Abstract
Thy-1, a marker of hematopoietic stem cells, has been reported to be expressed by oval cells proliferating during stem cell-mediated regeneration in rat liver, suggesting a relationship between the two cell populations. Consequently, Thy-1 has become an accepted cell surface marker to sort hepatic oval cells. In the present study we used the well-characterized 2-acetylaminfluorene/partial hepatectomy model to induce transit-amplification of hepatic oval cells in the regenerating liver and characterized Thy-1 expression using Northern hybridization, quantitative reverse transcriptase-polymerase chain reaction analysis, immunofluorescence confocal microscopy, and immunoelectronmicroscopy. We found that Thy-1 expression was induced during transit-amplification of the oval cell population, but Thy-1 mRNA was not present in the α-fetoprotein-expressing oval cells. Thy-1 protein was consistently present outside the basement membrane surrounding the oval cells. It overlapped frequently with smooth muscle actin staining. A similar cellular localization of the Thy-1 protein was found on human liver specimens with ductular reactions obtained from patients with fulminant liver failure. Furthermore, Thy-1 was expressed by myofibroblasts in experimental liver fibrosis models without oval cell proliferation. We conclude that Thy-1 is not a marker of oval cells but is present on a subpopulation of myofibroblasts/stellate cells.
Thy-1 (CD-90) is a rather promiscuous molecule. It is expressed by several different cell types, and, among others, it is present on the surface of the bone marrow stem cells. It was also reported to be present in the rat liver on the oval/progenitor cells in stem cell-mediated liver regeneration.1,2,3,4 Later, a precursor-product relationship was described between bone marrow cells and oval cells/hepatocytes in several experimental models1,3,5,6 as well as in humans,7 raising the very exiting possibility of liver cells being derived from hematopoietic cells. Several groups confirmed the Thy-1 expression in oval cells,1,2,3,4 resulting in the extensive use of Thy-1 as a cell surface marker to sort out liver progenitor cells. However, the issue of stem cell transdifferentiation has subsequently been one of the most debated issues in hepatic pathobiology, and most of these observations can now be explained by cell fusion and not transdifferentiation. The most comprehensive review of this topic recently concluded that although “data are sufficient to indicate that mesodermal hematopoietic cells can generate hepatocytes at a very low frequency, this is not an effective pathway under most conditions.”8 At the same time, others described cells coexpressing Thy-1 and smooth muscle actin (SMA) in similar experimental settings,9 questioning the identity of the Thy-1-expressing cells in the liver. To resolve this contradiction we performed detailed morphological expression analysis to identify the location of Thy-1 in the normal liver and in damaged liver with and without oval cell proliferation.
Materials and Methods
Animal Experiments
Male F-344 rats (160 to 180 g) were used for all experiments and were kept under standard conditions. Animal protocols were approved by the Danish Council for Supervision with Experimental Animals.
AAF/PHx Experiment
The animals received 2-acetylaminofluorene (AAF) (suspended in 1% dimethylcellulose) at 4.5, 9, 12, or 18 mg/kg/day administered daily for 4 consecutive days by gavage. Traditional two-thirds partial hepatectomy (PHx)10 was performed on the 5th day, followed by four additional AAF treatments. Groups of three animals were sacrificed 1, 5, 9, 14, and 21 days after PHx. Controls included untreated animals and rats subjected to a PHx or a sham laparotomy only. After resection of the liver, samples were taken for histological examinations and the rest snap-frozen in liquid nitrogen for RNA extraction.
Bile Duct Ligation
Ligation of the common bile duct was done according to Cameron and Oakley.11 The rats were sacrificed 2 weeks after the operation.
CCl4 Fibrosis
Twenty percent CCl4 (0.5 ml/kg, dissolved in vegetable oil) was administered by gavage to rats twice a week while the animals were kept on 0.05% phenobarbital in the drinking water. The experiment was terminated after 8 weeks.12
Human Tissue
Snap-frozen human liver specimens for immunohistochemical examination were obtained from two patients who underwent orthotopic liver transplantation because of fulminant liver failure of unknown etiology. The procedure was approved by the ethical committee of the Semmelweis University.
Isolation of Oval Cells for Northern Blot Analysis
Isolation of oval cells was performed using control liver, and animals were treated according to the AAF/PHx protocol (18 mg/kg/day) and sacrificed at day 9 after PHx. The isolation and enrichment procedure has been described in detail.13 In brief, liver cells were released by a three-step perfusion procedure in situ. Viable nonparenchymal cell populations were purified by centrifugation through a two-step Percoll gradient. Kupffer cells were removed by selective adherence to plastic tissue culture dishes. Removal of macrophages, endothelial cells, and red blood cells was achieved by selective panning using the mouse monoclonal antibody OX43 (catalog no. MCA276; Serotec, Oxford, UK). Cell preparations were snap-frozen in liquid nitrogen and stored at −70°C until processed for total RNA isolation and Northern blot analysis.
