Abstract
Amphiphysin 1 is involved in clathrin-mediated endocytosis. In this study, we demonstrate that amphiphysin 1 is essential for cellular phagocytosis and that it is critical for actin polymerization. Phagocytosis in Sertoli cells was induced by stimulating phosphatidylserine receptors. This stimulation led to the formation of actin-rich structures, including ruffles, phagocytic cups, and phagosomes, all of which showed an accumulation of amphiphysin 1. Knocking out amphiphysin 1 by RNA interference in the cells resulted in the reduction of ruffle formation, actin polymerization, and phagocytosis. Phagocytosis was also drastically decreased in amph 1 (−/−) Sertoli cells. In addition, phosphatidylinositol-4,5-bisphosphate–induced actin polymerization was decreased in the knockout testis cytosol. The addition of recombinant amphiphysin 1 to the cytosol restored the polymerization process. Ruffle formation in small interfering RNA-treated cells was recovered by the expression of constitutively active Rac1, suggesting that amphiphysin 1 functions upstream of the protein. These findings support that amphiphysin 1 is important in the regulation of actin dynamics and that it is required for phagocytosis.
INTRODUCTION
Phagocytosis is a form of endocytosis in which large molecules are engulfed by the plasma membrane and internalized for digestion (Greenberg and Grinstein, 2002; Niedergang and Chavrier, 2004). In vertebrates, macrophages and testicular Sertoli cells are the predominant phagocytes. Phagocytosis contributes to removal of foreign bodies, bacteria, and microbe-infected and apoptotic cells. Binding of these objects to the receptors, such as Fcγ receptors or phosphatidylserine receptors, on the surface of the macrophage initiates membrane remodeling and reorganization of the actin cytoskeleton. Cytoskeletal changes cause the cells to extend pseudopods that then engulf the objects (Anderem and Underhill, 1999).
Sertoli cells participate in the maturation of germ cells and the release of sperm (Russell et al., 1990). These cells are highly phagocytic and are responsible for eliminating the residual cytoplasm of spermatids (Chemes, 1986; Clermont et al., 1987; Morales and Clermont, 1991). The phagocytosis is also used to remove apoptotic germ cells (Shiratsuchi et al., 1997, 1999; Kawasaki et al., 2002). Phagocytosis in Sertoli cells is initiated by the recognition of phosphatidylserine (PS) found on the germ cell surface by PS receptors (Shiratsuchi et al., 1997, 1999; Blanco-Rodriguez and Martinez-Garcia, 1999; Kawasaki et al., 2002). The class B scavenger family receptors SR-BI and CD36 are found on the surface of Sertoli cells where they function in PS recognition (Shiratsuchi et al., 1997, 1999; Kawasaki et al., 2002; Gillot et al., 2005). In the testis, Sertoli cells express high levels of amphiphysin 1 (Watanabe et al., 2001; Kamitani et al., 2002). The expression of amphiphysin, along with that of dynamin 2, increases with sexual development (Watanabe et al., 2001); thus, it is hypothesized that amphiphysin 1 may play a role in spermatogenesis.
Amphiphysin 1 is found mainly in the neuronal synapse and in the testis. In the synapse, amphiphysin 1 acts as a linker between clathrin-coated proteins and dynamin 1 to assist in clathrin-mediated endocytosis (Slepnev et al., 2000). Amphiphysin 1 and dynamin 1 are physiological binding partners, and they play a major role in the fission process of clathrin-mediated endocytosis (Schmid et al., 1998; Takei et al., 1999). Amphiphysin 1 has three functional domains, a highly conserved amino-terminal region composed of ∼250 amino acids called the Bin/amphiphysin/Rvs (BAR) domain, a carboxy-terminal Src homology (SH)3 domain that binds dynamin 1 (David et al., 1996; Wigge and McMahon, 1998), and clathrin/AP-2-binding sites (CLAP) at the central variable region (Slepnev et al., 2000).
