Abstract
Several lines of evidence suggest that an increase in aldehyde-modified proteins is associated with development of atherosclerosis. Acrolein and 4-hydroxynonenal (HNE) are reactive aldehydes generated during active inflammation as a consequence of lipid peroxidation; both react with protein thiols, including thioredoxin-1 (Trx1), a protein recently found to regulate antioxidant function in endothelial cells. The present study examined whether acrolein or HNE modification of Trx1 could potentiate monocyte adhesion to endothelial cells, an early event of atherosclerosis. We examined the function of acrolein and HNE-modified Trx1 in the regulation of the early events of atherosclerosis using cultured aortic endothelial cells as a vascular model system, for in vitro enzymatic assay, and in mass spectrometry analysis. Our data show that acrolein and HNE at 1:1 ratios with Trx1 modified Cys-73 and inhibited activity. In endothelial cells, adducts were detected at concentrations as low as 1 μmol/L including conditions in which there was no detectable change in glutathione. Acrolein and HNE modification of Trx1 was associated with increased production of reactive oxygen species. Microinjection of acrolein- and HNE-modified Trx1 into endothelial cells stimulated monocyte adhesion. Chemical modification of Trx1 by common environmental and endogenously generated reactive aldehydes can contribute to atherosclerosis development by interfering with antioxidant and redox signaling functions of Trx1.
Atherosclerosis is a complex process including proinflammatory and pro-oxidative events, which recruit monocytes and lymphocytes to adhere to the surface of the injured endothelium as the first observable event. Epidemiological studies have revealed numerous risk factors for atherosclerosis including genetic and environmental risk factors, such as smoking, diet, infection, and air pollution, which interestingly have also been known to induce oxidative stress.
The thioredoxin-1 (Trx1) system plays a key role in modulating redox signaling pathways regulating physiological as well as pathophysiological processes such as atherosclerosis development.1,2 Trx1 is a major substrate for thioredoxin reductase-1 (TrxR1), serving as an electron carrier to reduce peroxiredoxins, redox factor-1 (Ref1), and other proteins. A dithiol (Cys-32, Cys-35) in the active site of Trx1 undergoes reversible oxidation to the disulfide during the transfer of reducing equivalents. In addition to the redox regulatory function of the active-site cysteines (Cys-32, Cys-35), post-translational modifications of the other cysteines by oxidation (Cys-62, Cys-69), S-nitrosylation (Cys-69), and glutathionylation (Cys-73) have a significant effect on Trx1 function.3,4,5 Modification of thiols in Trx1 interrupts signaling mechanisms involved in cell growth, proliferation, and apoptosis.3,4
Previous studies show that common products of lipid peroxidation, including acrolein and 4-hydroxy-2-nonenal (HNE) react with and modify functions of various cellular proteins. Acrolein and HNE are electrophilic lipids that target a number of redox-sensitive proteins, inducing multiple cellular responses through several mechanisms.6 The cytoprotective role of acrolein and HNE in cellular mechanisms has been demonstrated at low concentrations. For example, HNE induces cytoprotective antioxidants, HO-1 and glutathione (GSH), in bovine aortic endothelial cells (BAECs)7 and activates thioredoxin reductase (TrxR)-1 via Nrf2 activation.8 Many cytotoxic effects are mainly attributed to the alkylating properties and strong reactivity of both acrolein and HNE, resulting from the conjugated double-bond system (CH2=CH-CHO). Yang and colleagues9 demonstrated that acrolein inhibited Trx1 activity without affecting total cellular Trx1 protein amount in A594 cells. However, the mechanism of acrolein-induced inhibition of cellular Trx1 is currently unknown, and a specific role of acrolein-modified Trx1 in cardiovascular disease has not been identified. HNE, a product of lipid peroxidation, modified Cys32 and Cys35 of Escherichia coli Trx and inhibited catalytic activity.10 Increased HNE concentrations can be a potent biomarker for numerous diseases and conditions, including atherosclerosis, ischemia, diabetes, inflammation, and neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease. Accumulation of HNE and acrolein under pathological conditions are toxic and can modify cellular proteins and DNA.10,11,12
The present study shows that acrolein and HNE inhibit Trx1 activity through direct modification of a nonactive site thiol. Acrolein reacts with the thiol residue Cys-73 in Trx1, which was identified by mass spectrometry and Western blot analyses under native conditions,5,13 with modification of Trx1 occurring at lower concentration in endothelial cells. Microinjection of acrolein- or HNE-modified Trx1 into endothelial cells elevated monocyte binding to endothelium, showing that modification of Trx1 by reactive aldehydes is sufficient to stimulate critical early events of atherosclerosis.
Materials and Methods
Cell Culture, Acrolein Treatment, and Western Blot Analysis
BAECs purchased from American Type Culture Collection, Bethesda, MD, were maintained with 10% fetal bovine serum in Dulbecco’s modified Eagle’s medium. Confluent BAECs were treated with acrolein or HNE for 1 hour, washed with Hanks’ balanced salt solution three times, replenished with fresh Dulbecco’s modified Eagle’s medium including 10% fetal bovine serum for 5 hours, and then used for each experiment. 6-Carboxy-2′,7′-dichlorofluorescein diacetate, calcein AM, and Alexa Fluor 568 were purchased from Molecular Probes (Eugene, OR), acrolein and iodoacetic acid (IAA) were from Sigma-Aldrich (St. Louis, MO), and HNE was from Cayman Chemical (Ann Arbor, MI). Purified human Trx1 and TrxR1 were obtained from Labfrontier (Seoul, Korea) and American Diagnostica (Greenwich, CT), respectively. Mutant human Trx1 proteins (C32,35S and C62,69,73S) were obtained as reported previously.5 Antibodies were from BD Pharmingen (San Diego, CA) for Trx1; Santa Cruz Biotechnology (Santa Cruz, CA) for phospho-IκBα, ICAM1, and P-selectin; and Cell Signaling Technology (Beverly, MA) for phospho-cJun. To examine protein expression level, cells treated with or without acrolein were washed with ice-cold phosphate-buffered saline (PBS) three times, lysed with ice-cold buffer (10 mmol/L Tris, pH 7.4, 100 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 2 mmol/L Na3VO4, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 10% glycerol, and 0.5% deoxycholate), sonicated for 10 seconds on ice, and heated at 95°C. Fifty μg of protein were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE), followed by Western blot with an antibody described above and an Alexa Fluor 680 or 800 secondary antibody (Molecular Probes).5,13 Corresponding protein bands were visualized using an Odyssey scanner using the Odyssey 2.1 software (LI-COR, Lincoln, NE) and quantified by densitometry. For measurement of gene expression, total cell mRNA was isolated and used for real-time polymerase chain reaction (PCR).14
Determination of Modifications of Trx1 by Western Blotting under Native Condition
To examine thiol modifications in Trx1, BAECs and purified human Trx1 proteins (WT and mutants) treated with acrolein, HNE, dithiothreitol, or H2O2 were prepared following the procedures as described.5 In brief, cells or purified proteins suspended in the buffer including 6 mol/L guanidine and 50 mmol/L IAA (pH 8.3) were incubated at 37°C for 30 minutes (note: excess dithiothreitol was always removed by G-25 spin column before adding IAA) followed by G-25 column process. The modified forms of cellular and purified Trx1 were analyzed by native PAGE followed by Western blot with mouse anti-human Trx1 and an Alexa Fluor 680 anti-mouse secondary antibody for Trx1 (Molecular Probes).5,13 Bands corresponding to Trx1 were visualized using an Odyssey scanner using the Odyssey 2.1 software (LI-COR).
Preparation of Acrolein- and HNE-Modified Trx1 [Wild Type (WT), Mutant (C62,69,73S)] and Activity Assays for Trx1 (WT, Mutant) and TrxR1
Acrolein- or HNE-modified Trx1 was prepared fresh for each experiment. Purified human Trx1 WT and mutant proteins (mutation of Cys 62,69,73 to Ser), fully reduced by TCEP or dithiothreitol, were purified using Microspin G-25 columns (GE Health Care, Little Chalfont, Buckinghamshire, UK) before further treatment with acrolein or HNE. After 1 hour, activated thiol-Sepharose (Sigma-Aldrich) was added to remove unreacted acrolein and HNE, samples were centrifuged, and the supernatant fractions, including acrolein- or HNE-modified Trx1, were collected for additional experiments including activity assays, mass spectrometry analysis, and microinjection. Trx1 and TrxR1 activities in a cell-free system were measured by the insulin reduction assay.5,15
Mass Spectrometry
For matrix-assisted laser desorption ionization/time of flight (MALDI-TOF)-mass spectrometry (MS), purified Trx1 (WT, mutant) proteins were prepared by tryptic digestion (Promega, Madison, WI) and desalting with Ziptip (Millipore, Bedford, MA). Positive-ion MALDI-TOF MS analysis was performed using Reflex III delayed-extraction MALDI-TOF mass spectrometer (Bruker Daltonics, Billerica, MA) equipped with a 337-nm nitrogen laser; α-cyano-4-hydroxycinnamic acid (Agilent Technology, Santa Clara, CA) was used as the matrix. MALDI-TOF/TOF MS/MS was performed on a 4700 Proteomics Analyzer (Applied Biosystems, Foster City, CA) MALDI-TOF/TOF instrument. The instrument was operated in the positive ion mode. Laser intensity was adjusted manually to obtain the optimal fragmentation.
