Abstract
The recently identified endogenous peptide apelin and its specific apelin receptor (APJ) are currently being considered as potential regulators in vascular tissue. Previously, we reported apelin mediates phosphorylation of myosin light chain and elicits vasoconstriction in vascular smooth muscle. In this study, physiological roles of the apelin-APJ system were investigated on atherosclerosis. In APJ and apolipoprotein E double-knockout (APJ−/−ApoE−/−) mice fed a high-cholesterol diet, atherosclerotic lesions were dramatically reduced when compared with APJ+/+ ApoE−/− mice, in the absence of an effect of cholesterol levels. Immunohistochemical detection of smooth muscle cells, using a smooth muscle α-actin antibody, showed greatly reduced staining for these cells in lesions of APJ−/−ApoE−/− mice fed a high-cholesterol diet. Vascular production of superoxide radicals and the expression of nicotinamide-adenine dinucleotide phosphate oxidase subunits were decreased in APJ−/−ApoE−/− mice compared with APJ+/+ApoE−/− mice fed a standard normal diet. In vascular smooth muscle cells, apelin induced nicotinamide-adenine dinucleotide phosphate oxidase subunit expression. Apelin also induced vascular smooth muscle cell proliferation, which was inhibited by superoxide dismutase or diphenylene iodonium. The apelin-APJ system is a mediator of oxidative stress in vascular tissue, and thus we propose it to be a critical factor in atherogenesis under high-cholesterol dietary conditions. APJ deficiency is preventative against oxidative stress-linked atherosclerosis.
Apelin receptor (APJ) is a G-protein-coupled receptor with seven transmembrane domains, and its endogenous ligand, apelin has been recently identified.1,2 The structures of APJ and apelin are highly conserved among species, and both are highly expressed in the cardiovascular system.3,4 In the vascular system, APJ and apelin are known to be expressed in endothelium and vascular smooth muscle cells (VSMCs). Histological studies in rat show that the VSMCs of the medial layer of the aorta and pulmonary artery display intense staining for APJ-like immunoreactivity.4
The vascular actions of apelin-APJ system may be complex. Under physiological conditions, the apelin-APJ system shows transient hypotension. The baseline blood pressure of APJ and angiotensin-type 1 receptor double-knockout mice was significantly elevated compared with that of angiotensin-type 1 receptor knockout mice,5 although APJ knockout (APJ−/−) mice did not show any significant changes in cardiovascular parameters. In spontaneously hypertensive rats, APJ and apelin expression in both heart and aorta were markedly depressed compared with Wistar-Kyoto rats.6 In aortae from type 2 diabetic db/db mice, APJ and apelin expression were also less than in db/+ mice.7 In clinical studies, APJ is the most significantly up-regulated gene after placement of a left ventricular assist device.8 These data suggest that the apelin-APJ system is important in the regulation of cardiovascular function.
Apelin in humans has a vasoconstrictor role in endothelium-denuded vessels.3 In a previous study on murine vascular smooth muscle, we have demonstrated that apelin stimulates myosin light chain phosphorylation via APJ, which is a potential mediator of apelin-mediated vasoconstriction.9 At the present time, the effects of apelin on vascular tissues remain to be clarified. In addition, the role of the apelin-APJ system in atherosclerosis has not yet been examined. In the present study, we studied the effect of APJ deficiency on atherogenesis. We also investigated whether the apelin-APJ system mediates oxidative stress in VSMCs.
Materials and Methods
Animal Studies
Apolipoprotein E knockout (ApoE−/−) mice (The Jackson Laboratory, Bar Harbor, ME) were backcrossed more than 10 generations into the C57BL/J strain. APJ−/− mice were generated by gene targeting as described previously.5,9 APJ−/− mice were back-crossed more than seven generations into the ICR strain. APJ+/+ApoE+/+, APJ−/−ApoE+/+, APJ+/+ApoE−/−, and APJ−/−ApoE−/− mice on the F2 background of an F1 (C57BL/J × ICR) heterozygote intercross were analyzed to gain the equivalent effect of other gene backgrounds with the exception of the APJ and ApoE genes. Animals were housed under a 12-hour day/night cycle at a temperature of 25°C. Tap water was provided ad libitum. Five-week-old female mice were maintained on either a standard normal diet (ND) (0.075% cholesterol) or a high-cholesterol diet (HCD) (1% cholesterol; Oriental Yeast, Tokyo, Japan) for 15 weeks. Plasma, aorta, and other tissues were collected at the time of sacrifice. Plasma triglycerides, total cholesterol, HDL-C, LDL-C, aspartate aminotransferase, alanine aminotransferase, and free fatty acids were quantified. Serum apelin-12 (Phoenix Pharmaceuticals, Belmont, CA) or monocyte chemoattractant protein-1 (MCP-1) (R&D Systems, Minneapolis, MN) were measured by enzyme-linked immunosorbent assay. Superoxide release in intact aortic segments was determined by L-012 chemiluminescence (Wako Chemicals, Saitama, Japan) as described previously.10 Aortae were carefully excised and placed in ice-cold modified Krebs’ solution (118 mmol/L NaCl, 4.7 mmol/L KCl, 1.18 mmol/L KH2PO4, 1.17 mmol/L MgSO4, 2.5 mmol/L CaCl2, 5.0 mmol/L HEPES, and 11.1 mmol/L d-glucose, pH 7.4). Fat and connective tissue were then removed from the preparation, and aortae were cut into 2-mm segments. Aortae were transferred into scintillation vials containing modified Krebs’ solution and were incubated for 5 minutes with 100 μmol/L L-012. Chemiluminescence was then assessed for 15 minutes in a scintillation counter at 1-minute intervals. The vessel segments were then dried, and dry weight was determined. Superoxide release was expressed as relative chemiluminescence per mg of aortic tissue. Experiments were conducted under the guidelines for animal experiments set by the Animal Experiment Committee of Yokohama City University School of Medicine.