The BAR domain of amphiphysin 1 is a banana-shaped α-helical dimer that senses highly curved membranes (Peter et al., 2004). It also contains an amphipathic helix at the amino terminus called N-BAR, which associates primarily with acidic phospholipids (Dawson et al., 2006). This property enables the N-BAR proteins to induce membrane deformation. It has recently been reported that N-BAR–containing proteins, such as the Drosophila amphiphysin isoform or endophilin function as a linker between the plasma membrane and the actin cytoskeleton (Dawson et al., 2006). Phagocytosis requires actin dynamics; the actin polymerization underneath plasma membrane is thought to generate the driving forces of pushing (extension) or pulling (invagination) of the plasma membrane through a linker protein (Small et al., 2002; Dawson et al., 2006; Smythe and Ayscouh, 2006).
Amphiphysin has been previously interrelated with the actin cytoskeleton. Yeast amphiphysin isoforms Rvs 167 and Rvs 161 are involved in two actin-dependent processes, cellular polarity, and endocytosis (Munn et al., 1995; Sivadon et al., 1995; Kaksonen et al., 2005). In neurons, amphiphysin 1 is colocalized with dynamin 1 at the filopodia formed in growth cones. The suppression of amphiphysin 1 by antisense oligonucleotides in the neurons decreases filopodia formation and leads to the collapse of the growth cones (Mundigl et al., 1998; Yoo et al., 2002). In the mature synapse, amphiphysin 1 localizes in proximity to actin cytomatrix (Bauerfeind et al., 1997); however, the precise role of amphiphysin in actin dynamics is still not defined.
In this study, we analyze the function of amphiphysin 1 in actin dynamics during phagocytosis in Sertoli cells. We demonstrate a novel function for amphiphysin in the stimulation of actin polymerization in phagocytosis.
MATERIALS AND METHODS
Animals
Three-week-old male Wister rats, 3-wk-old and 20-wk-old wild-type mice were purchased from Shimizu Laboratory Supplies Co. (Kyoto, Japan). Amphiphysin 1 knockout mice [amph 1 (−/−)] were generated by gene targeting in embryonic stem cells as described previously (Di Paolo et al., 2002). Twenty-week-old wild-type or amph 1 (−/−) mice were used for harvest of cytosol and hematoxyline-eosin staining, and 2–3-wk-old mice were used for primary culture. All animals were maintained in clean conditions with free access to food and water. They were allowed to adapt to their environment for more than 1 wk before initiating the experiments.
Cell Culture
Preparation of Sertoli cells and spermatogenetic cells from 3-wk-old rats or mice was done as described previously (Shiratsuchi et al., 1997). Cells were cultured at 32°C under 5% CO2. Mixture of Sertoli cells and germ cells were plated on collagen-coated culture dishes. After 24 h, the residual germ cells were removed by hypo-osmotic shock in 20 mM Tris-HCl, pH 7.4, for 3 min at room temperature. After 2 d of culture, the Sertoli cell-enriched culture consisted of ∼95% Sertoli cells as established by immunofluorescent methods for the cell markers, Mullerian hormone, or amphiphysin 1 (Tran et al., 1987; Watanabe et al., 2001). Cells were plated in monolayers and experiments were performed. Ser-W3 cells were cultured with DMEM containing 10% fetal bovine serum at 37°C under 5% CO2 (Prognan et al., 1997). Apoptosis of the germ cells were induced by treatment with 2 μM camptothecin at 37°C for 8 h. Apoptosis was determined by analysis of fluorescein isothiocyanate (FITC)-annexin binding or chromatin condensation with 4,6-diamidino-2-phenylindole (DAPI) staining (Zhang et al., 1998).