Monocyte Adhesion and Reactive Oxygen Species Measurement
Confluent BAECs, plated in six-well plates were exposed to acrolein for 1 hour, washed, incubated in fresh culture medium at 37°C for 5 hours, and used for monocyte adhesion studies. THP1 monocyte cells (5 × 105/plate) were prelabeled with green calcein AM and then coincubated with BAECs for 30 minutes. Plates were washed three times with PBS to remove unbound THP1 cells and observed at ×20 magnification of fluorescent microscope. For quantification of monocyte binding, BAECs in 96-well plates were treated with acrolein or HNE and prepared as described above. Attached THP1 were quantified by measuring fluorescence intensity on a M2 plate reader (Molecular Devices, Sunnyvale, CA). Reactive oxygen species (ROS) detection was determined by dichlorofluorescein oxidation of BAECs plated onto 96-well plates incubated with acrolein or HNE for 1 hour and assayed as previously described.14
Glutathione Analysis and Redox Potential (Eh) Calculation
GSH and GSSG were quantified by high-performance liquid chromatography with fluorescence detection, expressed as molar concentrations based on cell volume, which were used to calculate the steady-state redox potential values using the Nernst equation.16
Determination of Gene Expression Levels by Real-Time RT-PCR
Total cellular mRNA was isolated from BAECs 5 hours after being treated, washed, and incubated with fresh medium. An equal amount of mRNA from each sample was used to generate cDNAs by reverse transcription (BD Bioscience, Franklin Lakes, NJ). For quantitative PCR, amplication was performed in triplicate on an iCycler IQ Multicolor RT-PCR detection system (Bio-Rad, Hercules, CA) for 35 cycles as follows: 95°C for 30 seconds, 62°C for 30 seconds, and 72°C for 1 minute. Quantification and melting curves were analyzed with iCycler software relative to a standard curve for each gene. The primers used for real-time PCR are as follows: ICAM forward, 5′-CTGAGGTCTCAGAATGGACTACTG-3′; ICAM reverse, 5′-GCCACGTCCAGTTTCCCGGGCAAT-3′; PECAM-1 forward, 5′-GTCCAACGTGGAGTCCTCCAGACC-3′; PECAM-1 reverse, 5′-GCCTCACTGCTGAGGCTGTTGTGT-3′; P-selectin forward, 5′-GACATGTCCTGCAGCAAGCAAGGA-3′; P-selectin reverse, 5′-GCTGCAGCTGGACTGGTGCTGGAA-3′; and MCP-1 forward, 5′-AACAGCTTCCCGCTGAAAC-3′; MCP-1 reverse, 5′-TCTGCACATAACTCCTTGCC-3′.
Microinjection
BAECs grown in 35-mm glass-bottom dishes were coinjected with Trx1 or acrolein- or HNE-modified Trx1 and cell membrane impermeable Alexa Fluor 568. Microinjection (Eppendorf FemtoJet with glass microcapillary) was performed under a fluorescence microscope (Ultraview ERS; Perkin-Elmer, Emeryville, CA) enclosed in a heated chamber with CO2 perfusion (5% CO2 at 37°C). Injection parameters were Pi: 45 hPa, Ti: 0.5 s, Pc: 15 hPa. BAECs were microinjected with modified or unmodified Trx1 (Trx1, Acr-Trx1, HNE-Trx1) and with the red fluorescent Alexa Fluor 568 as injection control. Cultures were then incubated for 5 hours at 37°C and 5% CO2, before the monocyte adhesion assay.
Electrophoretic Mobility Shift Assay
Cells (1 × 107) with or without acrolein treatment were prepared for nuclear extraction using a nuclear extraction kit (Panomics, Fremont, CA) following the manufacturer’s protocol. To examine NF-κB activity, electrophoretic mobility shift assay was performed using an electrophoretic mobility shift assay gel-shift kit (Panomics) by incubating a biotin-labeled or unlabeled probe containing an NF-κB DNA-binding consensus sequence (5′-AGTTGAGGGGACTTTCCCAGGC-3′) with a nuclear extract (5 μg) for 30 minutes at 20°C. The samples were analyzed by a 6% nondenaturing PAGE, electroblotted for 30 minutes, and signals were detected by chemiluminescence imaging according to the manufacturer’s protocol (Panomics).
Results
Acrolein and HNE Resulted in Thiol Modification and Inhibited Trx1 Activity
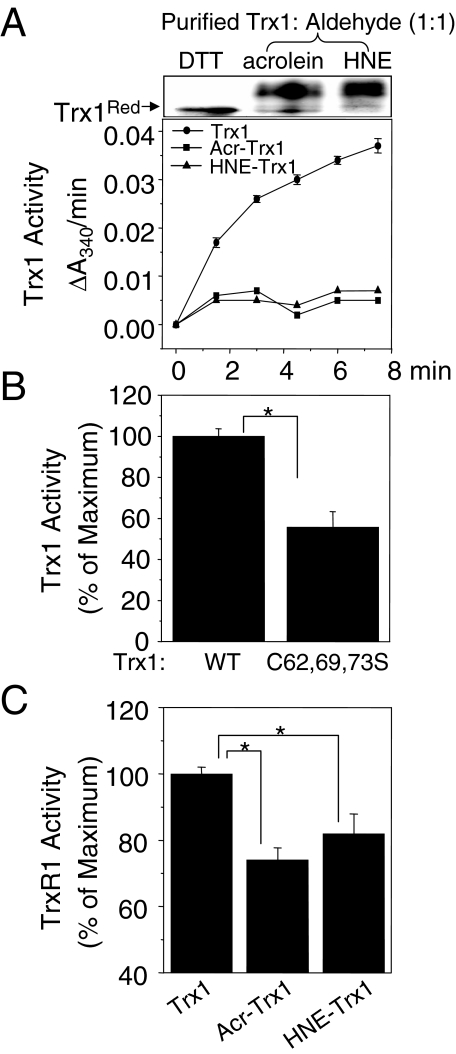
Previously, Yang and colleague9 showed that acrolein inhibited Trx1 and TrxR1 activities using cell lysates extracted from acrolein-treated human lung adenocarcinoma cell line A549. Another recent study demonstrated that HNE inhibits E. coli Trx activity examined by using purified E. coli Trx protein.10 We examined whether acrolein or HNE reacted with purified human Trx1 and caused inhibition of Trx1 activity (Figure 1A). Purified human Trx1 protein was reduced by dithiothreitol (2 mmol/L) and excess dithiothreitol was removed by G-25 column before incubation with each aldehyde for 1 hour at room temperature. Remaining aldehyde was removed by thiol-conjugated agarose beads before performing Trx1 activity assay. Addition of acrolein or HNE to purified Trx1 at molar ratios of 1:1 (Trx1/aldehyde) showed complete inhibition of activity (Figure 1A, Acr-Trx1 and HNE-Trx1) compared with untreated, control Trx1, indicating a high specificity of reaction, probably with a critical residue. Figure 1B shows that mutation of the nonactive site disulfide of Trx1 (C62,69,73S) inhibits catalytic activity of Trx1 (WT, 100 ± 3.79; C62,69,73S, 55.7 ± 7.59) consistent with our previous report demonstrating inhibitory function of C62,69S.5 To examine whether modified Trx1 by acrolein or HNE inhibits TrxR1 function, we measured TrxR1 activity in a cell-free system under conditions with excess unmodified Trx1.15 As shown in Figure 1C both acrolein- and HNE-modified Trx1 significantly inhibited TrxR1 activity by 26% and 18%, respectively [TrxR1 activity, 73.9 ± 3.8 (by acr-Trx1); 81.8 ± 6.1 (by HNE-Trx1); 100 ± 2.2 (by Trx1 control)].
Figure 1.
