Abstract
The present study examined the effects of cartilage oligometric matrix protein angiopoietin-1 (COMP-Ang1) on the revascularization of mice skin grafts. Full-thickness skin grafts were autotransferred into BALB/c mice. The donor grafts were soaked in COMP-Ang1 protein (50 μg/ml, n = 10) or in bovine serum albumin (BSA) (50 μg/ml, n = 10) dissolved in 1 ml of sterile, phosphate-buffered saline for 5 minutes before transfer. Revascularization of the grafts was monitored using an intravital microscope on postoperative days 3, 4, and 5. Morphological and immunohistochemical analyses were performed to evaluate platelet-endothelial cell adhesion molecule-1 and survivin expression and apoptotic signal in the transplanted grafts. Grafts soaked in COMP-Ang1 (COMP-Ang1 group) showed significantly increased revascularization compared with grafts soaked in BSA (BSA group) on intravital microscopy and platelet-endothelial cell adhesion molecule-1 staining. The COMP-Ang1 group showed a significant increase of survivin expression in the endothelial cells and a reduction of apoptotic signal in comparison to the BSA group. Therefore, we believe that COMP-Ang1 provides the therapeutic benefit of enhancing the survival of vascular endothelial cells during transplantation of skin graft.
Skin grafting is a common surgical procedure that achieves recuperative wound coverage, and its success rate depends on the level of eventual revascularization. The vascular endothelium of grafted skin has the function such as coagulation, vascular permeability, vascular tonus and remodeling, and is one of the most critical sites for the control of apoptosis in vascular injury and vascular remodeling.1,2 During revascularization the vascular endothelial cells may undergo oxidative stress, after which apoptotic processes may occur because of the inadequate blood supply.3,4 If the apoptosis of graft dermal vascular endothelial cells caused by oxidative stress from the transplantation can be prevented, we believe that vascularization of the graft dermal vascular layer after the inosculation may be facilitated.
Angiopoietin-1 (Ang1) is a specific growth factor that helps to generate a stable and functional vasculature, and it has potential therapeutic applications for inducing angiogenesis, enhancing endothelial cell survival, and preventing vascular leakage.5,6 In several studies7,8 it has been described that Ang1 inhibits the apoptosis of nonproliferating endothelial cells and induces the expression of survivin, an inhibitor of apoptosis protein in endothelial cells. Theoretically, Ang1 could provide the therapeutic benefit to endothelial cells of the dermal layer in grafted skin.
Recently, a soluble, stable Ang1 variant, cartilage oligometric matrix protein (COMP)-Ang1, was developed with a more potent effect than native Ang1.9 Cho and colleagues10,11,12 reported that COMP-Ang1 could protect from radiation-induced apoptosis in microcapillary endothelial cells of intestinal villi and could produce an angiogenic effect with subsequent nonleaky neovessel formation. Kim and colleagues13 recently described how COMP-Ang1 treatment could preserve renal endothelial cells and how it decreased the progression of renal fibrosis in a unilateral ureteral obstruction model. Although there has been a report about the anti-apoptotic effect of COMP-Ang-1 on an in vivo radiation-induced model,12 there have been no reports of how COMP-Ang1 affects vascular endothelial cell survival in an animal transplantation model. In this study, we examined whether COMP-Ang1 affects the survival of vascular endothelial cells in an animal model to evaluate the apoptosis induced by oxidative stress during transplantation.
Materials and Methods
Animals
Twenty-six BALB/c mice were purchased from Central Laboratory Animal Inc., Seoul, Korea, and were bred in our pathogen-free animal facility. Mice 7 to 8 weeks of age were used for this study. All mice lived in a system equipped with day-night light cycling and were provided with standard mouse chow. Experimental procedures were performed under approval from the Animal Care Committee of the Wonkwang University.
Surgery and Treatment
Mice were anesthetized using an intramuscular injection of a combination of anesthetics (80 mg/kg ketamine and 12 mg/kg xylazine) before the surgery. The mice were placed in a prone position on a standard surgical platform. The dorsum of the mice was outlined as a 1.5-cm2 area with a marker after shaving. This area was prepared and draped using the standard sterile surgical technique. Preoperative antibiotics (30 mg/kg cefazolin sodium; Chong-kun-dang Pharm., Seoul, Korea) were administered by intramuscular injection. The skin incision was made along the previously outlined mark and was then trimmed down to a 1.0-cm2 area. The loose connective tissue under the panniculus carnosus was excised. The dermal appearance of the incised skin was photographed using a digital camera (D200; Nikon, Tokyo, Japan). The recipient site was carefully dissected.
All grafts were soaked in 50 μg of COMP-Ang1 recombinant protein (n = 10) or bovine serum albumin (BSA) (n = 10) dissolved in 1 ml of sterile phosphate-buffered saline (PBS) for 5 minutes before transfer. The procured skin grafts were sutured onto the dorsal recipient beds using a nonabsorbable material (6-0 Ethicon; Ethicon, Inc., Somerville, NJ). Multiple, traction-interrupted sutures were placed to secure the grafts to the underlying fascia and to prevent seroma or hematoma formation. The mice were kept warm postoperatively.