cDNA Constructs and Transfection
The cDNAs encoding full-length human amphiphysin 1 and its truncation constructs were prepared by polymerase chain reaction (PCR) amplification by using specific primers (Yoshida et al., 2004). Full-length amphiphysin 1, amph 1-626aa (ΔSH3), and 226-695aa (ΔBAR) were subcloned into the plasmid pEF-BOS-myc or pGEX-6P vector as BamHI–EcoRI fragments. To prepare amph D322-386aa (ΔCLAP), amph D1-321aa was subcloned into a pGEX-6P vector as a BamHI–EcoRI fragment. EcoRI-EcoRI fragments containing amph 387-695aa were then inserted into the EcoRI site. Amph D322-386aa (ΔCLAP) was subcloned into a pEF-BOS-myc vector as BamHI–EcoRI fragments. Full-length amphiphysin 1 containing the BamHI and EcoRI restriction sites was subcloned into a pEGFP-C1 vector (Clontech, Mountain View, CA). The nucleotide sequences of the constructs were verified using DNA sequence analysis. The plasmids pEF-BOS-myc-N17Rac1, V12Rac1, N17Cdc42, and N17Rho were a gift from Dr. Toshiki Itoh (Kobe University, Japan). The constructs were transfected into the cells using a Lipofectamine 2000 transfection system (Invitrogen, Carlsbad, CA). The efficiency of transfection was ∼90% in Ser-W3 cells determined by the levels of green fluorescence protein (GFP) expressed in the cells. Twenty-four hours after transfection, cells were subjected to phagocytic analysis.
Preparation for Liposomes and Lipid-coated Beads
Liposomes containing 70% phosphatidylcholine (PC) and 30% phosphatidylserine (PS) were prepared as described previously (Shiratsuchi et al., 1997). For the actin polymerization assay, liposomes containing 46% PC, 30% PS, 20% cholesterol, 4% phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2] were prepared by sonication as described in Ma et al. (1998b). To prepare the PS-coated styrene beads, liposomes composed of 30% PS, 60% PC, and 10% biotinylated phosphatidylethanolamine (PE) (Invitrogen) were first made by sonication in serum-free DMEM. Two milligrams of the liposomes was incubated with 100 μl of styrene beads coated with streptavidin (2 μm in diameter; Polysciences, Warrington, PA) for 2 h at room temperature. The slurry was centrifuged at 5000 × g for 5 min, and the beads were resuspended with 4 ml of serum-free DMEM.
Phagocytic Analysis
When indicated, cells (1 × 104 cells/coverslip, 22 × 22 mm) were pretreated with 2 μM cytochalasin D (Cyt D), or with the equivalent amount of dimethyl sulfoxide for 30 min at 32 or 37°C in serum-free DMEM. To measure phagocytosis, cells were incubated with 300 μl of bead suspension on each coverslip and incubated at 32 or 37°C for the various time points. Because the kinetics of phagocytosis in Sertoli cell is relatively slow (Filippini et al., 1989), cells were incubated for 6 h to ensure internalization of the beads. Cells were gently washed four times with phosphate-buffered saline (PBS) containing 1.5 mM CaCl2 and 1 mM MgCl2 [PBS (+)]. To distinguish between internalized beads and those attached to the cell surface, the later were labeled with FITC-annexin (BioVision, Mountain View, CA), which specifically binds to PS. For this purpose, cells were incubated with FITC-annexin at room temperature for 10 min. Then, cells were fixed with 2% paraformaldehyde (PFA) in PBS (+). When necessary, cells were permeabilized with 100 μM digitonin to avoid detachment of FITC-annexin from the beads. Mutant cells were determined by immunofluorescence for myc. To quantitate phagocytosis, the annexin-positive and negative beads on the cell surface were counted using phase contrast and fluorescent microscopy. The number of internalized beads was counted in 50 cells randomly chosen from more than 10 independent fields. Phagocytic indices were presented as the number of annexin-negative beads per Sertoli cell. An attachment index was also determined by counting the number of annexin-positive beads per cell.
