH, respectively (Figure 5). Overall, 13 of 20 (65.0%) cases exhibited 18q LOH at one or more microsatellite markers. The relationship between Cables expression by immunohistochemistry and 18q LOH showed that cases with LOH in the Cables region (D18S44 and D18S1107) showed higher rates of loss of Cables (six of seven, 85.7%) compared with cases with any 18q LOH (all six markers) (9 of 13, 69.2%) and 18q distal LOH (D18S55, D18S56, D18S67, and D18S487) (6 of 10, 60%) (Table 2). Three cases (nos. 7, 12, and 15) showed LOH only in the region of Cables (D18S44 and D18S1107) without LOH of distal 18q markers (D18S55, D18S56, D18S67, and D18S487) (Figure 6A, below). These three cases all showed loss of Cables expression by immunohistochemistry.
Figure 5.
Loss of heterozygosity at 18q11-12 in colon tumors. A representative example of an LOH study using the D18S44 microsatellite marker is shown. N, paired normal control. T, tumor sample. Arrow highlights loss of allele in the tumor sample.
Table 2.
Relationship between 18q LOH and Cables Expression Detected by Immunohistochemistry in 20 Fresh Colon Tumor Samples
| Case no. | Immunohistochemistry of Cables
|
P value* | ||
|---|---|---|---|---|
| Negative | Positive | |||
| 18q LOH* | 0.356 | |||
| Negative | 7 | 3 | 4 | |
| Positive | 13 | 9 | 4 | |
| 18q LOH distal† | 1.000 | |||
| Negative | 10 | 6 | 4 | |
| Positive | 10 | 6 | 4 | |
| 18q Cables LOH¶ | 0.158 | |||
| Negative | 13 | 6 | 7 | |
| Positive | 7 | 6 | 1 | |
LOH for one of D18S44, D18S55, D18S56, D18S67, D18S487, or D18S1107.
LOH for one of D18S55, D18S56, D18S67, or D18S487.
LOH for one of D18S44 or D18S1107.
Figure 6.
A: Cables promoter methylation, Cables immunohistochemistry, and 18q LOH status in 20 primary colon adenocarcinoma tissues. Cables promoter methylation was determined by using direct bisulfite DNA sequencing method. The extent of methylation of individual CpG sites is shown in the left panel. Number of column and row represents ordinal number for the 16 CpG sites between −628 and −307 bp from transcription start of Cables and the case numbers of 20 fresh colon tumors, respectively. Cables expression was assessed by immunohistochemistry (IHC). Strong Cables nuclear staining in more than 10% of tumor cells was considered to be positive and less than 10% to be negative (middle panel). 18q LOH status with the D18S44, D18S1107, D18S55, D18S56, D18S67, and D18S487 markers is shown in the right panel. B: Direct sequencing result of fresh colon tumor samples 2 (top) and 5 (bottom) showing three CpG sites. Extent of CpG methylation was estimated as ratio of cytosine peak to the cytosine plus thymine peak signals using direct bisulfite DNA sequencing method.
Hypermethylation of the Cables Promoter Is Associated with Silencing of Cables Gene Expression in Colon Cancer Cell Lines
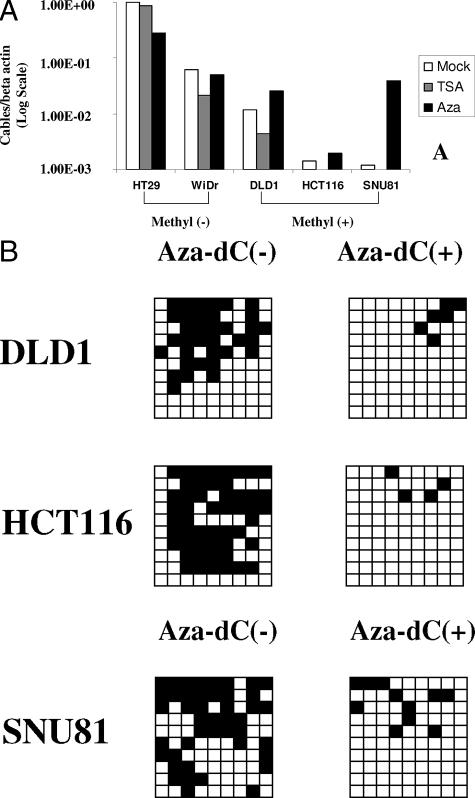
To assess further the mechanism of Cables gene inactivation in colorectal cancer, we first sequenced all 10 exons of the Cables gene in 20 fresh colon tumors and six colon cancer cell lines. We found several polymorphisms (data not shown), but no mutations. To examine further the potential mechanisms of Cables loss, we confirmed the Cables expression status of colon cancer cell lines by real-time RT-PCR and immunocytochemistry. Expression of Cables was decreased in SNU81, HCT116, DLD1, and LoVo cell lines, but HT-29 and WiDr showed strong expression of Cables (Figure 7, A and B). Therefore, we investigated the methylation status of the promoter of Cables gene in these cell lines. We analyzed CpG site methylation between −762 and +663 bp from the transcriptional start site. There were 107 CpG sites between −762 and + 663 bp from the transcription start site, and we identified methylation of CpG islands in 16 CpG sites between −628 and −307 bp from the transcription start site (Figure 7C). There was no methylation at other 91 CpG sites. Methylation mapping by bisulfite sequencing in the colon cancer cell lines demonstrated that Cables was methylated in HCT116, SNU81, and DLD1 cells; partially methylated in LoVo cells; and unmethylated in HT-29 and WiDr cells (Figure 7C). The methylation status of these cell lines correlated well with the results of Cables expression in each cell line; these 16 CpG sites were methylated in colon cancer cells that had decreased Cables expression, and colon cancer cells in which the promoter was not methylated exhibited high levels of Cables expression.
Figure 7.
Cables expression levels in colon cancer cell lines by immunocytochemistry (A) and real-time RT-PCR (B). Expression of Cables was high in HT-29 and WiDR and decreased in LoVo, DLD1, HCT116, and SNU81. C: Cables gene promoter methylation status in colon cancer cell lines. Top: CpG sites are represented by vertical lines spanning from −1.5 kb to the end of exon 1 (+1238). Bottom: The Cables gene promoter was methylated in SNU81 cells, HCT116, and DLD1; partially methylated in LoVo cells; and unmethylated in WiDR and HT-29 cells. The Cables gene promoter methylation status correlated with expression levels of each cell lines. Part of this figure was made based on MethPrimer21 (top). Ten clones were evaluated from each cell line for 16 CpG sites between −628 and −307 bp from the transcription start site. Each column and row represents individual CpG site and clone, respectively. Middle panel: ▪, methylated CpG sites; □, represent unmethylated CpG sites. Original magnification: ×400 (A).
To confirm the role of epigenetic modification in down-regulation of Cables gene expression, colon cancer cell lines were treated with 5-aza-dC, a demethylating agent, and trichostatin A, a histone deacetylase inhibitor. Treatment with 5-aza-dC induced an increase of Cables expression in low Cables-expressing methylated cell lines (Figure 8A), whereas trichostatin A did not. We confirmed that 5-aza-dC-treated cell lines revealed decrease of methylation in CpG sites confirmed by cloning sequencing (Figure 8B). To assess promoter activity of the 5′ flanking region of Cables gene, we performed transient transfection of various promoter constructs into colon cancer cell lines. Luciferase activity was decreased after transfection with pGL3-355, which lacks the promoter region spanning −607 to +37 (Figure 9). These findings suggested that the promoter region located between −607 and +37 bp from the transcription start site is an important regulatory region of the Cables gene promoter, and this finding is consistent with the methylation data.
Figure 8.
Demethylation analysis with 5-aza-dC induced an increase in Cables expression in methylated colon cancer cell lines but not in unmethylated colon cell lines. A: Trichostatin A treatment showed no increase of Cables in methylated and unmethylated colon cancer cell lines. For real-time RT-PCR, the Cables expression was normalized to that of β-actin. All experiments were repeated a minimum of three times independently. B: 5-Aza-dC-treated cell lines revealed demethylation in CpG sites confirmed by cloning sequencing. Each column represents methylation status for the 9 CpG sites between −628 and −409 bp from transcription start of Cables (A). ▪, methylated CpG sites; □, unmethylated CpG sites. Ten clones were evaluated from selected cell line.
Figure 9.
Cables promoter reporter assay. Various lengths of upstream promoter region of Cables around the transcriptional start site were transiently transfected into colon cancer cell lines HT29 (top) and SNU81 cell lines (bottom). Luciferase activity was markedly decreased when the region between −607 (pGL3-1K) and + 37 (pGL3-355) was deleted.