Morphometric Analysis of the Skin Grafts
For morphometric analysis of the skin grafts, the dermal and epidermal appearance of the grafted skin was photographed using a digital camera on postoperative day (POD) 5. The areas of epidermal necrosis and epidermal pinkness in the skin grafts were calculated as a percentage of the total graft area using photographic analysis on IMAGEJ software (National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij). We defined grafted skin as healthy when the areas were soft, pink-white, and normal in texture and as necrotic when the areas were black, rigid, and did not bleed when cut.
Intravital Microscopic Observation
For in vivo microscopic observation of the papillary dermal vasculature of the grafted skin, the mice were immobilized in a microscopic stage after intramuscular anesthesia using a combination of anesthetics (80 mg/kg ketamine and 12 mg/kg xylazine). As a control, we observed the vasculature of the papillary dermis of the back skin in three BALB/c mice using intravital microscopy. For evaluation of the revascularization process, we obtained serial images of papillary dermal vessels in three skin-grafted mice on PODs 2, 3, 4, and 5. When the papillary dermal vessels were seen, we obtained serial images at 10-minute intervals on the same grafted site. In all of the skin grafts soaked in COMP-Ang1 or BSA, we obtained serial images of papillary dermal vessels on PODs 3, 4, and 5.
In brief, high-molecular mass (2000 kDa) dextran labeled with fluorescein isothiocyanate (Sigma-Aldrich, St. Louis, MO) was administered into the tail vein (0.1 ml, 25 mg/ml). After injection of fluorescein isothiocyanate-labeled dextran (contrast enhancement by staining of blood plasma), the grafted skin was observed on a fluorescence intravital microscope (BX51WI; Olympus, Tokyo, Japan). The microscopic images were recorded by a charge-coupled device video camera (Roper Scientific, Tucson, AZ). The microvessel density of the graft was calculated as a percentage of the microvasculature area in the nine-image area of the graft. The analysis of microvessel density was performed using MATLAB 7.1 software (The MathWorks, Inc., Natick, MA).
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from grafted skin using TRIzol reagent (Invitrogen, Carlsbad, CA). Concentration of the RNA was detected by the absorbance at 260 nm, and the integrity was verified by electrophoresis on formaldehyde gels. Total RNA was reverse-transcribed into cDNA, which was subjected to PCR for measurement of mRNA. The product of PCR was checked by 2% agarose gel electrophoresis for a single band of the expected size. The abundance of each mRNA was detected and normalized to that of GAPDH mRNA. The sequences of all primers used in this project are SURVIVIN: sense, 5′-GTACCTCAAGAACTACCGCATC-3′; and reverse, 5′-GTCATCGGGTTCCCAGCCTTCC-3′; and for GAPDH: sense, 5′-CATGACCACAGTCCATGCCATCAC-3′; and antisense 5′-TGAGGTCCACCACCCTGTTGCTGT-3′.
Western Blot Analysis
Grafted skin was disrupted by grading and sonication in 1 ml of RIPA buffer at 4°C, and the clarified lysate was obtained by centrifugation. Protein concentrations were measured with a Micro BCA protein assay reagent kit (Sigma-Aldrich). After being boiled in electrophoresis sodium dodecyl sulfate sample buffer, 50 μg of protein were separated on 15% polyacrylamide gel and transferred onto nitrocellulose membranes. Nonspecific binding sites were blocked with blocking buffer (5% fat-free skimmer milk with 0.1% Tween 20) for 30 minutes. The membranes were subsequently washed with TBST buffer and were incubated overnight with the particular primary antibodies diluted in the blocking buffer at 4°C. The membranes were then washed with TBST buffer and were incubated with secondary antibodies. The proteins were detected by enhanced chemiluminescence reagent according to the manufacturer’s instructions. The abundance of each protein was detected and normalized to that of β-actin.
Histological and Immunostaining Examination
On POD 5, the grafted skin was removed and embedded in paraffin [for hematoxylin and eosin (H&E) stain or for immunostain]. Paraffin sections (30-μm thickness) were prepared and incubated for 1 hour at room temperature with blocking solution containing 5% normal goat serum (Jackson ImmunoResearch, West Grove, PA) in PBS with 0.3% Triton X-100 (PBST). The graft sections were incubated for 2 hours at room temperature with 1:300 primary antibodies, anti-platelet-endothelial cell adhesion molecule-1 (PECAM-1) antibody (hamster clone 2H8; Chemicon International, Temecula, CA) for blood vessels and survivin antibody (FL-142, rabbit polyclonal antibody; Santa Cruz Biotechnology, Santa Cruz, CA) for survivin expression. After several washes in PBS, the sections were incubated for 1 hour at room temperature with secondary antibodies, Cy3-conjugated anti-hamster IgG antibody, 1:500, and with fluorescein isothiocyanate-conjugated anti-rabbit IgG antibody, 1:500 (Jackson ImmunoResearch).