Microscopy
Sertoli cells (1 × 104 cells/coverslip) were fixed with 2% PFA/PBS (+) at room temperature, permeabilized, and subjected to immunocytochemistry (Krauss et al., 2003). The samples were examined using a spinning disk confocal microscope system (CSU10; Yokogawa Electric, Kanazawa, Japan) combined with an inverted microscope (IX-71; Olympus Optical, Tokyo, Japan) and a CoolSNAP-Pro camera (Roper Industries., Sarasota, FL). The Z-positioning was accomplished by a piezo-electric motor (Olympus Optical) mounted underneath the objective lens. The system was steered by MetaMorph software (Molecular Devices, Sunnyvale, CA). Z-series images were taken at 0.2-μm increments. Vertical images were reconstituted from the Z-series of images by MetaMorph. For live imaging, cells (1 × 104 cells/coverslip) were cultured at 37 or 32°C for 3 d on collagen type I-coated coverslips (12 mm in diameter), and then they were transfected with human amphiphysin 1-GFP at the concentration of 1 μg per coverslip. Live-cell confocal time-lapsed images were taken using a spinning disk confocal microscope system (CSU10) combined with an inverted microscope (IX-71) and a CoolSNAP-Pro camera (Tadakuma et al., 2001). Images were automatically captured every 30 s. Vertical images were reconstituted from a Z-series of images by MetaMorph, as described above. When necessary, images were further processed using Adobe Photoshop and Illustrator software (Adobe Systems, Mountain View, CA).
Small-interfering RNA (siRNA)-mediated Interference
Preannealed siRNA for rat amphiphysin 1, and negative control siRNA were synthesized and purified by Ambion (Austin, TX). The sequences for siRNA are as follows: rat amphiphysin 1, 5′-GGCAGAUGAAACAAAAGAUtt-3′ for oligo 1, 5′-GGUAUGCAGGAGGCCUCAAtt-3′ for oligo 2, and 5′-GGAGAACAUCAUCAAUUUCtt-3′ for oligo 3. Scrambled RNA that has no significant sequence homology to mouse, rat, or human gene sequences was used as the negative control. The day before transfection, cells were plated in six-well plates (5.0 × 104 cells/well). Two hundred picomoles of duplex siRNA was transfected into the cells using 4 μl of Lipofectamine 2000 (Invitrogen). After 48 h, the cells were subjected to the different experiments. We confirmed that all three transfections of siRNA for amphiphysin 1 were effective, and we obtained essentially the same results.
Quantification of Membrane Ruffle Formation
Liposomes were added to Ser-W3 cells (1 × 104 cells/coverslip) at 0.25 mM in serum-free DMEM and incubated at 37°C for 10 min. The cells were then washed with PBS (+) three times. Stimulated or control Sertoli cells were fixed, permeabilized, and stained with anti-amphiphysin 1 antibodies (mab 3; provided by Dr. De Camilli, Yale University) or anti-c-myc antibodies and Alexa488- or rhodamine-phalloidin. Cells with peripheral ruffles were characterized as cells with thick peripheral actin filament accumulation according to Suetsugu et al. (2003). When counting the cells with ruffles, cells with free edges were selected. To quantify ruffling efficiency, cells that had no ruffles were scored as negative, whereas cells that had one or more ruffles were considered to be positive. The number of cells that were positive for ruffles was counted and expressed as a percentage of total number of cells analyzed. At least 100 cells in different areas of the wells were counted in each experiment.