Acrolein and HNE resulted in thiol modification and inhibited Trx1 activity. Purified recombinant human Trx1 protein was reduced by 2 mmol/L dithiothreitol (or TCEP), passed through a G-25 spin column to remove excess dithiothreitol, and reacted with acrolein or HNE (Trx1; aldehyde, 1:1) for 1 hour. After removal of unreacted acrolein and HNE, Trx1 derivatized by thiol-reactive IAA was separated by 15% native PAGE followed by Coomassie staining (A, top; dithiothreitol, control Trx1; Acr-Trx1cc acrolein-reacted Trx1; HNE-Trx1, HNE-reacted Trx1). Purified Trx1 after treating with acrolein (Acr-Trx1) or HNE (HNE-Trx1) with 1:1 ratio was examined to measure Trx1 activity (A: bottom line graph). A and B show Trx1-dependent insulin-reductase activity in a cell-free system measured by monitoring the oxidation of NADPH at 340 nm (A, line graph; Trx1, dithiothreitol-reduced Trx1; Acr-Trx1, acrolein-reacted Trx1l HNE-Trx1, HNE-reacted Trx1; B: bar graph; WT, dithiothreitol-reduced Trx1; C62,69,73S, dithiothreitol-reduced Trx1 mutant). C: TrxR1 activity was measured under conditions with excess unlimited amount of Trx1 control (Trx1), acrolein-reacted Trx1 (Acr-Trx1), or HNE-reacted Trx1 (HNE-Trx1). Data represent means ±SE; n = 3 different experiments. *P < 0.05 versus Trx1 control.
Identification of Reactive Aldehyde-Modified Thiol in Trx1
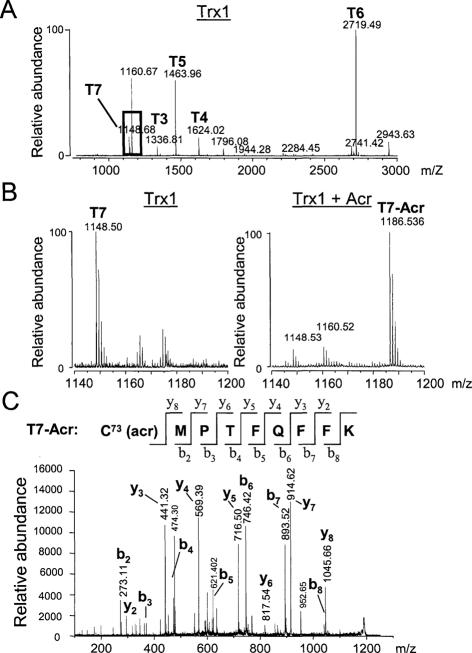
To identify acrolein- or HNE-modified residue(s) of Trx1, purified Trx1 and mutant Trx1 were reduced with Tris-(2-carboxyethyl)phosphine (TCEP) or dithiothreitol, purified with a G-25 column to remove excess dithiothreitol, and incubated at various ratios of acrolein or HNE to Trx1. After 1 hour, any excess free aldehyde was removed with activated thiol-containing agarose beads. Collected acrolein-Trx1 (WT, mutant) or HNE-Trx1 adduct was digested with trypsin and analyzed by MALDI-TOF mass spectrometry. At the bottom of Tables 1 and 2, predicted peptides are listed and labeled T1 through T12, with five cysteine (Cys) residues for Trx1 (WT) and two Cys and three serine (Ser) residues for the mutant (C62,69,73S) indicated by italicized and underlined letters, respectively. The mass spectrum shown in Figure 2A displays the tryptic fragments (T3 to T7) of control Trx1 (WT) larger than 1000 Da. All predicted peptides more than 1000 m/z were identified in the spectra including T4, T6, and T7, which contain all five cysteines, and T3 and T5, which do not contain cysteine residues (Table 1). Figure 2, B and C, shows mass spectra used to identify acrolein-modified thiol residues of Trx1. No m/z values were obtained corresponding to the acrolein modification of Cys-32 or Cys-35 in T4 or Cys-62 or Cys-69 in T6 of Trx1 under all experimental conditions by varying ratios of acrolein to Trx1 as analyzed by MALDI-TOF mass spectrometry (Trx1/Acr, 1:5, 1:10; data not shown). However, modification of Trx1 by low concentrations of acrolein (Trx1/Acr, 1:1) was observed as a new peak at m/z 1186 (Figure 2B, right), which was not detected in Trx1 controls (Figure 2B, left). The mass increase in formation of the acrolein-T7 adduct was 38, which was 18 less than expected from addition of the molecular weight of acrolein (56) and is probably attributable to water elimination. The T7 peptide contains both a thiol and free amine on the lysine residue, either of which could react with acrolein. However, if the lysines were modified by acrolein, trypsin would not hydrolyze the adjacent peptide bond connecting T7 and T8. Therefore, the 1186 m/z seemed to be attributable to reaction of acrolein with Trx1 Cys-73, with rearrangement and elimination of water on tryptic digestion. We also examined the possibility of acrolein modification of His residues. One His residue is contained in T5 peptide of Trx1. If His is modified by acrolein, an adduct should appear at 1519.7 m/z. The mass of this peak was not detected in MS data of acrolein-Trx1 (Table 1), suggesting that His is not modified by acrolein at the conditions used for the current study. In the present study, we have not investigated detailed chemical identification. Therefore, whether acrolein-modified Trx1 adducts are a result from the Michael addition or from the Schiff base is unknown. We conclude that Trx1 adduct formation based on expected masses and observed masses is through the acrolein modification adduct, which was formed after reaction with acrolein (56 Da) and subsequent elimination of H2O (18 Da) (Table 1).
Table 1.
Expected and Observed Monoisotopic Masses (MH+) of Tryptic Fragments from Acrolein-Reacted Trx1
| Fragment (Trx1 WT) | Expected mass (MH+) | Observed mass (MH+) |
|---|---|---|
| Cys-containing peptide | ||
| T4 dithiol (Cys32, Cys35) | 1624.9 | 1624.0 |
| T4-1Acr | 1680.9 (1624.9 + 56.0) | ND |
| T4-2Acr | 1736.9 (1624.9 + 112) | ND |
| T6 dithiol (Cys62, Cys69) | 2719.2 | 2719.4 |
| T6-1Acr | 2775.2 (2719.2 + 56.0) | ND |
| T6-2Acr | 2831.2 (2719.2 + 112) | ND |
| T7 thiol (Cys73) | 1148.5 | 1148.6 |
| T7-1Acr | 1204.5 (1148.5 + 56.0) | ND |
| 1186.5 [1204.5 − 18]: addition, elimination of H2O | 1186.5 | |
| Other identified peptides | ||
| T3 | 1336.6 | 1336.8 |
| T5 His (His43) | 1463.7 | 1463.9 |
| T5-1Acr | 1519.7 (1463.7 + 56) | ND |
| Amino acid sequences of the Trx1 tryptic fragments | ||
| 1 MVK(T1), QIESK(T2), TAFQEALDAAGDK(T3), | ||
| 22 LVVVDFSATWCGPCK(T4), MIKPFFHSLSEK(T5), | ||
| 49 YSNVIFLEVDVDDCQDVASECEVK(T6), | ||
| 73 CMPTFQFFK(T7), K(T8), GQK(T9), VGEFSGANK(T10), | ||
| 95 EK(T11), LEATINELV(T12) |
Trx1 (100 μmol/L) was reduced with dithiothreitol or TCEP, reacted with acrolein (100 μmol/L), digested with trypsin, and analyzed by MALDI-TOF. The amino acid sequences of the tryptic fragments are indicated (T1 to T12). T4, T6, and T7 are Cys-containing fragments. Acrolein-modified fragments by one addition (−1Acr) or two additions (−2Acr) of acrolein are indicated. Expected masses for adducts were shown by the adducts after acrolein reaction and after the addition with subsequent elimination of H2O. ND, not detected.
Table 2.