To identify the apoptotic endothelial cells, the sections were stained using the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) method according to the manufacturer’s protocol (Chemicon International). The microcapillary endothelial cells and the apoptotic cells in the dermal layer were viewed and photographed using a Zeiss LSM 510 confocal microscope (Carl Zeiss, Goettingen, Germany) equipped with argon and helium-neon lasers.
For histological comparison of both treated groups, the histological status of the grafts was scored according to the degree of inflammatory cell infiltrate, hair follicle number, and epidermal spongiosis. This scoring system was a modified system of that presented in the previous publication.14 All tissue slides were examined blindly by two pathologists. For each specimen, the pathologists were requested to evaluate the tissues for a number of morphological features grouped in the following three categories: inflammatory cell infiltrates, epidermal spongiosis, and dermal hair follicle changes. The inflammatory cells were counted at high-power magnification using a ×400 microscope view. The intensity of the infiltrates was scored qualitatively as 1 when they were not present or when the number of inflammatory cells was less than 5, 2 when the number of inflammatory cells was from 6 to 10, and 3 when the number of inflammatory cells was greater than 11. The predominant type of the inflammatory cells (small lymphocytes, reactive enlarged lymphocytes, eosinophils, or neutrophils) was noted. Changes in the epidermis were studied. Spongiosis was graded as 1 (mild) when it was only seen at high-power magnification, 2 (moderate) at mid-power magnification, and 3 (severe) when it was obvious at low-power magnification. The dermal hair follicles were counted at high-power magnification using a ×400 microscope view. The intensity of the dermal hair follicles was scored qualitatively as 1 when the number of the hair follicles was more than six, 2 when the number of the hair follicles was from two to five, and 3 when the number of the hair follicles was less than one (Table 1).
Table 1.
The Values of Histological Scoring
| Score | Inflammatory cell number/HPF (×400) | Epidermal spongiosis* | Hair follicle number/HPF (×400) |
|---|---|---|---|
| 1 | <5 | Mild | >5 |
| 2 | 5 to 10 | Moderate | 2 to 5 |
| 3 | >10 | Severe | <2 |
Epidermal spongiosis was graded as mild when seen only at high-power magnification (×400), moderate at mid-power magnification (×100), and severe when it was obvious at low-power magnification (×40).
To avoid area variation, histological examinations were performed in nine sections per graft. The histological samples were photographed with an Axioskop2 Plus microscope (Carl Zeiss) equipped with a ProgResC14 color charge-coupled device camera (Jenoptik, Jena, Germany) and monitor. Dermal area densities of the blood vessels, survivin expression, and TUNEL signals were measured by PECAM-1-, surviving-, and TUNEL-immunopositive areas, respectively, at a magnification of ×200 in three regions per section in nine sections per graft.
Statistical Analysis
Data were expressed as mean ± SD. Two independent investigators, who were unaware of the experimental conditions, calculated the data. Statistical significance was tested using an independent t-test using the software program SPSS version 11.5 (SPSS, Chicago, IL). The statistical significance was set at P < 0.05.
Results
COMP-Ang1 Effect on Morphometric Analysis of the Skin Graft
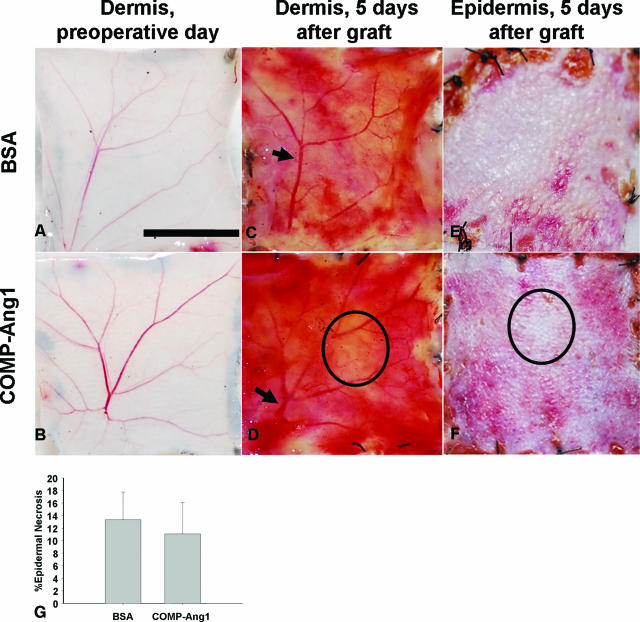
When the epidermal necrosis was compared, the COMP-Ang1 group had a lower percentage of graft epidermal necrosis than the BSA group (Figure 1). There was no significant difference between the two groups (11.08 ± 5.01% versus 13.31 ± 4.44%, P = 0.087).