Northern Blot Analysis
Northern blotting with cDNA probes was performed as previously described.14 The cDNA for rat Thy-1 encompassed nucleotides 46 to 531 (GenBank accession no. NM_012673), and for α-fetoprotein (AFP), nucleotides 101 to 329 (GenBank accession no. X02361). The filters were hybridized with rat S18 to assess the integrity and ensure equal loading of the RNA.
Quantitative Real-Time Reverse Transcriptase-Polymerase Chain Reaction Analysis
Frozen sections (8 μm) were fixed in acetone, dried at room temperature, and stained with RNase-free hematoxylin. Laser microdissection of oval cells was performed by using the PALM MicroBeam system, and 500 to 1000 cells were collected in RNA-Later. For whole liver quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR) analysis, frozen sections from normal and AAF/PHx-treated liver were collected in lysis buffer.
Total RNA was isolated by RNAqueous micro kit (catalog no. AM 1931; Ambion, Austin, TX). A high capacity cDNA reverse transcription kit (catalog no. 4368814; ABI) was used for cDNA synthesis as recommended by the supplier. PCR was performed by the ABI Prism 7300 sequence detection system (Applied Biosystems, Weiterstadt, Germany), using ABI TaqMan gene expression assays for AFP (assay ID: Rn00560661_m1), SMA (assay ID: Rn01759928_ g1), and Thy-1 (assay ID: Rn00562048_m1) according to the manufacturer’s instructions. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as endogenous control. All samples were run in triplicate, in a 20-μl reaction volume. Results were obtained as threshold cycle (CT) values. Expression levels were calculated using the ΔCT method. The values were calculated as the mean values of three independent measurements, and the expression levels of mRNA in all samples were defined as a ratio to GAPDH expression.
Morphological Analysis
Frozen sections (10 to 20 μm) were fixed in methanol and were incubated at room temperature (1 hour) with a mixture of the primary antibodies (Table 1) and with appropriate secondary antibodies afterward (Jackson ImmunoResearch, West Grove, PA). All samples were analyzed by confocal laser-scanning microscopy using the Bio-Rad MRC-1024 system (Bio-Rad, Richmond, CA). Negative controls were performed by replacing the primary antibodies with preimmune sera (data not shown).
Table 1.
Primary Antibodies Used for the Immunohistochemical Studies
| Antibody | Species | Manufacturer* | Catalog number | Dilution |
|---|---|---|---|---|
| Laminin | Rabbit polyclonal | DAKO | Z0097 | 1:200 |
| Anti-rat Thy-1 | Mouse monoclonal | BD Pharmingen | 554895 | 1:100 |
| FITC-labeled anti-rat Thy-1 | Mouse monoclonal | BD Pharmingen | 554897 | 1:50 |
| Anti-human Thy-1 | Mouse monoclonal | BD Pharmingen | 550402 | 1:100 |
| FITC-labeled anti-human Thy-1 | Mouse monoclonal | BD Pharmingen | 555595 | 1:50 |
| GFAP | Mouse monoclonal | BD Pharmingen | 556330 | 1:100 |
| Anti-human cytokeratin-19 | Mouse monoclonal | BioGenex | MU246-UC | 1:50 |
| Anti-rat cytokeratin-19 | Mouse monoclonal | Novocastra | NCL-CK19 | 1:50 |
| OV-6 | Mouse monoclonal | R&D Systems | MAB2020 | 1:100 |
| FITC-labeled cytokeratin | Mouse monoclonal | DAKO | F0859 | 1:10 |
| Desmin | Rabbit polyclonal | Neomarkers | RB-9014-P1 | 1:100 |
| Smooth muscle actin | Mouse monoclonal | DAKO | M0851 | 1:100 |
| OX-62 | Mouse monoclonal | Serotec | MCA1029G | 1:100 |
| Mononuclear phagocyte (rMPh/ED-1) | Mouse monoclonal | BD Pharmingen | 554954 | 1:100 |
| Lyve-1 | Rabbit polyclonal | Reliatech | 102-PA505 | 1:100 |
| CD45 | Mouse monoclonal | BD Pharmingen | 550566 | 1:100 |
DAKO, Glostrup, Denmark; BD Pharmingen, San Jose, CA; Biogenex, San Ramon, CA; Novocastra, Newcastle upon Tyne, UK; R & D System, Minneapolis, MN; Neomarkers, Fremont, CA; Serotec, Oxford, UK; Reliatech, Bvaunschweig, Germany.
Co-localization analysis was performed using the Image J program (National Institutes of Health, Bethesda, MD). The red (channel 1) and green (channel 2) images were acquired separately and sequentially to avoid bleed-through. The area fraction (%) occupied by red and green fields was determined by manual thresholding. Analysis of co-localized points (%) was determined using the co-localization plug-in.