Histology and Immunohistochemistry
Quantification of atherosclerotic lesions was performed as previously described.11,12 Serial sections were cut through the aorta at the origins of the aortic valve leaflets, and every fifth 4-μm section throughout the aortic sinus was stained with hematoxylin and eosin (H&E) or Masson trichrome. Mean lesion area was quantified from 12 digitally captured sections per mouse. For en face aorta analysis, mice were sacrificed and the aortic tree was perfused with phosphate-buffered saline and then fixed with formalin. The aorta was isolated by severing minor branching arteries and dissecting the adventitia. Fatty tissue deposited outside the aorta was carefully and completely removed. The specimen was removed en bloc from the root to the iliac bifurcation and then opened by a longitudinal cut along the ventral surface. After 24 hours of fixation in formalin, lipids were stained with Sudan IV (Wako Chemicals). The percentage of aortic area stained red was determined using image analysis software (MacSCOPE; Mitani Co., Fukui, Japan).11 Immunohistochemistry was performed with antibodies to F4/80 (1:200; Serotec, Oxford, UK), smooth muscle α-actin (1:600; Abcam, Cambridge, UK), and nitrotyrosine (1:50; Upstate Technology, Lake Placid, NY). Staining was visualized with the avidin-biotin immunoperoxidase reaction using diaminobenzidine (Nichirei, Tokyo, Japan).
Real-Time Quantitative RT-PCR
Real-time quantitative RT-PCR was performed to determine levels of rac1, Id3, and p47phox mRNA expression. Total RNA was isolated by the acid guanidinium thiocyanate-phenol-chloroform extraction method.13 RT reactions were performed using SuperScript III reverse transcriptase (Invitrogen, Burlington, ON, Canada). Quantitative PCR analysis was conducted by incubating RT product with TaqMan Universal PCR master mix and specific primer-probe sets (Applied Biosystems, Foster City, CA); the PCR reaction was run on an ABI Prism 7700 detection system using standard conditions. Each sample was added in triplicate. RNA quantity was expressed relative to an 18S endogenous control. Relative expression levels were expressed by the comparative threshold cycle (CT) method as described previously.14,15,16
Cell Culture
VSMCs from the thoracic aorta of 8-week-old female mice were prepared by the explant method and cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum as described previously.9,13,17 Subconfluent cells were serum-deprived for 24 hours and then stimulated with [Pyr1]-apelin-13 (Peptide Institute, Osaka, Japan) for the indicated time. For cell proliferation assays, VSMCs (6 × 103 cells/well) were plated on 96-well plates and were made quiescent by incubating for 72 hours with 0.1% fetal bovine serum. The cells were pretreated with various concentrations of apelin or angiotensin II (Sigma-Aldrich, St. Louis, MO) for 48 hours with or without superoxide dismutase (SOD) (100 U/ml) (Sigma-Aldrich) or diphenylene iodonium (10 μmol/L) (Sigma-Aldrich). For VSMC proliferation studies, 5-bromo-2′-deoxyuridine (BrdU) (Roche, Indianapolis, IN) or WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt] (TetraColor One; Seikagaku Co., Tokyo, Japan)15,18 incorporation experiments were performed using a microplate reader (Nalge Nunc International, Rochester, NY). BrdU or WST-8 incorporation was plotted as fold changes in untreated control values.
Statistical Analysis
Data are expressed as mean ± SEM. Statistical significance was determined by analysis of variance with P values <0.05 being deemed statistically significant.
Results
Serum Parameters in APJ and Apolipoprotein E Double-Knockout Mice
We directly tested the impact of APJ on atherogenesis. To achieve this, we generated double-knockout mice for APJ and apolipoprotein E by crossing APJ−/− and ApoE−/− mice. ApoE−/− mice are a well-established model used to study the pathogenesis of atherosclerosis; this model develops spontaneous atherosclerotic lesions at 20 weeks when on a ND.19 Typically, atherosclerotic lesion formation starts in the aortic sinus. Concomitantly, endothelium-dependent vasodilation is profoundly impaired because of increased vascular oxidative stress.20 In APJ+/+ApoE+/+, APJ−/−ApoE+/+, APJ+/+ApoE−/−, and APJ−/−ApoE−/− mice fed a ND, body weight, water intake, food intake, and blood pressure were not significantly different (data not shown). As shown in Table 1, total cholesterol and LDL cholesterol levels were markedly elevated in APJ+/+ApoE−/− mice compared with APJ+/+ApoE+/+ mice fed a ND. APJ−/−ApoE−/− mice showed modest elevations of total cholesterol and LDL cholesterol above levels in APJ+/+ApoE−/− mice, but these differences were not statistically significant. Total cholesterol and LDL cholesterol levels were markedly elevated in all mice fed a HCD; absence of the APJ gene did not affect the extent of the elevation. HDL cholesterol levels were markedly reduced in apolipoprotein E gene-knockout mice fed a ND or HCD. In APJ−/−ApoE+/+ mice fed a HCD, HDL cholesterol levels were elevated over those in APJ+/+ApoE+/+ mice. In ApoE−/− mice fed a HCD, APJ gene-knockout had a tendency toward elevated HDL cholesterol levels, which did not reach statistical significance.