Preparation for Testis Cytosol
Testis cytosol was prepared as described previously (Ma et al., 1998b). Briefly, 20 testis of amph 1 (+/+) or amph 1 (−/−) mice were homogenized in 5 ml of XB buffer (10 mM HEPES, 100 mM KCl, 2 mM MgCl2, 0.1 mM CaCl2, 5 mM EGTA, 50 mM sucrose, 1 mM dithiothreitol, 1 μg/ml leupeptin, 5 μg/ml pepstatin, and 0.4 mg/ml phenylmethylsulfonyl fluoride), pH 7.4. The homogenate was centrifuged at 3000 × g for 20 min and 10,000 × g for 20 min. The resultant supernatant was diluted with XB buffer to 4 times in volume and centrifuged at 400,000 × g for 1 h. The clear supernatant was carefully removed and concentrated to 4 times in volume in Centriprep-10 concentrators (Millipore, Billerica, MA). A final concentration of the cytosol was 40–50 mg/ml. Monomer actin in amph 1 (−/−) and amph 1 (+/+) cytosol was determined to be equal as estimated by Western blotting. In recovery experiments, recombinant amphiphysin 1 or mutants were purified as described previously (Yoshida et al., 2004), and added to amph 1 (−/−) cytosol.
In Vitro Actin Assembly Assay
For visual assay for actin assembly, cytosol (20 mg/ml) was incubated at room temperature with liposomes (0.15 mM) and rhodamine-actin (0.4 mg/ml/coverslip; Invitrogen). Samples were supplemented with ATP generating system (1 mM ATP, 8 mM creatine phosphate, and 8 U/ml phosphocreatine kinase), 1.3 mM MgCl2, and 0.1 mM EGTA. The mixture was examined by confocal microscopy. MetaMorph software controlled the microscope functions, and it was used for image processing.
For quantitative analysis of actin assembly, pyrene-actin assay was carried out according to Ma et al. (1998b) with slight modification. Ma et al. (1998b) first mixed all the components except liposomes and then added the liposomes. The sequence was altered to ensure all the components mixed uniformly in the cuvette. Briefly, 125 μl of XB buffer was incubated in a quartz cuvette at room temperature for 5 min. Then, an ice-cold mixture of liposomes (50 μM) and cytosol (8 or 16 mg/ml) supplemented with 0.4 mg/ml pyrene-actin (Cytoskeleton, Denver, CO), 1.3 mM MgCl2, 0.1 mM EGTA, and ATP generating system (1 mM ATP, 8 mM creatine phosphate, and 8 U/ml phosphocreatine kinase) was added to the buffer. Pyrene fluorescence was measured at 407 nm with excitation at 365 nm in an F-2500 fluorescence spectrophotometer (Hitachi, Tokyo, Japan) with a 10-nm slit width. Fluorescent intensity of the buffer alone was subtracted from that of samples with cytosol and liposomes.
Determination of Residual Bodies in Wild and Amphiphysin 1-deficient Mice
Spermatogenetic stages in mice seminiferous tubules were categorized into XII stages, and the spermatids maturation was classified into 16 steps as described previously (Oakberg, 1956; Abe et al., 1991). To quantify residual bodies in stage VIII, 30 seminiferous tubules each from amph 1 (+/+) or amph 1 (−/−) testis were randomly examined from 5 of amph 1 (+/+) or amph 1 (−/−) mice using hematoxylin and eosin (H&E) staining and light microscopy. Residual bodies and unreleased spermatids were observed in VIII stage, visualized with H&E staining (Beardsley et al., 2003). The size of the residual bodies in stage VIII seminiferous tubules was determined and the average of the areas was calculated. The quantification of the residual body size was determined on light microscopic images of H&E-stained samples. Twenty independent fields were analyzed by Mac Scope software (Mitani, Osaka, Japan).
Scanning Electron Microscopy
Mouse primary-cultured Sertoli cells (1 × 104 cells on coverslip) were incubated with PS coated styrene beads at 32°C for 90 min. After incubation, cells were extensively washed with PBS (+) three times, and one time with 0.1 M cacodylate buffer, pH 7.4. Pairs of coverslips were fixed for 1 h at room temperature with 2% glutaraldehyde in 0.1 M cacodylate buffer, containing 6.8% sucrose (Diakonova et al., 2002). Coverslips were then postfixed with 1% osmium tetroxide in 0.1 M cacodylate buffer for 1 h at 4°C and washed with 0.1 M cacodylate buffer twice. After dehydration with a series of ascending concentrations of ethanol, the coverslips were coated with osmium tetroxide by using an ion coater. Cells were observed with a scanning electron microscope (S900; Hitachi).