Preparation of liver tissue for immunoelectronmicroscopy was described by Paku and colleagues.15 Cryosections were rinsed in phosphate-buffered saline and incubated with the primary antibody Thy-1 (dilution 1:100, 3 hours), followed by peroxidase-conjugated anti-mouse antibody (dilution 1:500, catalog no. 715-035-1500; Jackson ImmunoResearch). Semithin sections were slightly stained by 0.5% toluidine blue (pH 8.5), and unstained ultrathin sections were analyzed on a Philips CM 10 electron microscope (Philips, Eindhoven, The Netherlands).
Results
Thy-1 Expression in the Normal Liver
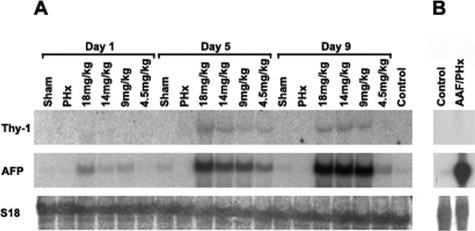
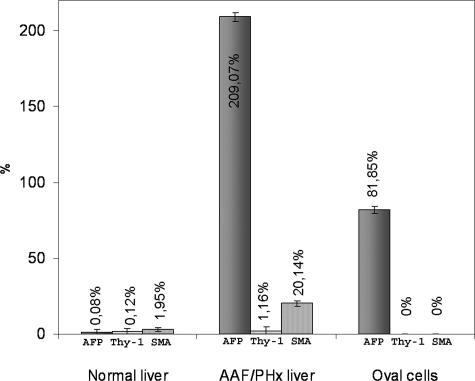
Transcripts for Thy-1 were not detected by Northern blot analysis in mRNA preparations from whole normal liver (Figure 1A) and were undetectable in preparations of nonparenchymal cells isolated from normal liver and enriched with a protocol for oval cells (Figure 1B). Likewise, qRT-PCR analysis detected low AFP, Thy-1, and SMA expression in normal liver (Figure 2).
Figure 1.
Northern blot analysis for the expression of Thy-1, AFP, and S18. A: RNA was isolated from whole liver of normal, sham-operated (sham), partially hepatectomized (PHx), and AAF/PHx-treated animals. The numbers refer to the daily dose of AAF. Animals were sacrificed at 1, 5, and 9 days after PHx. B: RNA was isolated from the oval cell fraction from control and AAF/PHx (AAF dose, 18 mg/kg/day)-treated rats. The strong AFP band in the second lane confirms the presence of oval cells in the enriched cell population. Notice the lack of Thy-1 expression in this cell population.
Figure 2.
qRT-PCR analysis of AFP, Thy-1, and SMA mRNA in whole liver of normal and AAF/PHx (9 days after PHx)-treated animals and in microdissected oval cell populations. The relative expression levels of AFP, Thy-1, and SMA were determined by comparing with that of GAPDH expression level (100%).
Thy-1 expression by immunohistochemistry was detectable and confined to the periportal region (Figure 3, A, B, C, and E; and Supplemental Figure 1A available at http://ajp. amjpathol.org). There was some faint cloudy staining around the major interlobular bile ducts (Figure 3A, and Supplemental Figure 1A available at http://ajp.amjpathol.org). Thy-1 antibody decorated more intensely and sharply the cross sections of peripheral nerves (Figure 3A, and Supplemental Figure 1A available at http://ajp.amjpathol. org). These nerves were also positive for synaptophysin (data not shown). Scattered undefined cells inside the portal areas also expressed Thy-1 (Figure 3, C and E).
Figure 3.
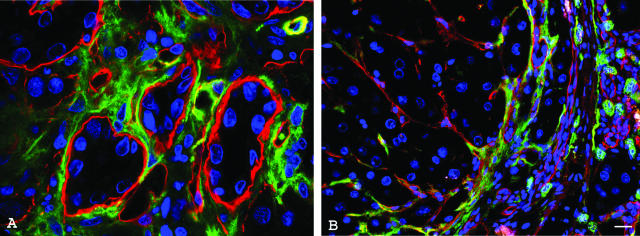
Thy-1 expression examined by confocal microscopy. A–E: Normal rat liver. F and G: Rat liver from an AAF/PHx experiment, 14 and 21 days after PHx. H: A human liver with ductular reaction. C–E: Serial sections of an interlobular bile duct and its neighborhood. A: The section is triple labeled for Thy-1 (green), cytokeratin-19 (red), and laminin (blue). The portal vein, the hepatic artery branch, and the major interlobular bile duct are surrounded by laminin-positive (blue) basement membrane. Thy-1-positive nerve fibers (arrowheads) are present around the vessels. There is a fine Thy-1 staining around the larger interlobular bile ducts (large arrow), but it cannot be observed around the small bile duct branches (small arrows). B: The section is triple labeled for Thy1 (green), GFAP (red), and laminin (blue). GFAP-positive stellate cells are present only in the liver parenchyma. Thy-1 and GFAP staining show separate structures in normal liver. C: The sections are stained for Thy-1 (green) and desmin (red). Desmin positivity can be observed in the wall of hepatic artery branches and in the capillaries of peribiliary plexus. Note the co-localization of desmin and Thy-1 in the wall of peribiliary vascular plexus (arrowheads). Desmin-positive cells can also be discerned within the portal area and in the liver parenchyma. There are scattered Thy-1-positive cells in the portal area (arrow). The nuclei are stained with toto-3 (blue). D: The section is stained for SMA (green) and desmin (red). SMA and desmin co-localize in hepatic artery branches and in the wall of capillaries of the peribiliary plexus (arrowheads). SMA-positive cells cannot be found in the liver parenchyma. The nuclei are stained with toto-3 (blue). E: The section is stained for Thy1 (green) and SMA (red). Note the co-localization of SMA