Table 1.
Serum Parameters
| Parameters | Diet | APJ+/+ApoE+/+ | APJ−/−ApoE+/+ | APJ+/+ApoE−/− | APJ−/−ApoE−/− |
|---|---|---|---|---|---|
| TC (mg/dl) | ND | 80 ± 13 | 82 ± 9 | 387 ± 28* | 479 ± 81† |
| HCD | 136 ± 8‡ | 148 ± 6‡ | 834 ± 91*‡ | 868 ± 65†‡ | |
| HDL-C (mg/dl) | ND | 48 ± 5 | 49 ± 5 | 12 ± 1* | 13 ± 2† |
| HCD | 58 ± 3 | 68 ± 4* | 8 ± 2* | 9 ± 1† | |
| LDL-C (mg/dl) | ND | 5 ± 1 | 4 ± 1 | 56 ± 4* | 72 ± 11† |
| HCD | 16 ± 1‡ | 20 ± 2‡ | 197 ± 19*‡ | 199 ± 14†‡ | |
| TG (mg/dl) | ND | 70 ± 5 | 67 ± 10 | 49 ± 6 | 41 ± 12 |
| HCD | 37 ± 6‡ | 23 ± 3‡ | 40 ± 8 | 23 ± 5 | |
| FFA (μEQ/L) | ND | 2521 ± 296 | 2461 ± 301 | 2113 ± 98 | 2292 ± 611 |
| HCD | 2029 ± 110 | 1936 ± 12 | 2411 ± 190 | 2059 ± 63 |
TC, total cholesterol; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; TG, triglyceride; FFA, free fatty acids; ND, standard normal diet; HCD, high-cholesterol diet. Values are expressed as mean ± SEM from 7 to 13 experiments.
P < 0.05 versus APJ+/+ApoE+/+.
P < 0.05 versus APJ−/−ApoE+/+.
P < 0.05 versus ND.
Atherosclerotic Lesions in APJ and Apolipoprotein E Double-Knockout Mice Fed a HCD
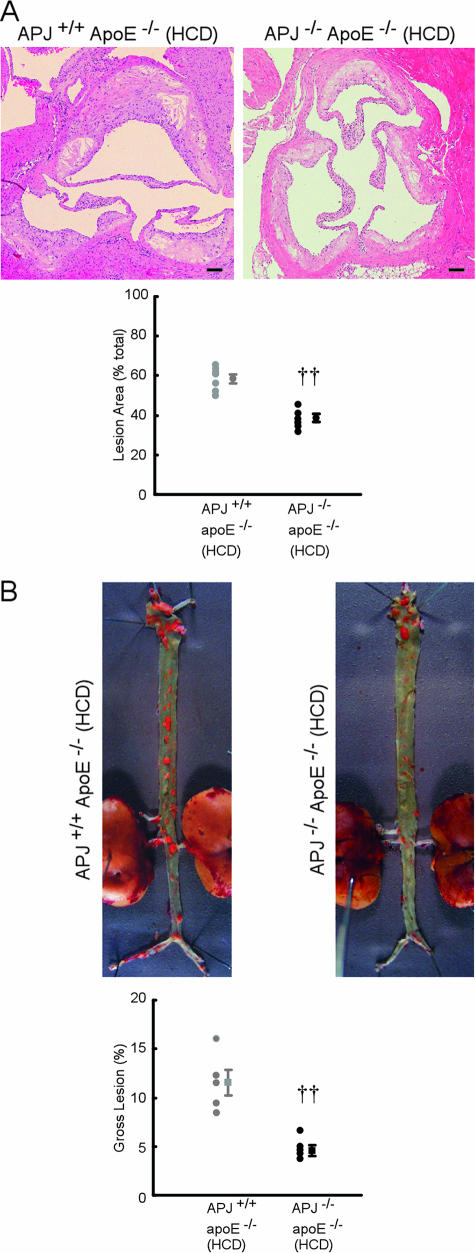
Analysis of aortic sinus atherosclerotic lesion formation in APJ−/−ApoE−/− mice fed a HCD revealed marked reductions in lesion size (Figure 1A). APJ−/−ApoE−/− mice showed a 34% decrease in lesion formation compared with their APJ+/+ApoE−/− counterparts (P < 0.01). Reduced evidence of atherosclerosis was also found along the length of the aorta in the absence of APJ, when analyzed en face. As shown in Figure 1B, there was markedly less Sudan IV staining of atherosclerotic lesions in APJ−/−ApoE−/− mice after a 15-week HCD. APJ−/− ApoE−/− mice showed a 61% decrease in atherosclerotic area compared with their APJ+/+ApoE−/− counterparts (P < 0.01). Furthermore, no overlap in lesion size was seen. Figure 1 and Table 1 indicates that APJ plays a pivotal role in early lesion development during atherogenesis, without affecting serum lipid profiles.