RESULTS
Amphiphysin 1 Is Involved in PS-dependent Phagocytosis
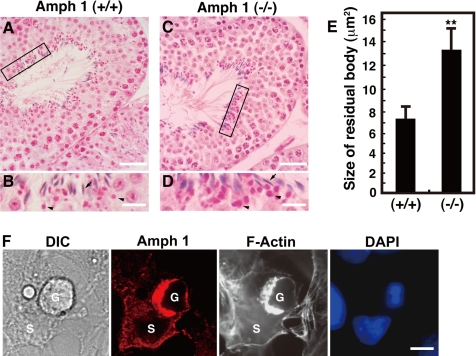
The last step of germ cell maturation includes the elimination of the spermatid cytoplasm before their release. This occurs at stage VIII of the seminiferous tubules. Sertoli cells use phagocytic mechanisms to internally incorporate the germ cell cytoplasm (Clermont et al., 1987). The incorporated cytoplasm, termed residual bodies, can be seen in the Sertoli cells with hematoxylin and eosin staining (Beardsley and O'Donnell, 2003). We analyzed amph 1 (−/−) Sertoli cells to investigate whether amphiphysin 1 is implicated in the phagocytic process of the residual bodies. In stage VIII of the seminiferous tubules, we saw round eosinophilic residual bodies adjacent to mature spermatids along the luminal surface of the tubule (Figure 1, B and D). The residual bodies were clearly larger in the amph 1 (−/−) testis versus the wild type (Figure 1, B and D). Morphological analysis revealed that the average size of the residual bodies in amph 1 (−/−) is ∼1.8 times larger than that of amph 1 (+/+) (Figure 1E), suggesting that residual body uptake or phagosome formation is reduced in the amph 1 (−/−) Sertoli cells.
Figure 1.
Amphiphysin 1 is implicated in phagocytosis in Sertoli cells. Hematoxylin- and eosin-stained low magnification images of seminiferous tubules at stage VIII in amph 1 (+/+) (A) or amph 1 (−/−) (C). Area enclosed with rectangles are shown at high magnification A and C (B and D, respectively). Arrowheads indicate residual bodies. Arrows indicate nuclei of spermatids. Note the difference in size in the wild type versus the mutant. Bar, 40 μm (A and C) and 10 μm (B and D). (E) Morphological analysis of residual bodies in size (area). The average area of the residual bodies in amph 1 (−/−) is ∼1.8 times of that in wild type. Statistical significance was determined by Student's t tests (**p < 0.01, n = 200). (F) Primary rat cultured Sertoli cells (1 × 104 cells/coverslip) were incubated at 32°C for 30 min with germ cells (1 × 103 cells/coverslip) pretreated with camptothecin. The cells were fixed, permeabilized and stained with anti-amphiphysin 1 antibodies (mab 3) and Alexa488-phalloidin. Nuclei of the cells were visualized with DAPI. The Sertoli cells engulfed the apoptotic germ cells, whereas amphiphysin 1 accumulated at the phagocytic cup. G, germ cell; S, Sertoli cell. Bar, 10 μm.