and Thy-1 in the wall of peribiliary vascular plexus (arrowheads). Scattered Thy-1-positive cells are present in the portal area (arrows). F: AAF/PHx experiment, 14 days after PHx. Triple staining for Thy-1 (green), CK-19 (red), and laminin (blue). The CK-19-positive (red) oval cell-formed ductules (arrows) are surrounded by laminin-positive (blue) basement membrane. The oval cells are not labeled by the Thy-1 antibody. Thy-1-positive cells were situated exclusively among the ductules, strictly outside the basement membrane. G: AAF/PHx experiment, 22 days after PHx. Thy-1-positive (green) cells are clearly localized outside the basement membrane (laminin-positive, blue) of CK-19-positive (red) oval cell ductules (asterisks). H: Thy-1-positive (green) cells are outside the laminin-containing basement membrane (laminin, blue) surrounding the proliferating ductules (CK-19, red) in human liver. Scale bars: 20 μm (A–E, H); 200 μm (F); and 10 μm (G).
Desmin antibody reacted with nonparenchymal cells in the liver lobule in addition to the muscular wall of the blood vessels and scattered single cells in the periportal connective tissue (Figure 3, C and D). Conversely, no SMA-positive cells were seen inside the liver lobule; only the vessel walls were stained (Figure 3, D and E). Glial fibrillary acidic protein (GFAP), an established marker of hepatic stellate cells, decorated scattered single cells in the liver lobules. There was no overlap between the GFAP and Thy-1 reaction (Figure 3B).
Thy-1 Expression in the Stem Cell-Mediated Liver Regeneration
In rat liver treated according to the AAF/PHx protocol, oval cells formed ductules invading the liver lobules during the first 7 to 10 days. These ductules are surrounded by continuous basement membrane, and there are numerous stellate cells/myofibroblasts around them.15 The number of transit-amplifying oval cells depends on the dose of AAF as demonstrated by the levels of AFP transcripts—the most widely used marker for rat oval cells (Figure 1A). A similar expression pattern was found in whole liver for Thy-1, confirming that Thy-1 expression is induced during oval cell-mediated liver regeneration (Figure 1A). However, when isolated oval cells from AAF/PHx-treated animals were examined, no expression of Thy-1 was detectable despite increased levels of AFP transcripts (Figure 1B). qRT-PCR also failed to detect Thy-1 (and SMA) expression in RNA isolated from microdissected oval cells, while AFP RNA was present. However, Thy-1 and SMA expression could be demonstrated by qRT-PCR from whole liver sections. Therefore, we performed a thorough immunohistochemical analysis of the Thy-1 expression.
The staining pattern with the different antibodies was identical in all studied time points. The laminin-containing basement membrane surrounded the CK-19-positive oval cell ductules. The Thy-1 reaction was observed consistently outside the basement membrane (Figure 3, F and G; and Supplemental Figure 1, B and C, available at http://ajp.amjpathol.org). The antibody sometimes labeled round, cellular body-like elements, but frequently only stripes or cell processes were positive. Thy-1 immunoreactions were easily abolished by detergent pretreatment. If sections were pretreated for 5 minutes in 0.05% Triton X-100, the staining was faint whereas pretreatment for 10 minutes resulted in complete disappearance of the reaction (data not shown). Thy-1 antibody also decorated cellular elements and long processes outside the basement membrane in human livers with extensive ductular reactions because of fulminant liver failure (Figure 3H).
The pattern of Thy-1 reaction was reminiscent of stellate cell/myofibroblast architecture, which also could be found outside the basement membrane. Therefore, in the rat liver we performed co-staining of Thy-1 and SMA or desmin, the two most widely used stellate cell/myofibroblast markers. SMA-positive cells appeared very early in the experiment at the limiting plate and spread along the ductules formed by oval cells into the parenchyma. The desmin antibody reacted with scattered nonparenchymal cells throughout the liver lobule from the beginning of the experiment, but they became more frequent in the zone of the oval cells. In the co-staining experiments, Thy-1 showed frequent co-localization with SMA (Figure 4, A–C). Of the Thy-1-positive areas 80.3 ± 9.6% stained with SMA, but only 58 ± 9.3% of the SMA-positive field was decorated by Thy-1.
Figure 4.