Figure 1.
Effect of APJ deletion in atherosclerotic lesions. A: Representative photographs of aortic sinus from APJ+/+ApoE−/− and APJ−/−ApoE−/− mice fed a HCD (15-week treatment). Sections were stained with H&E. Scatter plots showing mean lesion sizes (percent total). Each symbol represents the measurements from a single mouse. Seven mice in each group were analyzed, and results are expressed as means ± SEM. ††P < 0.01 versus APJ+/+ApoE−/− mice. B: Representative photographs from en face analysis of aortae from APJ+/+ApoE−/− and APJ−/−ApoE−/− mice fed a HCD (15-week treatment). Scatter plots showing mean lesion sizes (percent total). Each symbol represents the measurements from a single mouse. Seven mice in each group were analyzed and results are expressed as means ± SEM. ††P < 0.01 versus APJ+/+ApoE−/− mice. Scale bars = 100 μm. Original magnifications, ×40 (A).
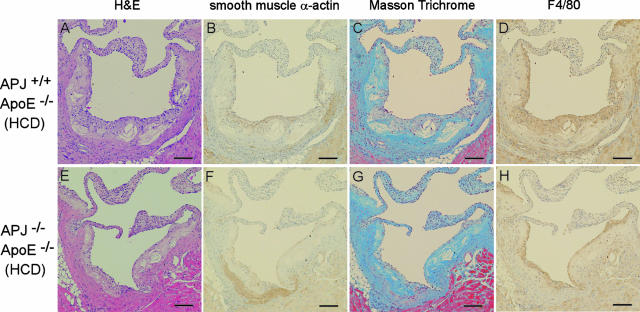
To facilitate additional comparisons of the lesions formed in APJ+/+ApoE−/− and APJ−/−ApoE−/− mice, we selected similar-sized atherosclerotic lesions from both groups and used them for histochemical and immunohistochemical characterization (Figure 2). Similar-sized lesion formation in the aortic sinus plaques of APJ+/+ApoE−/− and APJ−/−ApoE−/− mice was shown by H&E staining (Figure 2, A and E). Immunohistochemical detection of smooth muscle cells, using the smooth muscle α-actin antibody, showed greatly reduced staining for these cells in APJ−/−ApoE−/− lesions (Figure 2, B and F). Smooth muscle α-actin-positive cells were sparsely distributed in the sections from APJ−/−ApoE−/− mice. Masson trichrome staining and immunohistochemical detection for F4/80 revealed similar levels of collagen and macrophages/macrophage foam cells, independent of APJ expression (Figure 2, C, D, G, and H).
Figure 2.
Representative photographs of similar-sized aortic sinuses from APJ+/+ApoE−/− (top) and APJ+/+ApoE+/+ (bottom) mice fed a 15-week HCD. Sections were stained with H&E (A and E), antibody to smooth muscle α-actin (B and F), Masson trichrome (C and G), and antibody to F4/80 (D and H). Scale bars = 100 μm. Original magnifications, ×40.
Vascular Production of Superoxide Radicals and the Expression of Nicotinamide-Adenine Dinucleotide Phosphate (NADPH) Oxidase Subunits in APJ−/−ApoE−/− Mice and APJ+/+ ApoE−/− Mice
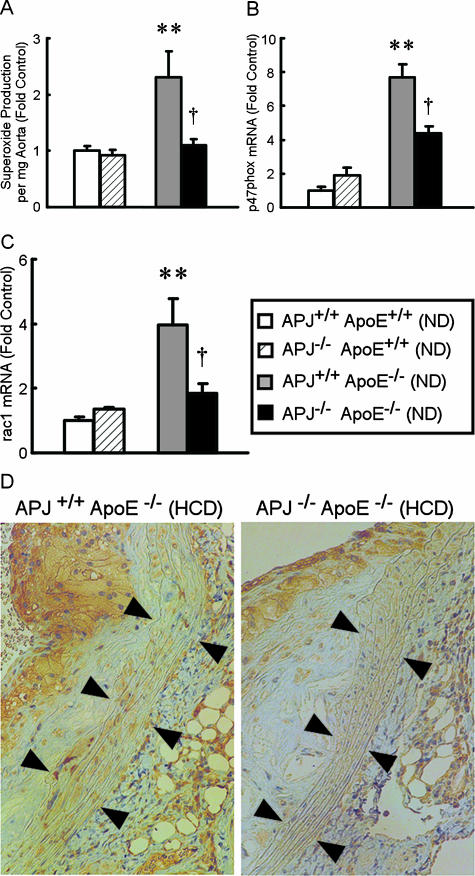
To examine further the mechanisms of reduced atherosclerosis in APJ−/−ApoE−/− mice, superoxide production was measured using aortae from 20-week-old mice fed a ND. The major source of superoxide anion in cardiovascular cells is NADH/NADPH oxidase, which transfers electrons from NADH or NADPH to molecular oxygen, producing superoxide anion.21 Superoxide anion is converted by SOD to H2O2, which is scavenged by catalase or peroxidases. Figure 3A shows that vascular superoxide was increased 2.3-fold in APJ+/+ApoE−/− mice fed a ND compared with APJ+/+ApoE+/+ mice (P < 0.01). This increase was virtually abolished in mice lacking the APJ gene (P < 0.05). Expression levels of p47phox and rac-1 mRNA, both of which are necessary for activation of NADPH oxidase,21 were increased 7.7-fold and 4-fold, respectively, in APJ+/+ApoE−/− mice fed a ND compared with APJ+/+ApoE+/+ mice (Figure 3, B and C). These expression levels were reduced significantly in APJ−/−ApoE−/− mice fed a ND compared with APJ+/+ApoE−/− mice. Lower levels of nitrotyrosine staining were detected in aortae of APJ−/−ApoE−/− mice compared with that seen in APJ+/+ApoE−/− mice (Figure 3D) treated with a HCD. Nitrotyrosine staining reflects reaction of superoxide with nitric oxide to yield peroxynitrite, which covalently modifies the amino acid tyrosine.