Figure 1.
Morphometric analysis of skin grafts. Grafts were soaked with 50 μg/ml of BSA (A, C, and E) or with COMP-Ang1 (B, D, and F). A and B: Preoperative dermal appearances. C and D: On the dermis of grafted skins, the recanalization of the reticular dermal vessels (arrow) is shown on POD 5. E and F: Epidermal appearance of grafted skin on POD 5. D and F: By comparing the dermal and epidermal black circle areas of the graft soaked with COMP-Ang1, we observed that a vacant pinkish area of dermis corresponded to a vacant pinkish area of epidermis. G: Bar graph demonstrating the insignificant difference determined by the t-test in mean percentages of epidermal necrosis between both treated groups (P = 0.087). Scale bar = 5 mm.
Skin Graft Findings and COMP-Ang1 Effect on Intravital Microscopy
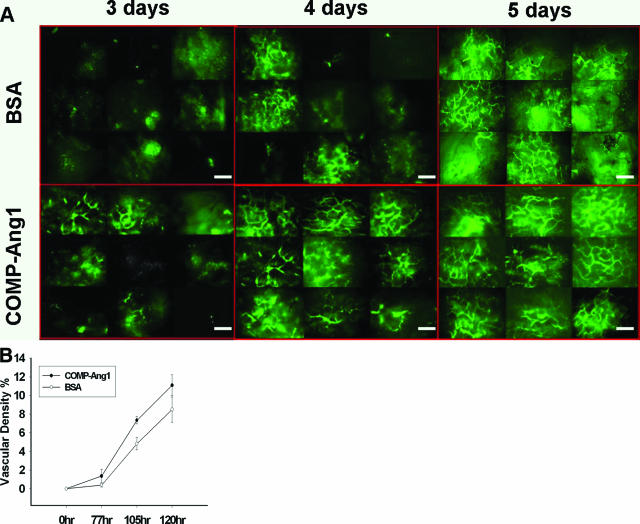
In the control mice, the vascular diameter of the papillary dermis of the back skin was 2 to 4 μm on the immediate preoperative day. After transplantation, the diameter increased and was measured as 10 to 12 μm at POD 5. When we obtained serial images of the papillary dermal vessels to evaluate the revascularization process, the papillary dermal vessels were not seen until 2 days after transplantation. On POD 3, we observed numerous, dot-like vessels that were suggestive of connecting vessels between the papillary dermal vessels and the vessels of the reticular dermis. On the serial images at 10-minute intervals, we found that the recanalization process was occurring through the pre-existing papillary dermal vessels from the connecting vessels (Figure 2). When comparing both treated groups, the COMP-Ang1 group showed more increased microvascular densities in the papillary dermal layer than those of the BSA group on PODs 3, 4, and 5 (Table 2 and Figure 3). The calculated microvascular densities of both treated groups were statistically significantly different (P < 0.05).
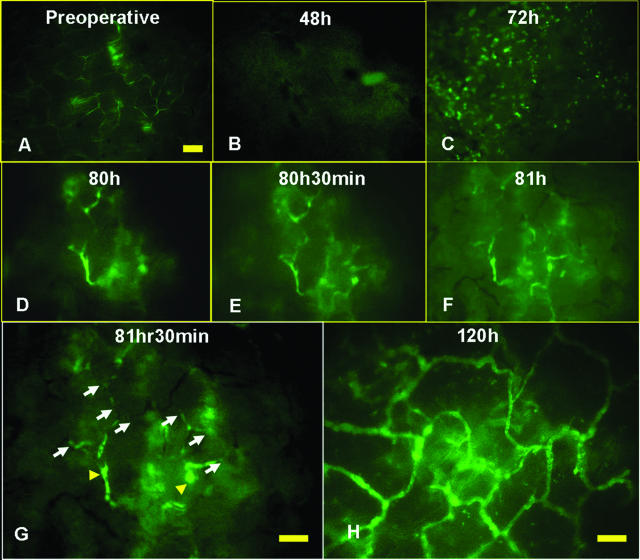
Figure 2.
Observation of skin grafts on intravital microscopy. A: Preoperative skin appearance. Papillary dermal vessels size: 3 to 5 μm. B: At 48 hours postoperatively, the graft area did not show vessels. C: At 72 hours postoperatively, the graft area had a dot-like appearance. D–G: The same skin-graft site was observed serially. Revascularization of the grafted skin increased through recanalization of pre-existing papillary dermal vessels. At 81.5 hours postoperatively, the points entering into the papillary dermal vessel showed up as visible dots with enhanced contrast (arrowheads). The recanalization of small vessels could also be seen to extend through the pre-existing dermal vessels (arrows). H: The graft appearance at 120 hours postoperatively. The papillary dermal vascular network was seen as the well-vascularized area. Papillary dermal vessels size: 10 to 30 μm. Scale bar = 100 μm.
Table 2.