Expected and Observed Monoisotopic Masses (MH+) of Tryptic Fragments from Acrolein-Reacted Trx1 Mutated Protein (C62,69,73S
| Fragment (Trx1 mutant) | Expected mass (MH+) | Observed mass (MH+) |
|---|---|---|
| Cys-containing peptide | ||
| T4 dithiol (Cys32, Cys35) | 1624.9 | 1624.6 |
| T4-1Acr | 1680.9 (1624.9 + 56.0) | ND |
| 1662.9 [(T4–1Acr) − 18] | ND | |
| T4-2Acr | 1736.9 (1624.9 + 112) | ND |
| 1700.9 [(T4–2Acro) − 36]; addition, elimination | 1700.8 | |
| of 2H2O | ||
| T6 from dithiol to Ser62, Ser69 | From 2719 (2Cys) to 2687.2 (2Ser) | 2687.9 |
| T6-1Acr | 2775.2 (2719.2 + 56.0) | ND |
| T6-2Acr | 2831.2 (2719.2 + 112) | ND |
| T7 from thiol to Ser73 | From 1148.5 (Cys) to 1132.5 (Ser) | 1132.6 |
| T7-1Acr | 1204.5 (1148.5 + 56.0) | ND |
| 1186.5 [(T7-Acr) − 18]; addition, elimination of H2O | ND | |
| Other identified peptides | ||
| T3 | 1336.6 | 1336.8 |
| T5 | 1463.7 | 1463.9 |
| Amino acid sequences of the Trx1 mutant tryptic fragments | ||
| 1 MVK(T1), QIESK(T2), TAFQEALDAAGDK(T3), | ||
| 24 LVVVDFSATWCGPCK(T4), MIKPFFHSLSEK(T5), | ||
| 49 YSNVIFLEVDVDDSQDVASESEVK(T6), | ||
| 73 SMPTFQFFK(T7), K(T8), GQK(T9), VGEFSGANK(T10), | ||
| 95 EK(T11), LEATINELV(T12) |
Acrolein-reacted Trx1 mutant (100 μmol/L) was prepared as described in Table 1 and analyzed by MALDI-TOF. The amino acid sequences of the Trx1 mutant tryptic fragments are indicated (T1 to T12). Only T4 is Cys-containing fragment. T6 and T7 fragments containing Ser instead of Cys were detected as expected masses. Acrolein-modified fragments by one addition (−1Acr) or two additions (−2Acr) of acrolein are indicated. Expected masses for adducts were shown by the adducts after acrolein reaction and after addition with subsequent elimination of H2O. ND, not detected.
Figure 2.
Mass spectral analysis of Trx1 and acrolein-modified Trx1. A: MALDI-TOF mass spectrum of tryptic fragments of Trx1 with identified fragments labeled (T3 to T7) as described in Table 1. A highlighted box indicates the T7 tryptic peptide (m/z = 1148) including Cys-73. B: MALDI-TOF mass spectra of m/z range for the T7 tryptic peptide reacted without (T7: m/z 1148.68, left) or with acrolein (T7-Acr: m/z 1186.5, right). Difference in m/z corresponds to the addition of an acrolein followed by the elimination of water (56 − 18 = 38 Da). C: MS/MS product ion spectra of presumed T7-Acr tryptic fragment (m/z 1186.5) from the tryptic digest of acrolein-treated Trx1 analyzed by MALDI-TOF/TOF. The observed b and y fragment ions clearly indicated that the acrolein modified the cysteine residue.
To confirm our interpretation that acrolein modified Cys-73 in the T7 peptide, the m/z 1186 peptide (T7-Acr) was further analyzed by MALDI TOF/TOF. Figure 2C shows MS/MS fragmentation data confirming that the cysteine residue (C-73) of T7 was modified by acrolein and resulted in 38 m/z increase in m/z 1148 of T7. Taken together, at the low 1:1 ratio of acrolein: Trx1, MALDI TOF/TOF and MS/MS analyses strongly suggest that none of the other Cys are modified except Cys-73. To support Cys-73 modification by acrolein, we also performed an experiment by utilization of Trx1 mutant protein, in which three regulatory Cys residues including Cys-73 were mutated to Ser (Table 2). All predicted peptides more than 1000 m/z were identified in the spectra including T4 containing two active cysteines, T6 containing two serines, T7 containing one serine, and T3 and T5, which do not contain cysteine residues (Table 2). The increase in mass after formation of the acrolein-T7 adduct (m/z 1186) shown from Trx1 (WT) with acrolein was not observed in the Trx1 mutant (expected mass, 1186.5 m/z; observed mass, ND; Table 2). However, a new peak (m/z 1700.8) was detected that was not observed in samples in which Trx1 (WT) was treated with acrolein. This is possibly an acrolein-T4 adduct involving the dithiol in the active site (C32,C35) because of the absence of thiol residues in T7 (C73S) and T6 (C62,69S) in the Trx1 mutant. The mass is consistent with addition of acrolein to each of the active site thiols, after elimination of water. However, this adduct needs further study for chemical identification.
To identify Trx1 modification by HNE, HNE-modified Trx1 was collected by the same treatment as described above for identification of Trx1-acrolein adduct. The result of MALDI-TOF analysis showed that HNE also targeted Cys-73 residue of Trx1, with a new peak (m/z: 1303.738, HNE + T7) in the spectra (data not shown). Thus, both acrolein and HNE modified the nonactive site Cys-73.
Reactive Aldehyde-Induced Irreversible Thiol Modification of Trx1 in Endothelial Cells
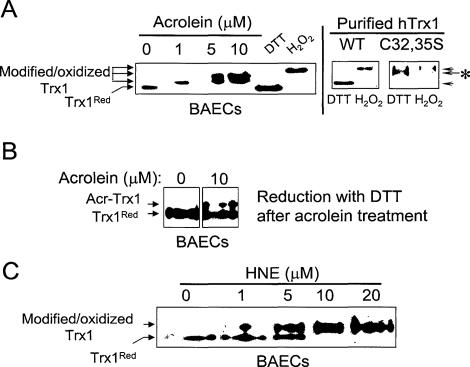
Acrolein- and HNE-induced thiol modifications in Trx1 were further examined by native and nonreducing PAGE combining Western blot analysis [Figure 3, A (left), B, and C]. Extracts are treated with IAA to introduce negative charges to thiols, allowing separation of thiol and disulfide forms of Trx1 by native gel electrophoresis.5 As previously shown for control Trx1,5 Trx1 pretreated with dithiothreitol before the addition of IAA had greater mobility than Trx1 pretreated with H2O2 (Figure 3A, left). H2O2-treated active site mutant Trx1 (C32,35S) had an identical mobility to that of WT Trx1 (Figure 3A, right; purified Trx1). However, with dithiothreitol treatment before IAA, the C32,35S-Trx1 had an intermediate mobility (arrow with asterisk). This result shows that C32,35S mutation affects Trx1 redox state examined by native, nonreducing PAGE combining Western blot analysis.
Figure 3.
Reactive aldehyde-induced thiol modification of Trx1 in endothelial cells. Confluent BAECs treated with acrolein (A, left) or HNE (C) at indicated concentrations for 1 hour were lysed and carboxymethylated with iodoacetic acid (IAA) in the presence of guanidine. B: Lysates from BAECs treated with acrolein as above (A) were reduced with dithiothreitol and spun with G-25 column to remove excess dithiothreitol before IAA addition. B: Acr-Trx1 indicates acrolein-modified Trx1 not lost by subsequent dithiothreitol treatment. Purified Trx1 control (WT) and mutant (C32,35S) treated with dithiothreitol (2 mmol/L) or H2O2 (1 mmol/L) for 30 minutes show that mutation of cysteines in active site affects Trx1 redox state analyzed by native, nonreducing PAGE (A, right). All prepared cell lysates with IAA were then separated by native PAGE, electroblotted, and probed with an antibody specific to Trx1. C: Dithiothreitol and H2O2 treatments indicate fully reduced and oxidized control Trx1, respectively.
To examine acrolein-induced thiol modification of Trx1 in a cellular system, BAECs were treated with acrolein for 1 hour at indicated concentrations (Figure 3A, left). With 1 μmol/L acrolein, Trx1 moved with slower mobility than that of Trx1 fully reduced form (Trx1Red). However, at 5 and 10 μmol/L, the majority of Trx1 migrated more slowly, indicating a dose-dependent modification (Figure 3A, left). The slower mobility of acrloein-Trx1 in cells was the same as seen for treatment of purified reduced Trx1 with acrolein (Figure 1A, top).
To examine if acrolein-induced Trx1 modification is irreversible, BAECs were treated with acrolein for 1 hour, washed, lysed, and incubated with dithiothreitol for 30 minutes. This treatment results in reduction of all disulfides in Trx1. Thus, acrolein-modified Trx1 (Acr-Trx1) can be resolved from unmodified Trx1 by the same procedure used above. As shown in Figure 3B, acrolein-modified and an irreversible form of Trx1 (Acr-Trx1) were observed after dithiothreitol treatment, whereas the oxidized, disulfide form of Trx1 was not shown any longer. The modification of Trx1 was still observed at 1 μmol/L or greater after dithiothreitol treatment (data not shown) suggesting acrolein-induced changes in Trx1 mobility represent irreversible modification. Analyses of HNE-treated BAECs showed that HNE similarly modified Trx1 (Figure 3C).