Figure 3.
Involvement of oxidative stress in the vascular phenotype. A: Vascular superoxide production. Aortae of 20-week-old mice fed a ND were excised, and vascular superoxide production in intact aortic segments was quantified by L-012 chemiluminescence assays. Superoxide release is expressed as relative chemiluminescence per mg of aortic tissue. B and C: p47phox (B) and rac-1 (C) mRNA expression in aorta fed a ND (20-week-old). Expression levels in APJ+/+ApoE+/+ mice were set at 1.0. Results are expressed as means ± SEM from six to nine experiments in each group. **P < 0.01 versus APJ+/+ApoE+/+ mice. †P < 0.05 versus APJ+/+ApoE−/− mice. D: Nitrotyrosine immunohistochemistry. Representative nitrotyrosine staining in APJ+/+ApoE−/− and APJ+/+ApoE+/+ mice fed a 15-week HCD. Vascular smooth muscle regions are indicated by arrows. Similar results were seen in six animals per condition.
Effect of Apelin on NADPH Oxidase Subunit Expression and Proliferation in VSMCs
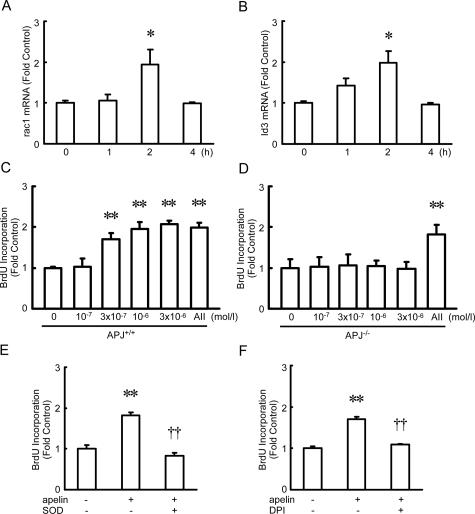
Cultured murine VSMCs were incubated with apelin, as a model system to evaluate its effect on vascular function in health and disease. Apelin stimulated rac-1 and Id3 mRNA expression, peaking at 2 hours (Figure 4, A and B). Apelin also stimulated Nox1, Nox2, Nox4, and p22phox mRNA expression, by 1.5, 4.2, 1.6, and 1.5 times, respectively, which were also NADPH oxidase subunits, peaking at 1 hour. Because Id3 is a requirement for reactive oxygen species (ROS)-induced cell proliferation,22 a cell proliferation assay was performed. When VSMCs were incubated with apelin for 48 hours, BrdU incorporation into VSMCs was significantly increased in a concentration-dependent manner (Figure 4C), whereas the same concentration of the peptide had no effect on VSMCs from APJ−/− mice (Figure 4D). Similar results were obtained when WST-8 was used instead of BrdU (data not shown). Apelin-induced cell proliferation was not observed on treatment with SOD, which catalyzes the dismutation of extracellular superoxide radicals, or on treatment with diphenylene iodonium, which inhibits NADH/NADPH oxidase (Figure 4, E and F). SOD or diphenylene iodonium alone had no effect on BrdU incorporation. These findings confirmed that apelin-induced extracellular ROS production promoted VSMC proliferation.
Figure 4.
Superoxide production and mitogenicity of APJ in VSMCs from APJ+/+ (A–C, E, and F) and APJ−/− mice (D). A and B: rac-1 (A) and Id3 (B) mRNA expression in response to apelin (10−6 mol/L). Expression of nontreated controls was set at 1.0. Results are expressed as means ± SEM from three experiments in each group. C and D: VSMCs were incubated in the presence of increasing concentrations of apelin for up to 48 hours, and then a BrdU incorporation assay was performed (n = 6). E and F: VSMCs were incubated in the presence of apelin (10−6 mol/L) with or without SOD (100 U/ml) or diphenylene iodonium (10−6 mol/L) for up to 48 hours, and then a BrdU incorporation assay was performed. Results are expressed as means ± SEM from six experiments in each group. *P < 0.05, **P < 0.01 versus control. ††P < 0.01 versus apelin (10−6 mol/L) group.