Mean Microvascular Densities of Papillary Dermal Layer on Grafts
| Number of treatment days | Soaked with BSA (n = 10, %) | Soaked with COMP-Ang1 (n = 10, %) | Statistics |
|---|---|---|---|
| 3 | 0.39 ± 0.20 | 1.36 ± 0.73 | P = 0.002 |
| 4 | 4.82 ± 0.67 | 7.38 ± 0.39 | P = 0.000 |
| 5 | 8.50 ± 1.36 | 11.09 ± 1.11 | P = 0.000 |
BSA, bovine serum albumin; COMP-Ang1, cartilage oligometric matrix protein angiopoietin-1.
Figure 3.
Time course observation of skin grafts using intravital microscopy. A: The graft papillary dermal microvasculatures were obtained 3, 4, and 5 days after surgery on one image consisting of nine selected regions. The skin graft was divided into nine subareas, after which the intravital microscopic images of these subareas were combined, three by three images. COMP-Ang1-soaked skin grafts showed faster inosculation after surgery than BSA-soaked skin grafts in POD 3 and greater revascularization in POD 4 and POD 5. B: The microvessel density of the graft was calculated as a percentage of the microvasculature area in the nine-image area of the graft. The dot graph demonstrating the significant difference, as demonstrated by the t-test in the mean percentages of the calculated microvascular densities in the papillary dermal layer in both groups, show a significant statistical difference (P < 0.05), ×100 on intravital microscopy. Scale bars = 300 μm.
Effect of COMP-Ang1 on Survivin Expression in the Grafted Skin on RT-PCR and Western Blot
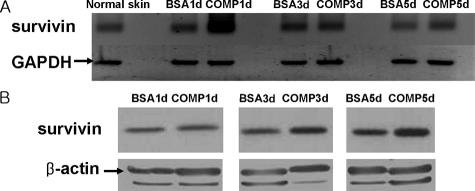
The expression of survivin in the grafted skin was detected by RT-PCR and Western blot. When comparing both treated groups, the mRNA levels of survivin of the COMP-Ang1 group were higher than those of the BSA group at POD 1 and the COMP-Ang1 group showed increased mRNA levels of survivin at POD 1, and the protein levels of survivin of the COMP-Ang1 group were higher than those of the BSA group on PODs 3 and 5 (Figure 4, A and B).
Figure 4.
Survivin expression in the grafted skins detected by RT-PCR (A) and Western blot (B). When comparing both treatment groups, mRNA levels of survivin of the COMP-Ang1 group were higher than that of the BSA group at POD 1 (A), and the protein levels of survivin of the COMP-Ang1 group were higher than that of the BSA group at POD 3 and POD 5 (B).
COMP-Ang1 Effect on Histological and Immunostaining Examinations
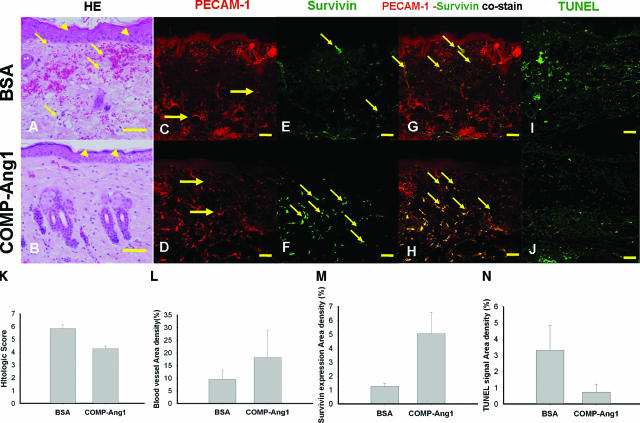
On histological scoring the COMP-Ang1 group had a better status than the BSA group in H&E stain (4.27 ± 0.21 versus 5.84 ± 0.32, P < 0.001) (Table 3 and Figure 5, A, B, and K). The overall blood vessel densities (PECAM-1-immunopositive areas/total dermal areas) in the COMP-Ang1 group were 1.91-fold greater than that in the BSA group (18.16 ± 10.84% versus 9.53 ± 3.68%, P = 0.047) (Figure 5, C, D, and L). The overall survivin expression densities on the endothelial cells (survivin and PECAM-1 co-immunopositive areas/total dermal areas) in the COMP-Ang1 group were 3.96-fold greater than that in the BSA group (5.04 ± 1.55% versus 1.27 ± 0.22%, P < 0.05) (Figure 5, G, H, and M). In addition, the overall apoptosis densities (TUNEL-immunopositive areas/total dermal areas) in the COMP-Ang1 group were 4.46-fold less than that in the BSA group (0.74 ± 0.49% versus 3.30 ± 1.54%, P < 0.05) (Figure 5, I, J, and N).
Table 3.
Histological Score of Both Treated Groups
| Soaked with BSA (n = 10, %) | Soaked with COMP-Ang1 (n = 10, %) | Statistics | |
|---|---|---|---|
| Histological scores | 5.84 ± 0.29 | 4.27 ± 0.21 | P < 0.001 |
Figure 5.