Methylation Status of Cables Promoter in Primary Colorectal Tumors
To examine the methylation status of the Cables gene in 20 fresh colon tumor samples, we performed direct sequencing after bisulfite treatment (Figure 6). Significant methylation of each CpG site was defined as at least 40% methylation quantified by the direct sequencing method. We defined methylation as significant if there was methylation of more than three CpG sites. In the 20 fresh colon tumor samples studied, there was a significant correlation between Cables methylation status and Cables down-regulation by immunohistochemistry (Table 3). Thus, hypermethylation of the promoter region of Cables also correlated with down-regulation of Cables in colon tumors.
Table 3.
Relationship between Methylation, Cables LOH, and Cables Expression Detected by Immunohistochemistry in 20 Fresh Colon Tumor Samples
| Case no. | Immunohistochemistry of Cables
|
P value* | ||
|---|---|---|---|---|
| Negative | Positive | |||
| Methylation status | ||||
| Unmethyl group | 8 | 2 | 6 | 0.019 |
| Methyl group | 12 | 10 | 2 | |
| Cables LOH or methylation† | ||||
| Negative | 5 | 0 | 5 | 0.004 |
| Positive | 15 | 12 | 3 | |
By Fisher probability exact test.
LOH for either one of D18S44 or D18S1107.
Of the 12 (of 20) examined primary colon tumor samples that showed decreased Cables expression, four cases exhibited both Cables promoter methylation and LOH of chromosome 18q, six cases showed only Cables promoter methylation, and two cases showed only LOH of chromosome 18q (Figure 6). There was a significant correlation between cases showing either Cables LOH or methylation and loss of Cables expression by immunohistochemistry (Table 3).
Discussion
In the present study, we analyzed expression of Cables in primary CRCs and studied possible mechanisms of inactivation of Cables. We found that Cables expression was decreased in 65% of primary CRCs. Loss of expression of Cables correlated with hypermethylation of the promoter region of Cables and with 18q LOH in cell lines and primary CRCs.
Cables interacts with multiple cyclin-dependent kinases and links the cdks and nonreceptor tyrosine kinases.13,14 Enhanced cdk2 tyrosine phosphorylation occurs in the presence of Cables, which can inhibit cellular proliferation. Cables also interacts with p53 and potentiates p53-induced cell death.22 Cables is lost in a variety of cancers, including head and neck, lung, endometrial, ovarian, and colon cancers.13,16,23,24 Our results show that Cables expression is frequently absent in malignant colonic epithelium. Cables expression by immunostaining was decreased or absent in 65% (105 of 160) of primary CRCs. A previous study localized Cables to human chromosome 18q11-12.13 Chromosome 18q deletions are observed in a high proportion of colorectal cancers.2 SMAD2, SMAD4, and DCC genes are located on chromosome 18q and are mutated in approximately 20% of colon cancers, not enough to account for the higher rates of 18q loss in colon cancer.25,26 In addition, the region of chromosome 18q loss is large, so more than one relevant gene may be lost.
In the present study, we found that overall 13 of 20 (65.0%) cases showed 18q LOH for at least one informative microsatellite marker. These data are in the range (45 to 81%) of 18q LOH in previously published data. Seven (35%) of the 20 fresh colon tumors showed LOH in the region of Cables, proximally on chromosome 18q, and Cables expression was decreased in most of these cases (six of seven). Furthermore, three of the seven cases showed LOH only in the region of Cables (D18S44 and D18S1107) without LOH of distal 18q markers (D18S55, D18S56, D18S67, and D18S487). The finding of proximal LOH without distal LOH supports the idea that 18q11-12 (the Cables region) is an important region that is lost in tumorigenesis and is not just a “chromosomal bystander effect.”
Overexpression of Cables in colon cancer cell lines showed tumor suppressor activity, including inhibition of colony formation and cell growth. Previous studies have shown that Cables−/− mice treated with carcinogen (1,2-dimethylhydrazine) develop more tumors compared with Cables+/+ mice, and primary mouse embryonic fibroblasts from Cables−/− mice displayed increased cellular proliferation.17,18 The present data and our previous observations support the hypothesis that Cables is a relevant tumor suppressor gene on 18q in colon cancer.