Phagocytosis of residual bodies is triggered by the recognition of PS moieties on the outer surface of germ cells (Blanco-Rodriguez and Martinez-Garcia, 1999). A similar mechanism is thought to be used when Sertoli cells phagocytose apoptotic germ cells (Shiratsuchi et al., 1997, 1999; Kawasaki et al., 2002). Therefore, we examined the mechanisms of the phagocytic processes in the presence of apoptotic germ cells. Apoptosis of germ cells was induced using camptothecin (Fusaro et al., 2003), and the germ cells were placed on primary cultured rat Sertoli cells. As shown in Figure 1F, amphiphysin 1 and F-actin accumulated at the phagocytic cup formed on the surface of the cells. Consistent with previous report (Kamitani et al., 2002), amphiphsyin 1 was absent in the germ cells. To further elucidate the function of amphiphysin 1 in phagocytosis, we measured the uptake of PS coated styrene beads (PS beads) by primary cultured rat Sertoli cells, or by Sertoli cell lines. We observed an accumulation of endogenous or exogenous amphiphysin 1 and F-actin at the phagosomes in Ser-W3 cells, Sertoli cell line (Figure 2A), and in TM4 cells, mouse Sertoli cell line (Supplemental Figure 1). Ser-W3 cells specifically associated to PS beads, but not to phosphatidylcholine (PC)-coated beads (Figure 2, B and C). The association of the PS beads with the cells was abolished by preincubation of the cells with PS liposomes or with anti-SR-BI antibodies that recognize the ectodomain of the receptor (Gu et al., 2000) (Figure 2, B and C). In this experimental system, we confirmed that phagocytosis is dependent on both PS and the PS receptor SR-BI.
Figure 2.
PS-dependent phagocytosis in Sertoli cells. (A) Amphiphysin 1 accumulates at phagosomes. Ser-W3 cells were transfected with amphiphysin 1-myc. After 24 h of transfection, the transfected cells were incubated with PS beads at 37°C for 90 min. They were fixed, permeabilized with digitonin, and stained with anti-amphiphysin 1 antibodies (mab 3) (top) and anti-myc antibodies (bottom) and Alexa488-phalloidin. Arrowheads indicate the incorporated beads surrounded by amphiphysin 1 and polymerized actin. Bar, 5 μm. (B and C) The association of beads with the Ser-W3 cells is PS and SR-BI dependent. Cells (1 × 104 cells on coverslips) were pretreated with 0.25 mM liposomes containing PS or PC at 37°C for 10 min. Then, cells were incubated in presence of beads coated with PS- or PC-liposomes at 37°C for 180 min. The cells were then washed, fixed, stained with DAPI, and analyzed by phase contrast microscopy. To block the binding of PS receptors to PS beads, cells were pretreated with anti-SR-BI antibodies or rabbit IgG as negative control at 100 μg/ml for 30 min (B). Bar, 10 μm. The number of beads associated with the cells was determined using phase contrast microscopy, and it is presented as the number of the beads per cell. To block the internalization, cells were pretreated with Cyt D at 2 μM for 30 min, and attached beads to the cells were analyzed. One hundred cells in 10 independent fields were counted in each experiment. All results are the mean ± SEM from three experiments. Statistical significance was determined by Student's t tests (**p < 0.01) (C).
PS-Liposomes Induce Amphiphysin 1-positive Peripheral Ruffles
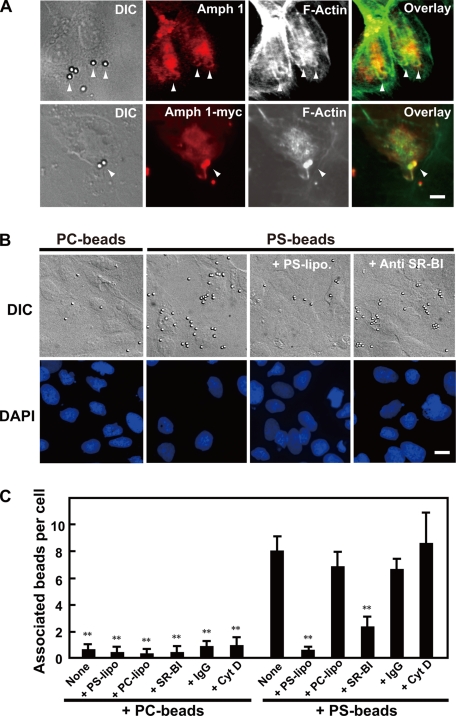
Phagocytosis requires actin polymerization, which causes dynamic membrane remodeling to form the characteristic membrane ruffling and pseudopods (Caron and Hall, 1998; Anderem and Underhill, 1999). We examined ruffle formation in PS liposome-stimulated Sertoli cells. The ruffles were readily observed as actin-rich protrusions at the cell periphery (Suetsugu et al., 2003). Approximately half of the Ser-W3 cells formed ruffles upon liposomal stimulation (Figures 3 and 4C). Vertical sectioning revealed that both amphiphysin 1 and the actin filaments were colocalized at the tip of leading edge of the ruffles (Figure 3A). In contrast, PC liposomes had no effect on cellular membranes (data not shown). Time-lapsed observations of amphiphysin 1-GFP revealed that amphiphysin 1-positive peripheral ruffles were visible within 60 s after the application of the PS liposomes. These peripheral ruffles occasionally bent up and moved backward as described previously (Figure 3B; Suetsugu et al., 2003).