H&E, PECAM-1, survivin, and TUNEL stains at POD 5. A and B: Representative photographs of H&E staining of grafts. The epidermis (arrowheads) of the BSA group showed more spongiosis than that of the COMP-Ang1 group. The inflammatory cells (arrows) were shown at the dermal layer on BSA group. The COMP-Ang1 group showed better results than the BSA group on histological status of PECAM-1 (red) immunostain (C and D) and survivin (green) immunostain (E and F). In PECAM-1 and survivin co-stains (G and H), the BSA group mainly showed co-staining points (arrows) of PECAM-1 and survivin in the reticular dermal vasculature, but the COMP-Ang1 group showed co-staining points (arrows) of PECAM-1 and survivin in the entire dermal vasculature. TUNEL stain (I and J) and the bar graph (N) show that the overall apoptosis densities (arrows) in the COMP-Ang1 group were 4.46-fold less than those observed in the BSA group. K: The bar graph showed the histological scoring of both treatment groups. The COMP-Ang1 group showed better results than the BSA group on histological status. L: The bar graph shows that the overall blood vessel densities in the COMP-Ang1 group were 1.91-fold greater than those observed in the BSA group. M: The bar graph shows that overall survivin expression densities on endothelial cells in the COMP-Ang1 group were 3.96-fold greater than those observed in the BSA group. Scale bars = 2 mm. Original magnifications, ×200 (C–F); ×400 (A and B).
Discussion
In this study, we investigated the biological role of COMP-Ang1 in promoting revascularization by improved survivin expression in the dermal endothelial cells of grafts. The endothelial cells of the graft are influenced by oxidative stress caused by ischemic damage until the skin graft becomes fully revascularized. Oxidative stress activates various signal transduction pathways that include Jun N-terminal kinase (JNK) and transcription factors such as nuclear factor-κB (NF-κB) that induce apoptosis.15,16 In particular, this apoptosis of endothelial cells often induces degeneration of the superficial graft vessels. The degeneration of a dermal vascular plexus that results in a delay of vascularization can contribute to secondary wound infection, thereby also increasing the risk of graft failure.
Several molecules have been used to increase the graft survival through the inhibition of ischemic damage. Recent reports have indicated that vascular endothelial growth factor administration increases the percentage of surviving tissue,17 induces the expression of survivin protein in endothelial cells,18 and protects the apoptosis of sinusoidal endothelial cells in liver tissue.19 Although these positive effects of vascular endothelial growth factor have been described, the exogenous administration of vascular endothelial growth factor for protection against the endothelial damage may often produce leaky, inflamed, and malformed vessels.20,21 To the contrary, Ang1 is a specific growth factor with potential therapeutic applications in inducing angiogenesis, preventing vascular leakage, and inhibiting the apoptosis of endothelial cells to oxidative stress through several pathways that include phosphatidylinositol-3 kinase/AKT activation and the up-regulation of survivin protein.5,6,7,8
The recently developed variant Ang1, COMP-Ang1, is more powerful than the native Ang1 in phosphorylating the Tie2 receptor and for signaling through Akt in primary cultured endothelial cells.9 In several studies with COMP-Ang1, the protein revealed a protective effect of apoptosis, enhancement of angiogenesis, and amelioration of fibrosis in animal models.9,10,11,12,13 Until now, there have been no reports regarding the effect of COMP-Ang1 on endothelial cell survival in a transplantation model.
In a previous publication,22 a concentration of 100 to 200 ng/ml COMP-Ang1 was used to stimulate human umbilical vein endothelial cells in vitro, and maximal receptor phosphorylation was seen at ∼600 ng/ml. To the contrary, in a recent study,10 concentrations of 100 μg/50 μl COMP-Ang1 were used in an in vivo tail wound model. The result indicated that topical COMP-Ang1 promotes wound healing by enhancing angiogenesis and blood flow in a tail-skin wound. We believe that the required concentrations in vivo will usually be higher than in an in vitro assay. Therefore, we tried an experimental study with various concentrations (1, 25, 50, and 100 μg/ml) of COMP-Ang1. When we used COMP-Ang1 with concentrations of 50 and 100 μg/ml, we obtained good study results (statistical data not shown). Therefore, we used a concentration of 50 μg/ml in this study.
To observe COMP-Ang1 effects, revascularization of the grafts was monitored with an intravital microscope during PODs 3, 4, and 5. To date, many researchers have used various ex vivo methods, eg, SEM and immunostain, to study vascularization.23,24 Although the penetration depth of intravital microscope is limited within a few millimeters, we were able to obtain serial time images of the papillary dermal layer of a skin graft in an in vivo state using IVM. We found that the recanalization process was occurring through the pre-existing papillary dermal vessels from the connecting vessels. In our study, the COMP-Ang1 group showed more increased microvascular densities in the papillary dermal layer than the BSA group on IVM observation. We used PECAM-1 immunostaining to support data of IVM. In addition, apoptosis-related factors also provided the supporting data to understand the reasons of dermal vessel increment.