In our study, 6 of 13 primary colon tumors without LOH exhibited lower levels of Cables expression. Thus, mechanisms other than 18q LOH are likely involved in the down-regulation of Cables. We did not find any mutations in the 10 exons of Cables in primary colon tumors. Other tumor suppressor genes that show LOH without mutations often have epigenetic modifications that contribute to silencing of tumor suppressor genes.27,28,29 Widespread genomic hypomethylation in benign adenomas and in malignant colon cancer is known.30 Interestingly, this global hypomethylation occurs in the setting of localized hypermethylation.29 These observations suggested that loss of Cables might be caused by promoter hypermethylation. Hypermethylation of CpG islands within promoter sequences of specific tumor suppressor genes can have a potent silencing effect and has been reported to silence p16 in 28 to 55% of colon cancers and RASSF2 in 42% of colon cancers.31,32,33,34 The upstream region of Cables contains a number of CpG islands. We identified methylation of 16 CpG sites between −628 and −307 bp from the transcription start site of Cables. There was a significant correlation between methylation of this CpG-rich cluster region and Cables expression in colon cancer cell lines and human colon cancer samples, and we confirmed the functional significance by a demethylation assay using 5-aza-dC. These findings suggest that promoter hypermethylation of Cables plays an important role in silencing of the Cables gene in CRCs. Furthermore, the CpG-rich cluster that showed hypermethylation overlaps the region that is defined in the Cables promoter reporter assay (−607 to 37 bp from transcription start site).
In the 12 (of 20) examined primary colon tumor samples that showed decreased Cables expression, 4 cases exhibited both promoter methylation and LOH of proximal chromosome 18q in the region of Cables, 6 cases showed only promoter methylation of the Cables gene, and two cases showed only LOH of proximal chromosome 18q in the region of Cables. We believe that down-regulation of Cables in colon cancers is likely to be related to hypermethylation of CpG islands in the promoter of Cables coupled with 18q LOH in some cases. In cases with only promoter methylation of Cables and no LOH, methylation of both alleles could be contributing to down-regulation of Cables. In the primary tumor samples, it is difficult to be certain whether the methylation is biallelic or monoallelic because of the admixture of normal cell types (fibroblasts, endothelial cells, and inflammatory cells) in the tissue. In this situation, we believe that methylation of both alleles is supported by finding greater than 50% methylated clones in conjunction with down-regulation of Cables by immunohistochemistry, especially in cases with only promoter methylation of Cables and no detectable Cables LOH (six cases in our study). In cases with only LOH, it is possible that haploinsufficieny can explain the reduced levels of Cables expression.35 Similar to Cables, a number of other tumor suppressor or putative tumor suppressor genes show allelic loss and hypermethylation, such as BRCA1 in ovarian cancer and RNX3 in hepatocellular carcinoma.36,37 Such reported associations between LOH and hypermethylation in these other cancer genes suggest that our findings of hypermethylation of the Cables gene plus 18q LOH in some cases are the most likely mechanism of gene inactivation.
The present data show that down-regulation of Cables in colon cancers is likely to be related to hypermethylation of CpG islands in the promoter of Cables coupled with 18q LOH. Decreased Cables expression likely provides a growth advantage to cells by allowing faster progression through the cell cycle and by inhibiting apoptosis. This is supported by slower growth of colon cancer cell lines when Cables is overexpressed, as well as by faster growth of Cables knockout mouse embryo fibroblasts and larger tumors in Cables knockout mice. Collectively, these findings support the hypothesis that Cables is a relevant tumor suppressor gene on chromosome 18q in CRC.
Footnotes
Address reprint requests to Lawrence R. Zukerberg, M.D., Department of Pathology, Massachusetts General Hospital, Warren 2, 55 Fruit St., Boston, MA 02114. E-mail: lzukerberg@partners.org.
Supported by National Institutes of Health grants RO1 CA 103883-01 (to L.R.Z.) and RO1 CA 98333 (to B.R.R.).
D.Y.P. and H.S. are co-first authors of this work.