Acrolein and HNE Enhance Monocyte Adhesion to Endothelium
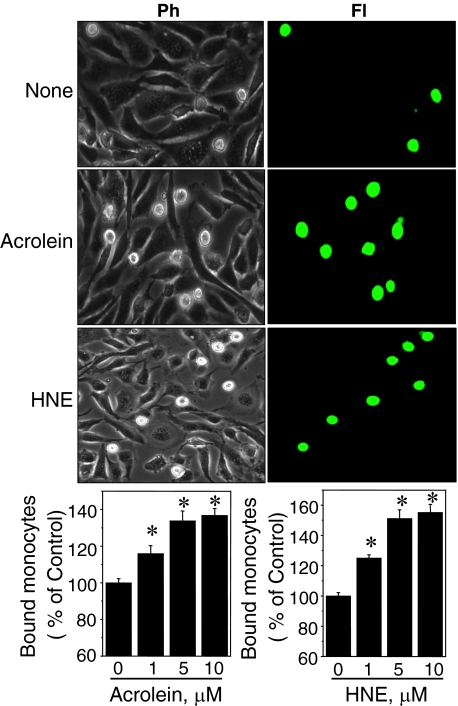
To examine acrolein or HNE stimulation of monocyte adhesion to the endothelium, we used an in vitro cell model consisting of THP1 cells and confluent BAECs. Confluent BAECs were exposed to 1, 5, or 10 μmol/L of acrolein or HNE in Hanks’ balanced salt solution for 1 hour, washed with Hanks’ balanced salt solution three times, and incubated in fresh culture medium (10% fetal bovine serum in Dulbecco’s modified Eagle’s medium) for 5 hours before monocyte addition. BAECs treated with either acrolein (Figure 4, middle) or HNE (Figure 4, bottom) showed a significantly higher degree of monocyte adhesion as determined by phase-contrast (Ph) and fluorescence (Fl) microscopy. Quantification of monocyte adhesion was obtained by measuring the fluorescence of attached monocytes labeled with calcein AM to BAECs with a fluorescence plate reader. Results showed that monocyte binding was increased in acrolein-treated (1 μmol/L, 116 ± 4.2; 5 μmol/L, 134 ± 5.3; 10 μmol/L, 137 ± 3.7) and HNE-treated (1 μmol/L, 125 ± 2.2; 5 μmol/L, 151 ± 5.7; 10 μmol/L, 155 ± 5.4) cells (Figure 4, bar graphs). We also examined if acrolein-induced cytotoxic effects were reversible. BAECs were incubated with complete media for 1 day after an initial 1-hour acrolein treatment. If acrolein-induced inflammatory effects are reversible in BAECs, increased monocyte adhesion should not be observed. The results showed that BAECs treated with 10 μmol/L acrolein increased cell death and loss of Trx1 protein significantly after 24 hours. Because of these results, cells with 10 μmol/L acrolein were not applicable for cell adhesion assay 24 hours after acrolein treatment. Cells treated with 1 or 5 μmol/L acrolein showed significant increase in cell adhesion examined 24 hours after acrolein exposure (data not shown), suggesting that acrolein-induced toxicity (cell death) and cell adhesion are not reversible.
Figure 4.
Acrolein and HNE enhance monocyte adhesion to endothelium. Confluent BAECs plated in six-well (first and second columns) or 96-well plates (bar graphs) were exposed to acrolein (5 μmol/L) or HNE (5 μmol/L) for 1 hour, washed with Hanks’ balanced salt solution, and examined for monocyte adhesion 5 hours after treatment. THP1 monocytes labeled with green calcein AM were added to BAECs, and adherent monocytes were examined. Representative photographs are shown as phase contrast (Ph) and fluorescent (Fl) images. Labeled THP1 cells attached to BAECs in 96-well plate were quantified by measuring fluorescence intensity. Data are means ±SE of data from eight wells (n = 8). *P < 0.05 versus control.
Microinjection of Reactive Aldehyde-Modified Trx1 Stimulated Monocyte Adhesion to BAECs
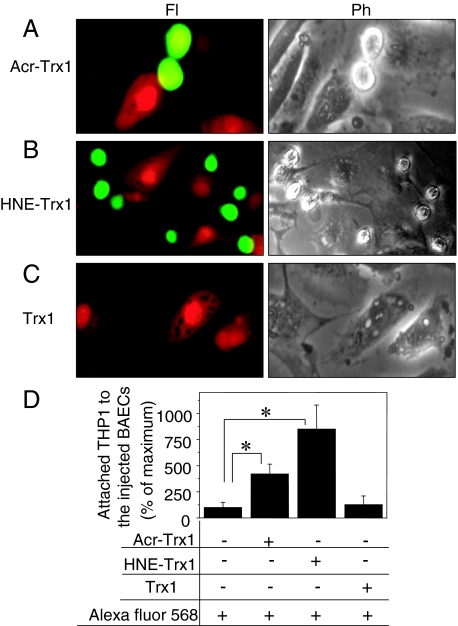
Because acrolein can have multiple cellular targets, the downstream effects that result directly from Trx1 modification cannot be determined by treating cells with acrolein or HNE. To specifically address Trx1 modification-related effects on monocyte adhesion, BAECs were grown in 35-mm plates and microinjected with Trx1, acrolein-modified Trx1, or HNE-modified Trx1, along with cell membrane-impermeant red fluorescent molecule (Alexa Fluor 568; Fl) to identify injected cells. After injection, cells were incubated at 37°C, 5% CO2 for 5 hours and then analyzed for monocyte adhesion assay as described above. THP1 monocytes (5 × 105) labeled by calcein AM (green-fluorescence after deacetylation) were added to BAEC monolayer cultures for 1 hour and then washed twice in the PBS to remove unbound THP1. Attached monocytes were observed using fluorescence microscopy (Olympus 1 × 81 with ×200 magnification). Red-fluorescent cells in Figure 5 are BAECs injected with acrolein-modified Trx1 (Figure 5A, Acr-Trx1, Fl), HNE-modified Trx1 (Figure 5B, HNE-Trx1, Fl), and Trx1 (Figure 5C, Trx1, Fl). THP1 (green) attached to the BAECs injected with acrolein-modified Trx1 and HNE-modified Trx1 are shown (Figure 5, A and B; top). The same images were taken to visualize all cells, including injected and uninjected cells, by phase-contrast microscopy (Ph; Figure 5, bottom). Monocytes attached to the injected BAECs with Trx1, Trx1-Acr, Trx1-HNE, or red-fluorescent dye alone were quantified as shown in Figure 5D. BAECs injected with acrolein-modified Trx1 (Acr-Trx1) and HNE-modified Trx1 (HNE-Trx1) showed between three and seven times more attached monocytes, respectively, than those in fluorescent dye alone (Alexa Fluor 568) or with injected Trx1 (Trx1).
Figure 5.
Microinjection of acrolein or HNE-modified Trx1 stimulated monocyte adhesion to BAECs. BAECs plated onto a 35-mm plate were microinjected with acrolein-modified Trx1 (A), HNE-modified Trx1 (B), or Trx1 (C) and simultaneously with Alexa Fluor 568 to identify injected cells. After 5 hours at 37°C and 5% CO2, calcein-labeled THP1 cells were added to BAECs. After 30 minutes, cells were washed to remove unbound THP1 and examined by fluorescence (Fl) and phase contrast (Ph) microscope. D: Calcein-labeled THP1 cells (green) attached to the injected BAECs (red) were quantified as shown in bar graph. Results are representative of three experiments, each performed in duplicate with ∼100 injected cells per treatment. Data are means ±SE for three independent experiments. *P < 0.05 versus control.
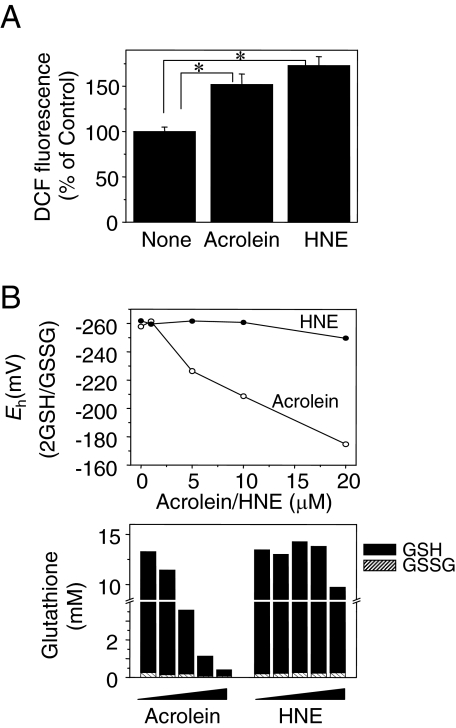
Acrolein and HNE Stimulate ROS Production and Oxidize GSH/GSSG Redox Potential in BAECs
Previous studies have shown that acrolein and HNE stimulate peroxide production in lung epithelial and lung endothelial cells by depletion of cellular thiols such as GSH.17,18,19 To determine whether acrolein and HNE stimulate ROS generation in BAECs, cultures were treated with acrolein (5 μmol/L) or HNE (5 μmol/L) for 1 hour, and dichlorofluorescein fluorescence was used to measure ROS (Figure 6A). ROS levels were elevated by both acrolein (152 ± 11.5%) and HNE (173 ± 9.8%) treatment compared with the control (Figure 6A). Amounts of reduced (GSH) and oxidized (GSSG) glutathione (Figure 6B: bottom left, acrolein; bottom right, HNE) and correlating redox potentials were measured in cells treated with acrolein and HNE (Figure 6B, top: filled circle, HNE; opened circle, acrolein). Oxidation of GSH/GSSG redox potential was significantly increased at 5 μmol/L or higher concentration of acrolein but not at 1 μmol/L. Oxidation of GSH/GSSG redox state was observed at 5 μmol/L acrolein, resulting in a 32 mV increase compared with control. Interestingly, there was no significant effect of HNE on the GSH/GSSG redox state.