Confocal microscopy of Thy-1 expression combined with stellate cell/myofibroblast markers SMA and desmin. Sections are from a rat liver treated according to the AAF/PHx protocol, 9 days after PHx. A and B: Double labeling for SMA (red) and Thy-1 (green). SMA-positive myofibroblast (A) and Thy-1-positive cells (B) are present among the oval cell ductules (asterisks). C: The white areas on the merged image show co-localization of SMA and Thy-1. D: Double immunofluorescence for Thy-1 (green) and desmin (red). The overwhelming majority of the green and red staining marks separate structures (arrows). There are only a few spots showing yellow signal (arrowheads), suggesting occasional co-localization of the two antibodies. E: Double-immunofluorescent staining for SMA (green) and desmin (red) labels different structures (arrows). F: Double labeling for Thy-1 (green) and Lyve-1 (red) in the area of proliferating ductules. Lyve-1-positive endothelium does not express Thy-1; it is localized outside the vessels. G: The CD45 (red) staining does not overlap with Thy-1 (green). Scale bar = 50 μm.
Thy-1 positivity hardly overlapped with desmin; the value of co-localization index was 6.8 ± 2.3% (Figure 4D and Supplemental Movie 1 available at http://ajp.amjpathol.org). This was surprising because SMA and desmin have been used to identify stellate cells/myofibroblasts. The co-localization index of these two markers was also negligible (7.16 ± 1.2%) (Figure 4E).
To investigate Thy-1 co-localization with other marker antigens, we performed further double-staining experiments. Lyve-1, a new marker for the endothelial cells of lymphatic vessels and hepatic sinusoids,16,17 did not show any co-staining with Thy-1 in the neighborhood of the oval cells (Figure 4F), a result that was similar to OX 62 and rMPh markers of hepatic dendritic18 and Kupffer cells (data not shown). In addition, CD45, a general leukocyte marker, did not stain the Thy-1-positive structures (Figure 4G).
Immunoelectron microscopic examination of Thy-1 expression also revealed long cell processes running clearly outside the basement membrane. Because of immunoelectronmicroscopic processing of the samples, the ultrastructure of labeled cells could not be examined in detail. However, our morphological evaluation suggested that the marked cells displayed features of stellate cells/myofibroblasts (Figure 5, A and B).
Figure 5.
Ultrastructural localization of Thy-1 in a rat liver treated according to the AAF/PHx protocol, 9 days after PHx. A: Black reaction product labels the surface of a periductal cell (asterisk). Numerous long cytoplasmic processes showing positive reaction for Thy-1 (arrowheads) can be observed close to the basal surface of the oval cell ductule (D). H, Hepatocyte; V, vessel lumen. B: Detail of A. Thy-1-positive cytoplasmic process (arrowhead) is localized outside the basement membrane (arrows) of the ductule (D). Scale bars: 2 μm (A); 1 μm (B).
Thy-1 Expression in Rat Liver Fibrosis Model
Thy-1 also decorated the myofibroblasts in two liver fibrosis models (bile duct ligation-induced cholangiofibrosis and CCl4/phenobarbital-induced cirrhosis), which were not characterized by oval cell proliferation (Figure 6).
Figure 6.
Thy-1 expression in other rat hepatic fibrosis models. A: Bile duct ligation-induced cholangiofibrosis. B: CCl4/phenobarbital cirrhosis model. A: Thy-1-positive (green) cells surrounding the large proliferating bile ducts. The nuclei are stained with toto-3 (blue); the basement membrane is marked by laminin (red). B: Thy-1-positive (green) cells are situated on the edge of the laminin-positive (red) cirrhotic septum. The nuclei are stained with toto-3 (blue). Scale bar = 20 μm.
Discussion
We have investigated Thy-1 expression in rat livers regenerating by the recruitment of oval/progenitor cells. The oval cells were not labeled by the Thy-1 antibody, but we observed a strong periductal reaction outside the basement membrane. There was a partial overlap between the Thy-1 and SMA staining, but no co-staining of Thy-1 and desmin could be observed. Furthermore, Thy-1 also marked myofibroblasts in two liver fibrosis models without oval cell reactions.
Thy-1 is a highly conserved protein anchored by a phosphatidylinositol to the cell membrane. Its exact function is unknown, but it has been proposed to be involved in cell recognition, adhesion, and lymphocyte activation.1 It is expressed in a wide variety of different tissues.1,19,20,21,22,23 Its expression has been extensively studied in the liver. Petersen and colleagues1 reported that hepatic oval cells expressed the hematopoietic stem cell marker Thy-1 in the rat. This observation led to further experiments suggesting that bone marrow cells can be the precursors of oval cells/hepatocytes. Hepatic progenitor cells in human fetal liver also have been reported to be Thy-1-positive.7 However, Hoppo and colleagues9 found in mouse that Thy-1-positive mesenchymal cells promoted the maturation of Thy-1-negative hepatic progenitor cells. A subpopulation of the Thy-1-positive cells also expressed SMA.