References
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Goel A, Arnold CN, Niedzwiecki D, Chang DK, Ricciardiello L, Carethers JM, Dowell JM, Wasserman L, Compton C, Mayer RJ, Bertagnolli MM, Boland CR. Characterization of sporadic colon cancer by patterns of genomic instability. Cancer Res. 2003;63:1608–1614. [PubMed] [Google Scholar]
- Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Fearon ER, Cho KR, Nigro JM, Kern SE, Simons JW, Ruppert JM, Hamilton SR, Preisinger AC, Thomas G, Kinzler KW, Vogenlstein B. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990;247:49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Thiagalingam S, Lengauer C, Leach FS, Schutte M, Hahn SA, Overhauser J, Willson JK, Markowitz S, Hamilton SR, Kern SE, Kinzler KW, Vogelstein B. Evaluation of candidate tumour suppressor genes on chromosome 18 in colorectal cancers. Nat Genet. 1996;13:343–346. doi: 10.1038/ng0796-343. [DOI] [PubMed] [Google Scholar]
- Keino-Masu K, Masu M, Hinck L, Leonardo E, Chan S, Culotti J, Tessier-Lavigne M. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- Gotley D, Reeder J, Fawcett J, Walsh M, Bates P, Simmons D, Antalis TM. The deleted in colon cancer (DCC) gene is consistently expressed in colorectal cancers and metastases. Oncogene. 1996;13:787–795. [PubMed] [Google Scholar]
- Fazeli A, Dickinson SLO, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli E, Keino-Masu K, Rayburn H, Simons J, Bronson RT, Gordaon JI, Tessier-Lavigne M, Weinberg RA. Phenotype of mice lacking functional deleted in colorectal cancer (DCC) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- Hahn SA, Schutte M, Hoque A, Schutte M, Moskaluk CA, Da Costa LT, Rosenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE. DPC4: a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Kohmura H, Futamura M, Kida H, Tanemura K, Kida H, Tanemura H, Shimokawa K, Saji S. Somatic alterations of the DPC4 gene in human colorectal cancers in vivo. Gastroenterology. 1996;111:1369–1372. doi: 10.1053/gast.1996.v111.pm8898652. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Kohmura H, Futamura M, Aoki S, Ymaguchi K, Kida H, Tanemura H, Shimokawa K, Saji S. Somatic alterations of the SMAD-2 gene in human colorectal cancers. Br J Cancer. 1998;78:1152–1155. doi: 10.1038/bjc.1998.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppert K, Scherer S, Ozcelik H, Pirone R, Hoodless P, Kim H, Tsui L, Bapat B, Gallinger S, Andrulis IL, Thompsen G, Wrana J, Attisano L. MADR2 maps to 18q21 and encodes a TGF-β-regulated MAD related protein that is functionally mutated in colorectal carcinoma. Cell. 1996;86:543–552. doi: 10.1016/s0092-8674(00)80128-2. [DOI] [PubMed] [Google Scholar]
- Wu CL, Kirley SD, Xiao H, Chuang Y, Chung DC, Zukerberg LR. Cables enhances cdk2 tyrosine 15 phosphorylation by Wee1, inhibits cell growth, and is lost in many human colon and squamous cancers. Cancer Res. 2001;61:7325–7332. [PubMed] [Google Scholar]
- Zukerberg LR, Patrick GN, Nikolic M, Humbert S, Wu CL, Lanier LM, Gertler FB, Vidal M, Van Etten RA, Tsai LH. Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase upregulation, and neurite outgrowth. Neuron. 2000;26:633–646. doi: 10.1016/s0896-6273(00)81200-3. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Matsuura Y, Semba K, Nishimoto I. Molecular cloning of a cyclin-like protein associated with cyclin-dependent kinase 3 (cdk 3) in vivo. Biochem Biophys Res Commun. 2000;273:442–447. doi: 10.1006/bbrc.2000.2965. [DOI] [PubMed] [Google Scholar]
- Zukerberg LR, DeBernardo RL, Kirley SD, D’Apuzzo M, Lynch MP, Littell RD, Duska LR, Boring L, Rueda BR. Loss of cables, a cyclin-dependent kinase regulatory protein, is associated with the development of endometrial hyperplasia and endometrial cancer. Cancer Res. 2004;64:202–208. doi: 10.1158/0008-5472.can-03-2833. [DOI] [PubMed] [Google Scholar]
- Kirley SD, D’Apuzzo M, Lauwers GY, Graeme-Cook F, Chung DC, Zukerberg LR. The Cables gene on chromosome 18Q regulates colon cancer progression in vivo. Cancer Biol Ther. 2005;4:861–863. doi: 10.4161/cbt.4.8.1894. [DOI] [PubMed] [Google Scholar]