Figure 6.
Acrolein and HNE stimulated ROS production, and acrolein oxidized GSH/GSSG redox potential. A: Dichlorofluorescein fluorescence as a measure of ROS generation was examined in BAECs exposed to acrolein (5 μmol/L) or HNE (5 μmol/L) for 1 hour. Data represent the means ±SE of eight determinations. B: Top: Cellular GSH and GSSG concentrations were measured in cell lysates after acrolein treatment. Bottom: Oxidation-reduction potentials (Eh) were calculated using concentration of GSH and GSSG as described in Materials and Methods. *P < 0.05 versus control.
The Transcriptional Levels of Adhesion Molecules PECAM-1, ICAM-1, MCP-1, and P-Selectin in BAECs Increase with Acrolein Treatment
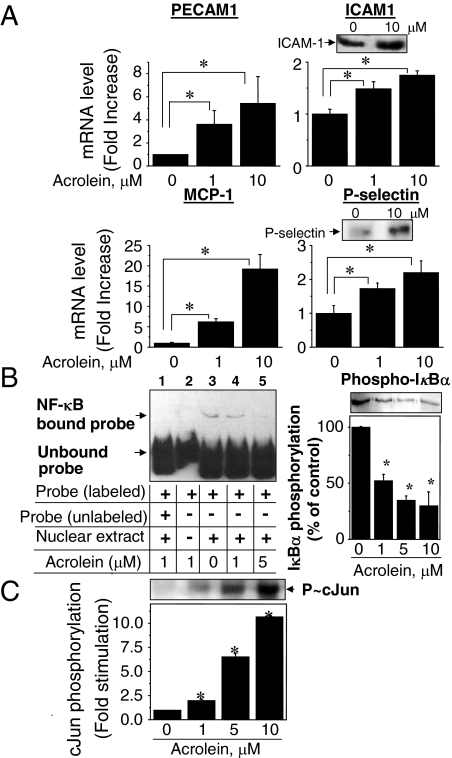
Increased cell adhesion in atherosclerosis is mediated by an increased expression of cell adhesion molecules in endothelial cells. Abundance of mRNA for members of the cell adhesion molecules and the selectin family, including ICAM-1, PECAM-1, P-selectin, E-selectin, and monocyte chemoattractant protein (MCP-1), was examined using real-time PCR. Expression levels for ICAM-1, PECAM-1, P-selectin, and MCP-1 were significantly increased in cells exposed to acrolein, even at 1 μmol/L (Figure 7A). The maximal increase in gene expression was ∼5.5-fold for PECAM-1, 1.7-fold for ICAM-1, 18-fold for MCP-1, and 2.2-fold for P-selectin by 10 μmol/L acrolein treatment, whereas mRNA for E-selectin was decreased by acrolein (data not shown). BAECs pre-exposed to acrolein for 1 hour followed by an additional 5-hour incubation with acrolein-free media enhanced gene expression levels as well as protein concentrations for cell adhesion molecules. To examine whether protein levels were elevated, P-selectin and ICAM-1 proteins were examined by immunoblotting (Figure 7A). Although fold increases in protein levels were not the same as mRNA, significant increases were observed in both ICAM-1 and P-selectin proteins (1.8- and 3.0-fold, respectively; Figure 7A). Collectively, the data show that exposure of endothelial cells to acrolein increased gene expression for cell-cell adhesion molecules, even at a concentration of acrolein (1 μmol/L) that did not cause detectable change in GSH/GSSG.
Figure 7.
Acrolein stimulated mRNA and protein levels of adhesion molecules and enhanced c-Jun phosphorylation but inhibited NF-κB activity. A: Quantification of gene transcripts for PECAM, ICAM-1, MCP-1, and P-selectin in acrolein-treated BAECs by real-time RT-PCR. Data are mean ±SEM for three independent experiments. *P < 0.05 versus control. Top panels in ICAM-1 and P-selectin show Western blotting probed with antibodies specific to ICAM-1 and P-selectin using cell lysates after treating without or with acrolein (10 μmol/L) for 1 hour and an additional 5 hours in the acrolein-free, fresh complete medium. B: Electrophoretic mobility shift assay for NF-κB binding activity to DNA. Lane 3 shows amount of NF-κB bound probe with relatively slow mobility compared with NF-κB-unbound probe for incubation with acrolein. Binding was prevented by unlabeled probe (lane 1). No slow mobility band was detected without nuclear extracts (lane 2). Binding of probe was decreased by increasing concentration of acrolein, indicating that acrolein inhibited NF-κB. Dose-dependent inhibition of IκBα phosphorylation by acrolein (B, right) confirmed that acrolein inhibited NF-κB activation. C: Acrolein stimulated c-Jun phosphorylation. BAECs treated with acrolein were examined by Western blot analysis. Densitometry was performed to quantify phospho-IkBα and phospho-cJun band intensities. Values in bar graph are means ±SE (n = 3). *P < 0.05 versus control.
Acrolein Inhibited NF-κB but Stimulated c-Jun Phosphorylation
NF-κB is a redox-sensitive transcription factor that functions in regulation of adhesion molecule expression on oxidative stimuli. To monitor the effect of acrolein on NF-κB activity, nuclear fractions of BAECs treated with acrolein for 1 hour were used for electrophoretic mobility shift assay (Panomics) (Figure 7B, left). Background level of binding activity of NF-κB to the biotin-labeled probe is shown and indicated as NF-κB-bound probe (Figure 7B, left, lane 3; acrolein, 0 μmol/L). Increasing concentrations of acrolein inhibited NF-κB binding to the probe, showing that acrolein inhibited rather than stimulated NF-κB function (Figure 7B, left; lanes 3 to 5).
As an independent approach to monitor effects of acrolein treatment on the NF-κB signaling, Western blot analysis was performed with an antibody specific to phosphorylated IκBα (Figure 7B, right). Phosphorylation of IκBα under control condition (acrolein: 0 μmol/L) was decreased by acrolein treatment in a dose-dependent pattern (Figure 7B, right; 1 μmol/L, 52.2 ± 5.8; 5 μmol/L, 34.7 ± 3.9; 10 μmol/L, 29.8 ± 12.7). Thus, the combination of approaches suggests that the activation of adhesion molecule expression (Figure 7A) in response to acrolein is not regulated through NF-κB activation.
AP-1 also mediates transcription of cell adhesion molecules, and acrolein has been shown to activate c-Jun in rat vascular smooth muscle cells.20 Consistent with this result, acrolein also increased phosphorylation of c-Jun in BAECs in a concentration-dependent manner (fold stimulation relative to control; 1 μmol/L,1.98 ± 0.16; 5 μmol/L, 6.5 ± 0.4; 10 μmol/L, 10.7 ± 0.2) (Figure 7C). Thus, the results suggest that acrolein-increased monocyte adhesion to vascular endothelial cells is associated with increased expression of adhesion molecules regulated by an AP-1-dependent mechanism.