In a previous publication, Cho and colleagues13 described that COMP-Ang1 treatment decreased monocyte/macrophage infiltration. In H&E staining of our study, the COMP-Ang1 group also showed reduction of inflammatory cell infiltration and better results than the BSA group on histological grading. A previous study also demonstrated the increment of survivin expression by Ang1.7 In our study, the COMP-Ang1 group showed the increment of survivin expression in the papillary and reticular dermal vasculature layers. To the contrary, the BSA group showed a survivin expression limited to the reticular dermal vasculature layer. A similar result was obtained by TUNEL staining for apoptosis in the COMP-Ang1 group. In RT-PCR and Western blot assay to detect the expression level of survivin mRNA and protein, the COMP-Ang1 group showed increased mRNA levels of survivin at POD 1 and increased protein levels of survivin on PODs 3 and 5. These results have supported the increased expression of survivin in the COMP-Ang1-treated grafted skin. Based on these results, we believe that COMP-Ang1 can facilitate inosculation-mediated recanalization by an inhibition effect to the apoptosis of dermal endothelial cells.
In general, despite the clear difference in vessel density and the speed of revascularization between the COMP-Ang1-treated group and the BSA group in immunohistochemical staining, the differences in terms of transplant necrosis are missed. We believe this occurs because the success rate of skin grafting is very high because of the small size and thin depth of skin grafts in general. Accordingly, our results provide supporting evidence that COMP-Ang1 would be helpful in enhancing revascularization of dermal vessels in the grafted skin model and have a potential for the therapeutic applications in other ischemic models.
In summary, we demonstrated that soaking grafts in COMP-Ang1 solutions before surgery could promote revascularization by increment of the survivin expression in the endothelial cells of dermal vessels in the grafted skin model. Therefore, we believe that COMP-Ang1 provides the therapeutic benefit of enhancing the survival of vascular endothelial cells during transplantation of skin graft.
Acknowledgments
We thank Bonnie Hami, M.A., for her comments and editing; and H.T. Chung, B.S. Lee, K.M. Kim, and E.S. Oh for their comments.
Footnotes
Address reprint requests to Kwon-Ha Yoon, M.D, Institute for Radiological Imaging Science and Department of Radiology, 344-2 Sinyong-dong, Wonkwang University School of Medicine, Iksan, Jeonbuk 570-749, South Korea. E-mail: khy1646@wonkwang.ac.kr.
Supported by the government of Korea Science and Engineering Foundation (KOSEF) funded by the Korea government (MOST) (no. M2-0415-01-0001).
References
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Karsan A, Harlan JM. Modulation of endothelial cell apoptosis: mechanisms and pathophysiological roles. J Atheroscler Thromb. 1996;3:75–80. doi: 10.5551/jat1994.3.75. [DOI] [PubMed] [Google Scholar]
- Wang N, Verna L, Hardy S, Zhu Y, Ma KS, Birrer MJ, Stemerman MB. c-Jun triggers apoptosis in human vascular endothelial cells. Circ Res. 1999;85:387–393. doi: 10.1161/01.res.85.5.387. [DOI] [PubMed] [Google Scholar]
- Sudoh N, Toba K, Akishita M, Ako J, Hashimoto M, Iijima K, Kim S, Liang YQ, Ohike Y, Watanabe T, Yamazaki I, Yoshizumi M, Eto M, Ouchi Y. Estrogen prevents oxidative stress-induced endothelial cell apoptosis in rats. Circulation. 2001;103:724–729. doi: 10.1161/01.cir.103.5.724. [DOI] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O’Connor DS, Li F, Altieri DC, Sessa WC. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- Kwak HJ, So JN, Lee SJ, Kim I, Koh GY. Angiopoietin-1 is an apoptosis survival factor for endothelial cells. FEBS Lett. 1999;448:249–253. doi: 10.1016/s0014-5793(99)00378-6. [DOI] [PubMed] [Google Scholar]
- Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, Yasunaga K, Kim KT, Kim I, Choi HH, Kim W, Kim SH, Park SK, Lee GM, Koh GY. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci USA. 2004;101:5547–5552. doi: 10.1073/pnas.0307574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho CH, Sung HK, Kim KT, Cheon HG, Oh GT, Hong HJ, Yoo OJ, Koh GY. COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proc Natl Acad Sci USA. 2006;103:4946–4951. doi: 10.1073/pnas.0506352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho CH, Kim KE, Byun J, Jang HS, Kim DK, Baluk P, Baffert F, Lee GM, Mochizuki N, Kim J, Jeon BH, McDonald DM, Koh GY. Long-term and sustained COMP-Ang1 induces long-lasting vascular enlargement and enhanced blood flow. Circ Res. 2005;97:86–94. doi: 10.1161/01.RES.0000174093.64855.a6. [DOI] [PubMed] [Google Scholar]