- Kirley SD, Rueda BR, Chung DC, Zukerberg LR. Increased growth rate, delayed senescense and decreased serum dependence characterize cables-deficient cells. Cancer Biol Ther. 2005;4:654–658. doi: 10.4161/cbt.4.6.1732. [DOI] [PubMed] [Google Scholar]
- Ogino S, Brahmandam M, Cantor M, Namgyal C, Kawasaki T, Kirkner G, Meyerhardt JA, Loda M, Fuchs CS. Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod Pathol. 2006;19:59–68. doi: 10.1038/modpathol.3800482. [DOI] [PubMed] [Google Scholar]
- Li LC, Chui R, Nakajima K, Oh BR, Au HC, Dahiya R. Frequent methylation of estrogen receptor in prostate cancer: correlation with tumor progression. Cancer Res. 2000;60:702–706. [PubMed] [Google Scholar]
- Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Mizumoto K, Yamochi T, Nishimoto I, Matsuoka M. Differential effect of ik3–1/cables on p53- and p73-induced cell death. J Biol Chem. 2002;277:2951–2957. doi: 10.1074/jbc.M108535200. [DOI] [PubMed] [Google Scholar]
- Tan D, Kirley S, Li Q, Ramnath N, Slocum HK, Brooks JS, Wu CL, Zukerberg LR. Loss of cables protein expression in human non-small cell lung cancer: a tissue microarray study. Hum Pathol. 2003;34:143–149. doi: 10.1053/hupa.2003.26. [DOI] [PubMed] [Google Scholar]
- Dong Q, Kirley S, Rueda B, Zhao C, Zukerberg L, Oliva E. Loss of cables, a novel gene on chromosome 18q, in ovarian cancer. Mod Pathol. 2003;16:863–868. doi: 10.1097/01.MP.0000084434.88269.0A. [DOI] [PubMed] [Google Scholar]
- Chung DC. The genetic basis of colorectal cancer: insights into critical pathways of tumorigenesis. Gastroenterology. 2000;119:854–865. doi: 10.1053/gast.2000.16507. [DOI] [PubMed] [Google Scholar]
- Bonifant CL, Waldman T. ‘Cables’ suspends cancer in mice. Cancer Biol Ther. 2005;4:864–865. doi: 10.4161/cbt.4.8.2012. [DOI] [PubMed] [Google Scholar]
- Presneau N, Manderson EN, Tonin PN. The quest for a tumor suppressor gene phenotype. Curr Mol Med. 2003;3:605–629. doi: 10.2174/1566524033479500. [DOI] [PubMed] [Google Scholar]
- Versteeg R. Aberrant methylation in cancer. Am J Hum Genet. 1997;60:751–754. [PMC free article] [PubMed] [Google Scholar]
- Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet. 1999;21:163–167. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- Goelz SE, Vogelstein B, Hamilton SR, Feinberg AP. Hypomethylation of DNA from benign and malignant human colon neoplasms. Science. 1985;228:187–190. doi: 10.1126/science.2579435. [DOI] [PubMed] [Google Scholar]
- Esteller M. CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene. 2002;21:5427–5440. doi: 10.1038/sj.onc.1205600. [DOI] [PubMed] [Google Scholar]
- Guan RJ, Fu Y, Holt PR, Pardee AB. Association of K-ras mutations with p16 methylation in human colon cancer. Gastroenterology. 1999;116:1063–1071. doi: 10.1016/s0016-5085(99)70009-0. [DOI] [PubMed] [Google Scholar]
- Liang JT, Chang KJ, Chen JC, Lee CC, Cheng YM, Hsu HC, Wu MS, Wang SM, Lin JT, Cheng AL. Hypermethylation of the p16 gene in sporadic T3N0M0 stage colorectal cancers: association with DNA replication error and shorter survival. Oncology. 1999;57:149–156. doi: 10.1159/000012023. [DOI] [PubMed] [Google Scholar]
- Akino K, Toyota M, Suzuki H, Mita H, Sasaki Y, Ohe-Toyota M, Issa JP, Hinoda Y, Imai K, Tokino T. The Ras effector RASSF2 is a novel tumor-suppressor gene in human colorectal cancer. Gastroenterology. 2005;129:156–169. doi: 10.1053/j.gastro.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Payne SR, Kemp CJ. Tumor suppressor genetics. Carcinogenesis. 2005;26:2031–2045. doi: 10.1093/carcin/bgi223. [DOI] [PubMed] [Google Scholar]
- Wang C, Horiuchi A, Imai T, Ohira S, Itoh K, Nikaido T, Katsuyama Y, Konishi I. Expression of BRCA1 protein in benign, borderline, and malignant epithelial ovarian neoplasms and its relationship to methylation and allelic loss of the BRCA1 gene. J Pathol. 2004;202:215–223. doi: 10.1002/path.1507. [DOI] [PubMed] [Google Scholar]
- Xiao WH, Liu WW. Hemizygous deletion and hypermethylation of RUNX3 gene in hepatocellular carcinoma. World J Gastroenterol. 2004;10:376–380. doi: 10.3748/wjg.v10.i3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]