Discussion
The present studies add to the accumulating evidence that the Trx1 system is critical to maintain normal endothelial function and protect against vascular diseases.2,4,21,22,23 For example, S-nitrosylation of Cys-69 in Trx1 stimulated its enzyme activity and antioxidant function in endothelial cells.4,21 On the other hand, nitration of Tyr-49 in Trx1 caused inactivation of Trx1 and resulted in cell death,23 and glutathionylation of Cys-73 also inhibited Trx1 activity.3
The level of acrolein in the sera of healthy persons was measured as high as 50 μmol/L and was measured at as high as 80 μmol/L in respiratory tract lining fluids in cigarette smokers.24 Acrolein and HNE are conjugated aldehydes10,25 that react with cellular thiols and primary amines.26 Acrolein found as protein conjugates in the plasma of patients was 180 μmol/L.24 HNE concentrations in the sera of healthy people were in the range 70 to 100 nmol/L,27 and liver tissue HNE levels were in the range 0.5 to 10 μmol/L.28 It is thought that the function of some proteins would be lost through the covalent linkage with these reactive aldehydes. Most proteins contain thiols and primary amines so that elucidation of critical targets of modification has proven difficult. However, thioredoxins and related proteins are common targets. Because Trx1 plays a central role in cell growth and survival signaling, modification and inhibition of Trx1 could have important pathological consequences. To support the present study, research is needed to determine whether acrolein- or HNE-modified Trx1 is actually present in diseased vascular tissues. Our initial studies of tissue samples show that the procedures described here should be suitable to detect such modifications when they occur. The present study shows that acrolein and HNE modify Trx1 in endothelial cells and stimulate inflammatory signaling events, including ROS generation, GSH/GSSG redox oxidation, elevated mRNA of cell adhesion molecules, and increased monocyte adhesion. Importantly, microinjection of acrolein- or HNE-modified Trx1 into the endothelial cells stimulated monocyte adhesion. We also performed experiments to test whether microinjection of reduced Trx1 can block acrolein-stimulated inflammatory signaling. Confluent BAECs pretreated with acrolein (1 μmol/L, 37°C) for 1 hour were used for microinjection of Trx1 in the complete medium after removal of acrolein. However, the experimental conditions combining acrolein treatment and microinjection (injection of reduced Trx1 protein plus dye or dye alone) stimulated cell death after an additional 5 hours of incubation at 37°C, 5% CO2 (95% of cell death in cells pretreated with acrolein followed by microinjection, data not shown). Because of the large amount of cell death, there were too few cells remaining to determine whether microinjection of Trx1 protected against acrolein-induced monocyte adhesion.
The active site Cys-32 residue of all thioredoxins including E. coli Trx has a low pKa and is very reactive under physiological conditions,29 but other Cys residues found in human Trx1, including Cys-62, -69, and -73, are also structurally and functionally important.5 In addition to a recent report suggesting that nitration of Tyr-49 is a novel posttranslational modification that inhibits Trx1 function,23 modification of cysteines by oxidation,5 S-nitrosylation (Cys-69),4,21 glutathionylation (Cys-73),3 or the experimental anticancer drug PX-12 (Cys-73) also inhibited activity.30,31 In addition to the previous study showing HNE-induced catalytic inhibition of E. coli Trx by modification of active site cysteines,10 we also examined E. coli Trx and found that Cys-32/Cys-35 were modified by acrolein (data not shown), which is consistent with the previous report by Fang and Holmgren.10 E. coli Trx is different from mammalian Trx1 structurally and functionally because E. coli Trx contains two cysteines in the catalytic site and does not have the other three regulatory cysteines observed in mammalian Trx1. We also examined mitochondrial Trx2, which only has active site cysteines, and found that this is also modified at the active site cysteines (data not shown). The present study demonstrates that low concentrations of acrolein and HNE preferentially modify Cys-73 of Trx1 rather than Cys-32 or Cys-35 in the active site, or the regulatory Cys-62 or Cys-69 sites. This may be attributable to Cys-73 being more accessible than Cys residues in active site, based on Trx1 structure.32 Previous reports showed that glutathionylation and dimerization of Trx1 occurs through Cys-73.33 The nature of the Cys-73 product detected by mass spectrometry is not clear but is likely to be derived from an initial acrolein-induced modification of the thiol group followed by reaction with the nucleophilic α-amino group on Cys-73, which becomes available for reaction after tryptic hydrolysis of the Lys-72-Cys-73 peptide bond. In addition to the spectral data, Western blots to detect chemical modifications by native PAGE showed that Trx1 is extensively modified and more sensitive than glutathione (Figure 6B) or mitochondrial Trx2 (data not shown).
Trx1 showed decreased electrophoretic migration at higher concentrations of acrolein (5 and 10 μmol/L) than at lower concentrations (1 μmol/L) as separated by native, nonreducing PAGE combining Western blot analysis, suggesting that the number of free thiols was decreased by increasing acrolein concentration in BAECs (Figure 3A). Mass spectrometry data using purified Trx1 protein showed that Cys-73 undergoes selective thiol modification and that this modification is independent of acrolein concentration throughout the range studied (Trx1/acrolein, 1:10; data not shown). Thus, additional low-mobility Trx1 bands at higher concentration of acrolein could be from disulfide formation resulting from increased ROS and oxidation of Trx1. This result could be attributable to inhibition of TrxR1 activity directly by acrolein and/or by acrolein-modified Trx1 as shown in Figure 1, which then results in Trx1 oxidation. This interpretation is consistent with earlier research showing that TrxR1 activity in human umbilical vein endothelial cells was significantly inhibited by acrolein treatment.34 Inhibition of TrxR1 activity has also been observed by HNE-induced modification of Cys-496/Sec-497 in the active site of TrxR1 in HeLa cells.10
A Trx1-regulated redox mechanism for activation of transcriptional factors, including NF-κB and AP-1, has been well studied. These transcription factors are redox-sensitive, regulate cell adhesion molecules, and are controlled by Trx1. In this study we found differential effects of acrolein on transcriptional factors such as inhibition of NF-κB and stimulation of c-Jun phosphorylation as a subunit of AP-1. Our data suggest that elevated expression of cell adhesion molecules (ICAM, PECAM, and P-selectin) and MCP-1 by acrolein is through an NF-κB-independent mechanism. Previous reports are somewhat confusing because AP-1 regulation by acrolein examined with c-Jun phosphorylation was controversial depending on cell types and exposure times.20,35 The present study shows that acrolein-induced c-Jun phosphorylation was increased in endothelial cells, suggesting that the acrolein-dependent monocyte adhesion is mediated by activation of AP-1.
In summary, stoichiometric amounts of acrolein or HNE selectively modified the nonactive site Cys-73 of Trx1. The modification resulted in loss of catalytic activity and inhibition of TrxR1. It seems that modification by acrolein or HNE is more complicated than oxidation or nitration because modified Trx1 not only lost its normal antioxidant function but also gained a biological function that has an adverse impact on monocyte-endothelial interaction, ie, stimulating monocyte binding. In cells, modification of Trx1 was detectable at 1 μmol/L whereas oxidation of GSH redox state was detectable at 5 μmol/L or higher concentrations. Elevated ROS levels by acrolein and HNE were also observed, indicating dysfunction of antioxidant systems and increased oxidative stress by these reactive aldehydes. Microinjection studies show that Cys-73 modification is sufficient to stimulate monocyte adhesion to endothelial cells. Therefore, chemical modification of Trx1 by reactive conjugated aldehydes may contribute to early events of atherosclerosis.
Footnotes
Address reprint requests to Dr. Dean P. Jones, Department of Medicine, 205 Whitehead Research Center, Emory University, Atlanta, GA 30322. E-mail: dpjones@emory.edu.
Supported by the National Institutes of Health (grants ES011195 and ES09047).