Our results in the rat are similar to this latter group’s observation. In our case the CK-19-positive, laminin-surrounded oval/progenitor cells were not decorated by the Thy-1 antibody. CK-19 is an established marker of oval cells. It is also generally accepted that the oval cells are surrounded by continuous basement membrane, which can be visualized by laminin immunohistochemistry. The co-staining of these three antigens on the same section combined by confocal microscopic analysis is a very reliable morphological examination. Thy-1-positive cells were also outside the proliferating ductules in human liver, confirming the observation of Crosby and colleagues.24 Furthermore, immunoelectronmicroscopy confirmed that the Thy-1-positive cells are outside the basement membrane. On traditional histological sections, it is very difficult to distinguish conclusively between the oval and the closely associated stellate cells/myofibroblasts. Serial sections stained by an oval cell marker, which was used by Petersen and colleagues,1 do not provide much help. Individual cells cannot be analyzed on serial sections, and the histological arrangement of the two cell populations is similar (spreading outward from the periportal region). Our Northern hybridization and qRT-PCR analysis also strongly support the morphological observations.
The Thy-1 molecule is bound weakly to the cell membrane.25,26 Triton X-100 pretreatment in our study also deleted the Thy-1 signal from the sections. Soluble Thy-l has been also described in the serum.27 Enzymatic digestion during the cell isolation procedures may cause detachment of the Thy-1 molecule, which might associate later to other cells causing misleading results in cell suspension.
To identify the Thy-1-expressing cells, we co-stained Thy-1 with several other marker antibodies. The negative results with OX-62, rMPh, and Lyve-1 excluded hepatic dendritic, Kupffer, and sinusoidal endothelial cells as the sources of Thy-1 expression. The position and shape of the Thy-1-positive cells refers strongly to the so-called stellate cells/myofibroblasts, which are well known to have close spatial relationship with the oval cells in all oval cell proliferation models. The partial co-staining with SMA supports this option, as well as the Thy-1 positivity of myofibroblasts in two hepatic fibrosis models. Thy-1-positive myofibroblasts were described in other tissues, and the ratio of the Thy-1+/− populations was a function of their activation stage.28,29,30,31
Surprisingly, co-staining of Thy-1 with desmin, another marker of stellate cells, could not be demonstrated. Desmin and SMA are alternatively used markers for stellate cells.32 These antibodies stain comparably located and shaped cell populations, but according to our result, the two reactions do not overlap. The origin and phenotype of hepatic stellate cells/myofibroblasts is one of the most controversial issues of liver pathobiology. It is not known if there are different differentiation/activation stages of the same cell population, or as Ramadori and Saile32 propose, there are two (or more) cell types with partially overlapping phenotype. Our observations on the normal liver and during the progression of oval cell proliferation in the rat are in support of the view of Ramadori and Saile.32 Scattered desmin/GFAP-positive cells were observed in the parenchyma of the normal liver, which might correspond to the (classical, perisinusoidal, vitamin A-storing) stellate cells. SMA-decorated cells were confined to the vessel walls in the normal liver, but they appeared in the periportal region very early after treatment with AAF alone (data not shown) and later spread along the oval cell ductules. This would imply that the SMA-positive cells would be myofibroblasts, derived from the periportal fibroblasts, as proposed by Ramadori and Saile32 and Beaussier and colleagues.33 As far as we know, co-staining for SMA and desmin has not been published in oval cell proliferation experiments. Considering the above data, the Thy-1-positive cells in the zone of the oval cells show the closest association with the myofibroblasts. The increased expression of Thy-1 in two liver fibrosis models also supports the myofibroblastic origin of this marker molecule.
The appearance of Thy-1-positive cells in the liver parenchyma can be explained if some of the myofibroblasts acquire the Thy-1 expression during the invasion of the liver lobule. Alternatively, it has been found that the mesenchymal stem cells in the bone marrow are Thy-1-positive and that this cell compartment can contribute to wound-healing processes34 including the fibrogenesis of the liver.35 It cannot be excluded that the Thy-1 antibody recognizes bone marrow-derived mesenchymal cells, which may participate in oval cell-mediated liver regeneration. At present, we cannot distinguish between these two possibilities, but transplantation experiments are under way to study the presence of bone marrow-originated cells among the stellate cells/myofibroblasts. Recently, Kisseleva and colleagues36 have described a unique CD45+ fibrocyte population in the liver. However, in accordance with our results, Kamo and colleagues37 could not demonstrate co-expression of Thy-1 and CD45.
In conclusion, we did not find Thy-1 expression in the hepatic oval/progenitor cell population in stem cell-mediated rat liver regeneration or in human ductular reactions. Instead, Thy-1 was localized to a subpopulation of stellate cells/myofibroblasts. Therefore, the use of Thy-1 as a cell surface marker for isolation of oval/progenitor cells from the liver is not recommended. The exact origin and function of Thy-1-expressing cells remains to be studied. Our results are in complete agreement with the recently published study of Dudas and colleagues.38
Supplementary Material
Acknowledgments
We thank Sándor Spisák for helping in microdissection.