Discussion
The important findings of the present study were that atherosclerotic lesion formation in APJ−/−ApoE−/− mice fed a HCD revealed marked reductions in lesion size than APJ+/+ApoE−/− mice in the absence of an effect of cholesterol levels. Apelin stimulates NADPH oxidase subunit expression and it induces oxidative stress-linked proliferation in VSMCs. Vascular production of superoxide radicals and the expression of NADPH oxidase subunits were decreased in APJ−/−ApoE−/− mice compared with APJ+/+ApoE−/− mice fed a standard ND. Immunohistochemical detection of smooth muscle cells, using the smooth muscle α-actin antibody, showed greatly reduced staining for these cells in lesions of APJ−/−ApoE−/− mice. These results demonstrate that APJ is involved in the development of hypercholesterolemia-associated atherosclerosis.
ROS are positively involved in the initiation and progression of atherosclerosis through their ability to reduce the bioavailability of nitric oxide (NO), to provoke oxidative modifications of DNA and proteins, lipid oxidation, enhanced mitogenicity and apoptosis of vascular cells, and increased expression and activation of pathophysiologically important genes.23 Smooth muscle cells are a principal source of ROS in the vasculature.24 Angiotensin II, which stimulates oxidative stress, also promotes atherosclerosis and endothelial dysfunction via AT1 receptor activation.14,25,26,27 Although there is no evidence for specific binding of angiotensin II to APJ, both apelin and angiotensin II stimulate Akt and eNOS phosphorylation in endothelium and they also stimulate myosin light chain phosphorylation in VSMCs. The present report has indicated that apelin induces NADPH oxidase subunit and cell proliferation in VSMCs. Furthermore, disruption of APJ reduces ROS formation and atherosclerotic area, as is also seen after disruption of the AT1 receptor.14 APJ is therefore a potential new therapeutic target for atherosclerosis. It will be necessary to develop APJ receptor antagonists for further investigations of this hypothesis.
It is reported that there is some cross talk between the apelin-APJ system and the renin-angiotensin system. Angiotensin II stimulation decreased cardiac apelin mRNA.28 The baseline blood pressure of APJ and angiotensin-type 1 receptor double-knockout mice was significantly elevated compared with that of angiotensin-type 1 receptor knockout mice.5 Apelin was hydrolyzed by angiotensin-converting enzyme-related carboxypeptidase (ACE2), the closest homolog of angiotensin I-converting enzyme.29 Further studies are needed to clarify how the renin-angiotensin system relates to the present study.
We have previously reported that apelin stimulation of APJ mediates phosphorylation of endothelial nitric-oxide synthase (eNOS) in cultured endothelial cells.5 The vasodilator function of eNOS-derived NO is protective because deletion of the eNOS gene in ApoE−/− mice results in hypertension and increased atherosclerosis.30 Thus, APJ can play a protective role against atherosclerosis by its actions in endothelium. On the other hand, according to the data presented above, APJ is a contributor to hypercholesterolemia-associated atherosclerosis. APJ has biphasic effects on blood vessels via endothelium-mediated vasodilatation and VSMC-dependent vasoconstriction.2,5,31 It is possible the role of apelin-APJ in healthy blood vessels may not be so obvious because APJ−/− mice did not show any significant changes in cardiovascular parameters including the serum lipid profile. However, pathophysiological situations such as declining influence of NO because of endothelial dysfunction in ApoE−/− mice fed with HCD may unmask the damaging effect of apelin on the vascular wall. The role of APJ in atherosclerosis resembles that of AT1: angiotensin II promotes atherosclerosis via AT1 receptor activation14 and also plays a role in maintaining vascular eNOS expression and activity.16,32,33,34
Progressive accumulation of macrophages, and their uptake of oxidized LDL, ultimately leads to the development of atherosclerotic lesions. Lipid-rich macrophages, known as foam cells, secrete inflammatory mediators that stimulate smooth muscle cell migration and proliferation. But in our study, immunohistochemical characterization of atherosclerotic lesions in similar-sized aortic sinus lesions also revealed similar levels of F4/80-positive cells, independent of APJ expression. Serum MCP-1 levels were increased in APJ+/+ApoE−/− mice compared with APJ+/+ApoE+/+ mice, whereas there was no difference in these levels between APJ+/+ApoE−/− mice and APJ−/−ApoE−/− mice (data not shown). Apelin also failed to induce p47phox and rac-1 expression in the cultured murine macrophage cell line, RAW264.7 cells (data not shown). Although further studies are needed to elucidate roles of APJ in monocytes/macrophages, APJ in monocytes/macrophages plays a minimal role in atherogenesis in our study.
Previous data support the view that as-yet-unidentified mechanisms contribute to atherosclerosis.35,36 Although further studies are needed to investigate the mechanisms of APJ action in atherogenesis, it is likely that APJ is involved in the development of hypercholesterolemia-associated atherosclerosis. Our results propose a possibility of therapeutic use of apelin-APJ system inhibition for patients with atherosclerosis. The development of a stable APJ receptor antagonist might provide us with a new therapeutic tool for cardiovascular disease.