- Cho CH, Kammerer RA, Lee HJ, Yasunaga K, Kim KT, Choi HH, Kim W, Kim SH, Park SK, Lee GM, Koh GY. Designed angiopoietin-1 variant, COMP-Ang1, protects against radiation-induced endothelial cell apoptosis. Proc Natl Acad Sci USA. 2004;101:5553–5558. doi: 10.1073/pnas.0307575101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Moon SO, Lee SY, Jang KY, Cho CH, Koh GY, Choi KS, Yoon KH, Sung MJ, Kim DH, Lee S, Kang KP, Park SK. COMP-angiopoietin-1 ameliorates renal fibrosis in a unilateral ureteral obstruction model. J Am Soc Nephrol. 2006;17:2474–2483. doi: 10.1681/ASN.2006020109. [DOI] [PubMed] [Google Scholar]
- Bejarano PA, Levi D, Nassiri M, Vincek V, Garcia M, Weppler D, Selvaggi G, Kato T, Tzakis A. The pathology of full-thickness cadaver skin transplant for large abdominal defects: a proposed grading system for skin allograft acute rejection. Am J Surg Pathol. 2004;28:670–675. doi: 10.1097/00000478-200405000-00016. [DOI] [PubMed] [Google Scholar]
- Minamino T, Yujiri T, Papst PJ, Chan ED, Johnson GL, Terada N. MEKK1 suppresses oxidative stress-induced apoptosis of embryonic stem cell-derived cardiac myocytes. Proc Natl Acad Sci USA. 1999;96:15127–15132. doi: 10.1073/pnas.96.26.15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont A, Hehner SP, Hofmann TG, Ueffing M, Droge W, Schmitz ML. Hydrogen peroxide-induced apoptosis is CD95-independent, requires the release of mitochondria-derived reactive oxygen species and the activation of NF-kappaB. Oncogene. 1999;18:747–757. doi: 10.1038/sj.onc.1202325. [DOI] [PubMed] [Google Scholar]
- Kryger Z, Zhang F, Dogan T, Cheng C, Lineaweaver WC, Buncke HJ. The effects of VEGF on survival of a random flap in the rat: examination of various routes of administration. Br J Plast Surg. 2000;53:234–239. doi: 10.1054/bjps.1999.3315. [DOI] [PubMed] [Google Scholar]
- Scalise A, Tucci MG, Lucarini G, Giantomassi F, Orlando F, Pierangeli M, Pugnaloni A, Bertani A, Ricotti G, Biagini G. Local rh-VEGF administration enhances skin flap survival more than other types of rh-VEGF administration: a clinical, morphological and immunohistochemical study. Exp Dermatol. 2004;13:682–690. doi: 10.1111/j.0906-6705.2004.00220.x. [DOI] [PubMed] [Google Scholar]
- Moriga T, Arii S, Takeda Y, Furuyama H, Mizumoto M, Mori A, Hanaki K, Nakamura T, Fujioka M, Imamura M. Protection by vascular endothelial growth factor against sinusoidal endothelial damage and apoptosis induced by cold preservation. Transplantation. 2000;69:141–147. doi: 10.1097/00007890-200001150-00024. [DOI] [PubMed] [Google Scholar]
- Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, Bunting S, Steinmetz HG, Gurtner GC. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–1947. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeano M, Deodato B, Altavilla D, Squadrito G, Seminara P, Marini H, Stagno DF, Colonna M, Calo M, Lo CP, Torre V, Giacca M, Venuti FS, Squadrito F. Effect of recombinant adeno-associated virus vector-mediated vascular endothelial growth factor gene transfer on wound healing after burn injury. Crit Care Med. 2003;31:1017–1025. doi: 10.1097/01.CCM.0000059435.88283.C2. [DOI] [PubMed] [Google Scholar]
- Saharinen P, Kerkela K, Ekman N, Marron M, Brindle N, Lee GM, Augustin H, Koh GY, Alitalo K. Multiple angiopoietin recombinant proteins activate the Tie1 receptor tyrosine kinase and promote its interaction with Tie2. J Cell Biol. 2005;169:239–243. doi: 10.1083/jcb.200411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T. Revascularisation of free full thickness skin grafts in rabbits: a scanning electron microscope study of microvascular casts. Br J Plast Surg. 1986;39:183–189. doi: 10.1016/0007-1226(86)90080-9. [DOI] [PubMed] [Google Scholar]
- Capla JM, Ceradini DJ, Tepper OM, Callaghan MJ, Bhatt KA, Galiano RD, Levine JP, Gurtner GC. Skin graft vascularization involves precisely regulated regression and replacement of endothelial cells through both angiogenesis and vasculogenesis. Plast Reconstr Surg. 2006;117:836–844. doi: 10.1097/01.prs.0000201459.91559.7f. [DOI] [PubMed] [Google Scholar]