Footnotes
Address reprint requests to Peter Nagy, First Department of Pathology and Experimental Cancer Research, Semmelweis University, Üllői út 26, Budapest, H-1085, Hungary. E-mail: nagy@korb1.sote.hu.
Supported by Országos Tudományos Kutatási Alap (Hungary) (grants T42674 and TS49887), Egészségüeyi Tudományos Tanács (Egészségügyi Minisztérium, Hungary) (grant 032/2006), and the Danish Medical Research Council (grants 22-03-0277 to H.C.B. and 2052-01-0045 to The Danish Stem Cell Research Centre).
H.C.B. and P.N. contributed equally as senior investigators of this work.
Supplemental material for this article can be found on http://ajp. amjpathol.org.
References
- Petersen BE, Goff JP, Greenberger JS, Michalopoulos GK. Hepatic oval cells express the hematopoietic stem cell marker Thy-1 in the rat. Hepatology. 1998;27:433–445. doi: 10.1002/hep.510270218. [DOI] [PubMed] [Google Scholar]
- Shu SN, Wei L, Wang JH, Zhan YT, Chen HS, Wang Y. Hepatic differentiation capability of rat bone marrow-derived mesenchymal stem cells and hematopoietic stem cells. World J Gastroenterol. 2004;10:2818–2822. doi: 10.3748/wjg.v10.i19.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurson J, Selden C, Hodgson HJ. Hepatocyte progenitors in man and in rodents—multiple pathways, multiple candidates. Int J Exp Pathol. 2005;86:1–18. doi: 10.1111/j.0959-9673.2005.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi L, Oh SH, Shupe T, Petersen BE. Role of connective tissue growth factor in oval cell response during liver regeneration after 2-AAF/PHx in rats. Gastroenterology. 2005;128:2077–2088. doi: 10.1053/j.gastro.2005.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theise ND, Krause DS. Bone marrow to liver: the blood of Prometheus. Semin Cell Dev Biol. 2002;13:411–417. doi: 10.1016/s1084952102001283. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- Masson NM, Currie IS, Terrace JD, Garden OJ, Parks RW, Ross JA. Hepatic progenitor cells in human fetal liver express the oval cell marker Thy-1. Am J Physiol Gastrointest Liver Physiol. 2006;291:G45–G54. doi: 10.1152/ajpgi.00465.2005. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson SS, Grisham JW. Hemopoietic cells as hepatocyte stem cells: a critical review of the evidence. Hepatology. 2006;43:2–8. doi: 10.1002/hep.21015. [DOI] [PubMed] [Google Scholar]
- Hoppo T, Fujii H, Hirose T, Yasuchika K, Azuma H, Baba S, Naito M, Machimoto T, Ikai I. Thy1-positive mesenchymal cells promote the maturation of CD49f-positive hepatic progenitor cells in the mouse fetal liver. Hepatology. 2004;39:1362–1370. doi: 10.1002/hep.20180. [DOI] [PubMed] [Google Scholar]
- Higgins GM, Anderson RM. Experimental pathology of the liver: restoration of liver of white rat following partial surgical removal. Exp Pathol. 1931;12:186–202. [Google Scholar]
- Cameron GR, Oakley CR. Ligation of the common bile duct. J Pathol Bacteriol. 1932;35:769–798. [Google Scholar]
- Proctor E, Chatamra K. High yield micronodular cirrhosis in the rat. Gastroenterology. 1982;83:1183–1190. [PubMed] [Google Scholar]
- Bisgaard HC, Santoni-Rugiu E, Nagy P, Thorgeirsson SS. Modulation of the plasminogen activator/plasmin system in rat liver regenerating by recruitment of oval cells. Lab Invest. 1998;78:237–246. [PubMed] [Google Scholar]
- Bisgaard HC, Müller S, Nagy P, Rasmussen LJ, Thorgeirsson SS. Modulation of the gene network connected to interferon-γ in liver regeneration from oval cells. Am J Pathol. 1999;155:1075–1085. doi: 10.1016/s0002-9440(10)65210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paku S, Schnur J, Nagy P, Thorgeirsson SS. Origin and structural evolution of the early proliferating oval cells in rat liver. Am J Pathol. 2001;158:1313–1323. doi: 10.1016/S0002-9440(10)64082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouta Carreira C, Nasser SM, di Tomaso E, Padera TP, Boucher Y, Tomarev SI, Jain RK. Lyve-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 2001;61:8079–8084. [PubMed] [Google Scholar]
- Brenan M, Puklavec M. The MRC OX-62 antigen: a useful marker in the purification of rat veiled cells with the biochemical properties of an integrin. J Exp Med. 1992;175:1457–1465. doi: 10.1084/jem.175.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arena G, Musto P, Cascavilla N, Carotenuto M. Thy-1 (CDw90) and c-kit receptor (CD117) expression on CD34+ hematopoietic progenitor cells: a five dimensional flow cytometric study. Haematologica. 1998;83:587–592. [PubMed] [Google Scholar]