Acknowledgments
We thank Prof. Kengo Funakoshi and Dr. Tetsuo Kadota, Department of Neuroanatomy; Mr. Masaichi Ikeda, Department of Pathobiology; and Ms. Emi Maeda and Ms. Hiroko Morinaga, Department of Medical Science and Cardiorenal Medicine, Yokohama City University Graduate School of Medicine and School of Medicine, for technical assistance, scientific discussions, and encouragement.
Footnotes
Address reprint requests to Minoru Kihara, Department of Medical Science and Cardiorenal Medicine, Yokohama City University Graduate School of Medicine and School of Medicine, Fukuura 3-9, Kanazawa-ku, Yokohama 234-0004, Japan. E-mail: stanesby@hotmail.co.jp.
Supported by The Japan Society for the Promotion of Science (The 21st Century Center Of Excellence Program); the Ministry of Education, Culture, Sports, Science, and Technology of Japan (grant-in-aid for scientific research no. 16590892; and grants-in-aid for young scientists nos. 16790425 and 19790537), the Japan Heart Foundation (grant for Research on Arteriosclerosis Update); the Yokohama Foundation for Advancement of Medical Science; and the Yokohama City University (Strategic Research Project grant W18009).
References
- O’Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–360. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- Katugampola SD, Maguire JJ, Matthewson SR, Davenport AP. [125I]-(Pyr1) apelin-13 is a novel radioligand for localizing the APJ orphan receptor in human and rat tissues with evidence for a vasoconstrictor role in man. Br J Pharmacol. 2001;132:1255–1260. doi: 10.1038/sj.bjp.0703939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther. 2005;107:198–211. doi: 10.1016/j.pharmthera.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Ishida J, Hashimoto T, Hashimoto Y, Nishiwaki S, Iguchi T, Harada S, Sugaya T, Matsuzaki H, Yamamoto R, Shiota N, Okunishi H, Kihara M, Umemura S, Sugiyama F, Yagami K, Kasuya Y, Mochizuki A, Fukamizu A. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem. 2004;279:26274–26279. doi: 10.1074/jbc.M404149200. [DOI] [PubMed] [Google Scholar]
- Zhong JC, Huang DY, Liu GF, Jin HY, Yang YM, Li YF, Song XH, Du K. Effects of all-trans retinoic acid on orphan receptor APJ signaling in spontaneously hypertensive rats. Cardiovasc Res. 2005;65:743–750. doi: 10.1016/j.cardiores.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Zhong JC, Yu XY, Huang Y, Yung LM, Lau CW, Lin SG. Apelin modulates aortic vascular tone via endothelial nitric oxidase phosphorylation pathway in diabetic mice. Cardiovasc Res. 2007;74:388–395. doi: 10.1016/j.cardiores.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Chen MM, Ashley EA, Deng DX, Tsalenko A, Deng A, Tabibiazar R, Ben-Dor A, Fenster B, Yang E, King JY, Fowler M, Robbins R, Johnson FL, Bruhn L, McDonagh T, Dargie H, Yakhini Z, Tsao PS, Quertermous T. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation. 2003;108:1432–1439. doi: 10.1161/01.CIR.0000091235.94914.75. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Kihara M, Ishida J, Imai N, Yoshida S, Toya Y, Fukamizu A, Kitamura H, Umemura S. Apelin stimulates myosin light chain phosphorylation in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2006;26:1267–1272. doi: 10.1161/01.ATV.0000218841.39828.91. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Czech T, van Eickels M, Fleming I, Bohm M, Nickenig G. Inhibition of diet-induced atherosclerosis and endothelial dysfunction in apolipoprotein E/angiotensin II type 1a receptor double-knockout mice. Circulation. 2004;110:3062–3067. doi: 10.1161/01.CIR.0000137970.47771.AF. [DOI] [PubMed] [Google Scholar]
- Fan J, Shimoyamada H, Sun H, Marcovina S, Honda K, Watanabe T. Transgenic rabbits expressing human apolipoprotein (a) develop more extensive atherosclerotic lesions in response to a cholesterol-rich diet. Arterioscler Thromb Vasc Biol. 2001;21:88–94. doi: 10.1161/01.atv.21.1.88. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Unoki H, Sun H, Shimoyamada H, Marcovina S, Shikama H, Watanabe T, Fan J. Lipoprotein(a) promotes smooth muscle cell proliferation and differentiation in atherosclerotic lesions of human apo(a) transgenic rabbits. Am J Pathol. 2002;160:227–236. doi: 10.1016/S0002-9440(10)64366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Kihara M, Sato K, Imai N, Tanaka Y, Sakai M, Tamura K, Hirawa N, Toya Y, Kitamura H, Umemura S. Heparin recovers AT1 receptor and its intracellular signal transduction in cultured vascular smooth muscle cells. FEBS Lett. 2005;579:281–284. doi: 10.1016/j.febslet.2004.11.093. [DOI] [PubMed] [Google Scholar]