References
- Takagi Y, Gon Y, Todaka T, Nozaki K, Nishiyama A, Sono H, Hashimoto N, Kikuchi H, Yodoi J. Expression of thioredoxin is enhanced in atherosclerotic plaques and during neointima formation in rat arteries. Lab Invest. 1998;78:957–966. [PubMed] [Google Scholar]
- Yamawaki H, Haendeler J, Berk BC. Thioredoxin: a key regulator of cardiovascular homeostasis. Circ Res. 2003;93:1029–1033. doi: 10.1161/01.RES.0000102869.39150.23. [DOI] [PubMed] [Google Scholar]
- Casagrande S, Bonetto V, Fratelli M, Gianazza E, Eberini I, Massignan T, Salmona M, Chang G, Holmgren A, Ghezzi P. Glutathionylation of human thioredoxin: a possible crosstalk between the glutathione and thioredoxin systems. Proc Natl Acad Sci USA. 2002;99:9745–9749. doi: 10.1073/pnas.152168599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haendeler J, Hoffmann J, Tischler V, Berk BC, Zeiher AM, Dimmeler S. Redox regulatory and anti-apoptotic functions of thioredoxin depend on S-nitrosylation at cysteine 69. Nat Cell Biol. 2002;4:743–749. doi: 10.1038/ncb851. [DOI] [PubMed] [Google Scholar]
- Watson WH, Pohl J, Montfort WR, Stuchlik O, Reed MS, Powis G, Jones DP. Redox potential of human thioredoxin 1 and identification of a second dithiol/disulfide motif. J Biol Chem. 2003;278:33408–33415. doi: 10.1074/jbc.M211107200. [DOI] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Ruiz E, Leake DS, Unoki H, Yamamoto M, Mann GE. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res. 2004;94:609–616. doi: 10.1161/01.RES.0000119171.44657.45. [DOI] [PubMed] [Google Scholar]
- Landar A, Zmijewski JW, Dickinson DA, Le Goffe C, Johnson MS, Milne GL, Zanoni G, Vidari G, Morrow JD, Darley-Usmar VM. Interaction of electrophilic lipid oxidation products with mitochondria in endothelial cells and formation of reactive oxygen species. Am J Physiol Heart Circ Physiol. 2006;290:H1777–H1787. doi: 10.1152/ajpheart.01087.2005. [DOI] [PubMed] [Google Scholar]
- Chen ZH, Saito Y, Yoshida Y, Sekine A, Noguchi N, Niki E. 4-Hydroxynonenal induces adaptive response and enhances PC12 cell tolerance primarily through induction of thioredoxin reductase 1 via activation of Nrf2. J Biol Chem. 2005;280:41921–41927. doi: 10.1074/jbc.M508556200. [DOI] [PubMed] [Google Scholar]
- Yang X, Wu X, Choi YE, Kern JC, Kehrer JP. Effect of acrolein and glutathione depleting agents on thioredoxin. Toxicology. 2004;204:209–218. doi: 10.1016/j.tox.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Fang J, Holmgren A. Inhibition of thioredoxin and thioredoxin reductase by 4-hydroxy-2-nonenal in vitro and in vivo. J Am Chem Soc. 2006;128:1879–1885. doi: 10.1021/ja057358l. [DOI] [PubMed] [Google Scholar]
- Kurtz AJ, Lloyd RS. 1,N2-Deoxyguanosine adducts of acrolein, crotonaldehyde, and trans-4-hydroxynonenal cross-link to peptides via Schiff base linkage. J Biol Chem. 2003;278:5970–5976. doi: 10.1074/jbc.M212012200. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Lin D, Yuan Q, Zhu X, Tang X. Protein adducts generated from products of lipid oxidation: focus on HNE and one. Drug Metab Rev. 2006;38:651–675. doi: 10.1080/03602530600959508. [DOI] [PubMed] [Google Scholar]
- Halvey PJ, Watson WH, Hansen JM, Go YM, Samali A, Jones DP. Compartmental oxidation of thiol-disulphide redox couples during epidermal growth factor signaling. Biochem J. 2005;386:215–219. doi: 10.1042/BJ20041829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Jones DP. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation. 2005;111:2973–2980. doi: 10.1161/CIRCULATIONAHA.104.515155. [DOI] [PubMed] [Google Scholar]
- Sasada T, Nakamura H, Ueda S, Sato N, Kitaoka Y, Gon Y, Takabayashi A, Spyrou G, Holmgren A, Yodoi J. Possible involvement of thioredoxin reductase as well as thioredoxin in cellular sensitivity to cis-diamminedichloroplatinum (II). Free Radic Biol Med. 1999;27:504–514. doi: 10.1016/s0891-5849(99)00101-x. [DOI] [PubMed] [Google Scholar]
- Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- Rahman I, Li XY, Donaldson K, Macnee W. Cigarette smoke, glutathione metabolism and epithelial permeability in rat lungs. Biochem Soc Trans. 1995;23:235S. doi: 10.1042/bst023235s. [DOI] [PubMed] [Google Scholar]
- Uchida K. Current status of acrolein as a lipid peroxidation product. Trends Cardiovasc Med. 1999;9:109–113. doi: 10.1016/s1050-1738(99)00016-x. [DOI] [PubMed] [Google Scholar]
- Usatyuk PV, Parinandi NL, Natarajan V. Redox regulation of 4-hydroxy-2-nonenal-mediated endothelial barrier dysfunction by focal adhesion, adherens, and tight junction proteins. J Biol Chem. 2006;281:35554–35566. doi: 10.1074/jbc.M607305200. [DOI] [PubMed] [Google Scholar]
- Ranganna K, Yousefipour Z, Nasif R, Yatsu FM, Milton SG, Hayes BE. Acrolein activates mitogen-activated protein kinase signal transduction pathways in rat vascular smooth muscle cells. Mol Cell Biochem. 2002;240:83–98. doi: 10.1023/a:1020659808981. [DOI] [PubMed] [Google Scholar]
- Haendeler J, Hoffmann J, Zeiher AM, Dimmeler S. Antioxidant effects of statins via S-nitrosylation and activation of thioredoxin in endothelial cells: a novel vasculoprotective function of statins. Circulation. 2004;110:856–861. doi: 10.1161/01.CIR.0000138743.09012.93. [DOI] [PubMed] [Google Scholar]
- Tao L, Gao E, Bryan NS, Qu Y, Liu HR, Hu A, Christopher TA, Lopez BL, Yodoi J, Koch WJ, Feelisch M, Ma XL. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: role of S-nitrosation [corrected]. Proc Natl Acad Sci USA. 2004;101:11471–11476. doi: 10.1073/pnas.0402941101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L, Jiao X, Gao E, Lau WB, Yuan Y, Lopez B, Christopher T, RamachandraRao SP, Williams W, Southan G, Sharma K, Koch W, Ma XL. Nitrative inactivation of thioredoxin-1 and its role in postischemic myocardial apoptosis. Circulation. 2006;114:1395–1402. doi: 10.1161/CIRCULATIONAHA.106.625061. [DOI] [PubMed] [Google Scholar]
- Sakata K, Kashiwagi K, Sharmin S, Ueda S, Irie Y, Murotani N, Igarashi K. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem Biophys Res Commun. 2003;305:143–149. doi: 10.1016/s0006-291x(03)00716-2. [DOI] [PubMed] [Google Scholar]
- Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, Suzuki D, Miyata T, Noguchi N, Niki E, Osawa T. Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci USA. 1998;95:4882–4887. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil L, Siems W, Mazurek B, Gross J, Schroeder P, Voss P, Grune T. Age-associated analysis of oxidative stress parameters in human plasma and erythrocytes. Free Radic Res. 2006;40:495–505. doi: 10.1080/10715760600592962. [DOI] [PubMed] [Google Scholar]
- Zamara E, Novo E, Marra F, Gentilini A, Romanelli RG, Caligiuri A, Robino G, Tamagno E, Aragno M, Danni O, Autelli R, Colombatto S, Dianzani MU, Pinzani M, Parola M. 4-Hydroxynonenal as a selective pro-fibrogenic stimulus for activated human hepatic stellate cells. J Hepatol. 2004;40:60–68. doi: 10.1016/s0168-8278(03)00480-x. [DOI] [PubMed] [Google Scholar]
- Kallis GB, Holmgren A. Differential reactivity of the functional sulfhydryl groups of cysteine-32 and cysteine-35 present in the reduced form of thioredoxin from Escherichia coli. J Biol Chem. 1980;255:10261–10265. [PubMed] [Google Scholar]
- Jordan BF, Runquist M, Raghunand N, Gillies RJ, Tate WR, Powis G, Baker AF. The thioredoxin-1 inhibitor 1-methylpropyl 2-imidazolyl disulfide (PX-12) decreases vascular permeability in tumor xenografts monitored by dynamic contrast enhanced magnetic resonance imaging. Clin Cancer Res. 2005;11:529–536. [PubMed] [Google Scholar]
- Kirkpatrick DL, Kuperus M, Dowdeswell M, Potier N, Donald LJ, Kunkel M, Berggren M, Angulo M, Powis G. Mechanisms of inhibition of the thioredoxin growth factor system by antitumor 2-imidazolyl disulfides. Biochem Pharmacol. 1998;55:987–994. doi: 10.1016/s0006-2952(97)00597-2. [DOI] [PubMed] [Google Scholar]
- Weichsel A, Gasdaska JR, Powis G, Montfort WR. Crystal structures of reduced, oxidized, and mutated human thioredoxins: evidence for a regulatory homodimer. Structure. 1996;4:735–751. doi: 10.1016/s0969-2126(96)00079-2. [DOI] [PubMed] [Google Scholar]
- Smeets A, Evrard C, Landtmeters M, Marchand C, Knoops B, Declercq JP. Crystal structures of oxidized and reduced forms of human mitochondrial thioredoxin 2. Protein Sci. 2005;14:2610–2621. doi: 10.1110/ps.051632905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YS, Misonou Y, Fujiwara N, Takahashi M, Miyamoto Y, Koh YH, Suzuki K, Taniguchi N. Induction of thioredoxin reductase as an adaptive response to acrolein in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2005;327:1058–1065. doi: 10.1016/j.bbrc.2004.12.104. [DOI] [PubMed] [Google Scholar]
- Biswal S, Acquaah-Mensah G, Datta K, Wu X, Kehrer JP. Inhibition of cell proliferation and AP-1 activity by acrolein in human A549 lung adenocarcinoma cells due to thiol imbalance and covalent modifications. Chem Res Toxicol. 2002;15:180–186. doi: 10.1021/tx015552p. [DOI] [PubMed] [Google Scholar]