- Boiret N, Rapatel C, Boisgard S, Charrier S, Tchirkov A, Bresson C, Camilleri L, Berger J, Guillouard L, Guerin JJ, Pigeon P, Chassagne J, Berger MG. CD34+CDw90(Thy-1)+ subset colocated with mesenchymal progenitors in human normal bone marrow hematon units is enriched in colony-forming unit megakaryocytes and long-term culture-initiating cells. Exp Hematol. 2003;31:1275–1283. doi: 10.1016/j.exphem.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Timper K, Seboek D, Eberhardt M, Linscheid P, Christ-Crain M, Keller U, Muller B, Zulewski H. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun. 2006;341:1135–1140. doi: 10.1016/j.bbrc.2006.01.072. [DOI] [PubMed] [Google Scholar]
- Saalbach A, Hildebrandt G, Haustein UF, Anderegg U. The Thy-1/Thy-1 ligand interaction is involved in binding of melanoma cells to activated Thy-1-positive microvascular endothelial cells. Microvasc Res. 2002;64:86–93. doi: 10.1006/mvre.2002.2401. [DOI] [PubMed] [Google Scholar]
- Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003;163:1291–1300. doi: 10.1016/S0002-9440(10)63488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby HA, Nijjar SS, de Goyet J, de V, Kelly DA, Strain AJ. Progenitor cells of the biliary epithelial cell lineage. Semin Cell Dev Biol. 2002;13:397–403. doi: 10.1016/s108495210200126x. [DOI] [PubMed] [Google Scholar]
- Heffer-Lauc M, Lauc G, Nimrichter L, Fromholt SE, Schnaar RL. Membrane redistribution of gangliosides and glycosylphosphatidylinositol-anchored proteins in brain tissue sections under conditions of lipid raft isolation. Biochim Biophys Acta. 2005;1686 3:200–208. doi: 10.1016/j.bbalip.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Heffer-Lauc M, Viljetic B, Vajn K, Schnaar RL, Lauc G. Effects of detergents on the redistribution of gangliosides and GPI-anchored proteins in brain tissue sections. J Histochem Cytochem. 2007;55:805–812. doi: 10.1369/jhc.7A7195.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalbach A, Wetzig T, Haustein UF, Anderegg U. Detection of human soluble Thy-1 in serum by ELISA: fibroblasts and activated endothelial cells are a possible source of soluble Thy-1 in serum. Cell Tissue Res. 1999;298:307–315. doi: 10.1007/s004419900079. [DOI] [PubMed] [Google Scholar]
- Sanders YY, Kumbla P. Enhanced myofibroblastic differentiation and survival in Thy-1(−) lung fibroblasts. Am J Respir Cell Mol Biol. 2007;36:226–235. doi: 10.1165/rcmb.2006-0178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudon-David F, Bouzeghrane F, Couture P, Thibault G. Thy-1 expression by cardiac fibroblasts: lack of association with myofibroblast contractile markers. J Mol Cell Cardiol. 2007;42:991–1000. doi: 10.1016/j.yjmcc.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Rege TA, Hagood JS. Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival, and cytokine/growth factor responses. Biochim Biophys Acta. 2006;1763:991–999. doi: 10.1016/j.bbamcr.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagood JS, Prabhakaran P, Kumbla P, Salazar L, MacEwen MW, Barker TH, Ortiz LA, Schoeb T, Siegal GP, Alexander CB, Pardo A, Selman M. Loss of fibroblast Thy-1 expression correlates with lung fibrogenesis. Am J Pathol. 2005;167:365–379. doi: 10.1016/S0002-9440(10)62982-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Saile B. Mesenchymal cells in the liver—one cell type or two? Liver. 2002;22:283–294. doi: 10.1034/j.1600-0676.2002.01726.x. [DOI] [PubMed] [Google Scholar]
- Beaussier M, Wendum D, Schiffer E, Dumont S, Rey C, Lienhart A, Housset C. Prominent contribution of portal mesenchymal cells to liver fibrosis in ischemic and obstructive cholestatic injuries. Lab Invest. 2007;87:292–303. doi: 10.1038/labinvest.3700513. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, Alison MR. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955–963. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, Brenner DA. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Kamo N, Yasuchika K, Fujii H, Hoppo T, Machimoto T, Ishii T, Fujita N, Tsuruo T, Yamashita JK, Kubo H, Ikai I. Two populations of Thy1-positive mesenchymal cells regulate the in vitro maturation of hepatic progenitor cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G526–G534. doi: 10.1152/ajpgi.00241.2006. [DOI] [PubMed] [Google Scholar]
- Dudas J, Mansuroglu T, Batusic D, Saile B, Ramadori G. Thy-1 is an in vivo and in vitro marker of liver myofibroblasts. Cell Tissue Res. 2007;329:503–514. doi: 10.1007/s00441-007-0437-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.