- Kihara M, Umemura S, Kadota T, Yabana M, Tamura K, Nyuui N, Ogawa N, Murakami K, Fukamizu A, Ishii M. The neuronal isoform of constitutive nitric oxide synthase is up-regulated in the macula densa of angiotensinogen gene-knockout mice. Lab Invest. 1997;76:285–294. [PubMed] [Google Scholar]
- Imai N, Hashimoto T, Kihara M, Yoshida S, Kawana I, Yazawa T, Kitamura H, Umemura S. Role for host and tumor angiotensin II type 1 receptor in tumor growth and tumor-associated angiogenesis. Lab Invest. 2007;87:189–198. doi: 10.1038/labinvest.3700504. [DOI] [PubMed] [Google Scholar]
- Sato K, Kihara M, Hashimoto T, Matsushita K, Koide Y, Tamura K, Hirawa N, Toya Y, Fukamizu A, Umemura S. Alterations in renal endothelial nitric oxide synthase expression by salt diet in angiotensin type-1a receptor gene knockout mice. J Am Soc Nephrol. 2004;15:1756–1763. doi: 10.1097/01.asn.0000130922.75125.b8. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Kihara M, Yokoyama K, Fujita T, Kobayashi S, Matsushita K, Tamura K, Hirawa N, Toya Y, Umemura S. Lipoxygenase products regulate nitric oxide and inducible nitric oxide synthase production in interleukin-1β stimulated vascular smooth muscle cells. Hypertens Res. 2003;26:177–184. doi: 10.1291/hypres.26.177. [DOI] [PubMed] [Google Scholar]
- Ishiyama M, Miyazono Y, Sasamoto K, Ohkura Y, Ueno K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta. 1997;44:1299–1305. doi: 10.1016/s0039-9140(97)00017-9. [DOI] [PubMed] [Google Scholar]
- Osada J, Joven J, Maeda N. The value of apolipoprotein E knockout mice for studying the effects of dietary fat and cholesterol on atherogenesis. Curr Opin Lipidol. 2000;11:25–29. doi: 10.1097/00041433-200002000-00004. [DOI] [PubMed] [Google Scholar]
- Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Baudler S, Muller C, Werner C, Werner N, Welzel H, Strehlow K, Bohm M. Redox-sensitive vascular smooth muscle cell proliferation is mediated by GKLF and Id3 in vitro and in vivo. FASEB J. 2002;16:1077–1086. doi: 10.1096/fj.01-0570com. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Nickenig G. Pathophysiological regulation of the AT1-receptor and implications for vascular disease. J Hypertens Suppl. 2006;24:S15–S21. doi: 10.1097/01.hjh.0000220402.53869.72. [DOI] [PubMed] [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Sugiyama F, Haraoka S, Watanabe T, Shiota N, Taniguchi K, Ueno Y, Tanimoto K, Murakami K, Fukamizu A, Yagami K. Acceleration of atherosclerotic lesions in transgenic mice with hypertension by the activated renin-angiotensin system. Lab Invest. 1997;76:835–842. [PubMed] [Google Scholar]
- Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Chen R, Li Z, Shiuchi T, Suzuki J, Ide A, Tsuda M, Okumura M, Min LJ, Mogi M, Horiuchi M. Deletion of angiotensin II type 2 receptor exaggerated atherosclerosis in apolipoprotein E-null mice. Circulation. 2005;112:1636–1643. doi: 10.1161/CIRCULATIONAHA.104.525550. [DOI] [PubMed] [Google Scholar]
- Iwanaga Y, Kihara Y, Takenaka H, Kita T. Down-regulation of cardiac apelin system in hypertrophied and failing hearts: possible role of angiotensin II-angiotensin type 1 receptor system. J Mol Cell Cardiol. 2006;41:798–806. doi: 10.1016/j.yjmcc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- Knowles JW, Reddick RL, Jennette JC, Shesely EG, Smithies O, Maeda N. Enhanced atherosclerosis and kidney dysfunction in eNOS(−/−)Apoe(−/−) mice are ameliorated by enalapril treatment. J Clin Invest. 2000;105:451–458. doi: 10.1172/JCI8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K, Takayama K, Zou MX, Kumaki I, Zhang W, Kumano K, Fujimiya M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept. 2001;99:87–92. doi: 10.1016/s0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- Pueyo ME, Arnal JF, Rami J, Michel JB. Angiotensin II stimulates the production of NO and peroxynitrite in endothelial cells. Am J Physiol. 1998;274:C214–C220. doi: 10.1152/ajpcell.1998.274.1.C214. [DOI] [PubMed] [Google Scholar]
- Kihara M, Sato K, Hashimoto T, Imai N, Toya Y, Umemura S. Expression of endothelial nitric oxide synthase is suppressed in the renal vasculature of angiotensinogen-gene knockout mice. Cell Tissue Res. 2006;323:313–320. doi: 10.1007/s00441-005-0058-3. [DOI] [PubMed] [Google Scholar]
- Ramchandran R, Takezako T, Saad Y, Stull L, Fink B, Yamada H, Dikalov S, Harrison DG, Moravec C, Karnik SS. Angiotensinergic stimulation of vascular endothelium in mice causes hypotension, bradycardia, and attenuated angiotensin response. Proc Natl Acad Sci USA. 2006;103:19087–19092. doi: 10.1073/pnas.0602715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Allayee H, Ghazalpour A, Lusis AJ. Using mice to dissect genetic factors in atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1501–1509. doi: 10.1161/01.ATV.0000090886.40027.DC. [DOI] [PubMed] [Google Scholar]