Figure 3.
PS-liposomes induce accumulation of amphiphysin 1 at ruffles in Sertoli cells. (A) Ser-W3 cells (1 × 104 cells/coverslip) were incubated with 0.25 mM PS liposomes at 37°C for 10 min, and then they were fixed, permeabilized, and stained with anti-amphiphysin 1 antibodies (mab3) (left) and Alexa488-phalloidin (middle). Vertical sections along a line in the overlay image (right) are shown in the bottom panels. The length of the z-axis is expanded by four times. Arrowheads indicate the ruffles. Note the colocalization of amphiphysin 1 and F-actin at the ruffles in stimulated cells. (B) Sequential images of Ser-W3 cells expressing amphiphysin 1-GFP under PS liposome stimulation are shown. After Ser-W3 cells were transfected for 24 h with amphiphysin 1-GFP, they were stimulated with PS liposomes, as described in A. Amphiphysin 1 accumulated at the peripheral membrane ruffles that originated at the lamellipodial edge (arrowheads at 150 s). Vertical sections along with lines are shown in the bottom panel. In the vertical sections, the length is expanded by 4 times.
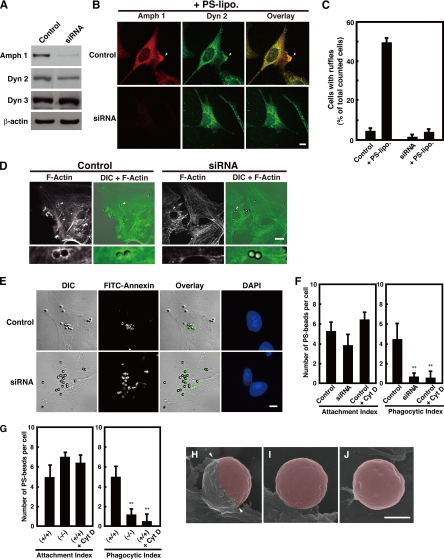
Figure 4.
Amphiphysin 1 is required for PS-stimulated membrane ruffling, actin polymerization and phagocytosis. (A) Control siRNA or siRNA for amphiphysin 1 was transfected into Ser-W3 cells. After 48 h, cells were lysed, and 20 μg of lysate was subject to Western blotting by using anti-amphiphysin 1 antibodies (mab3). Dynamin 2, dynamin 3, and β-actin were used as the controls. (B) Control cells and cells treated with amphiphysin 1 siRNA were stimulated with PS liposomes at 0.25 mM at 37°C for 10 min. Cells were labeled with anti-amphiphysin 1 (mab 3) (left) or dynamin 2 (middle) antibodies. The samples were analyzed by fluorescent confocal microscopy. Bar, 10 μm. (C) The number of cells, which were positive for ruffles, was determined and expressed as a percentage of the total number of cells analyzed. One hundred cells in 10 independent fields were counted in each experiment. All results represent the mean ± SEM from the three experiments. (D) Amphiphysin 1 RNAi inhibits actin polymerization during phagocytosis. Control cells or siRNA-treated cells were incubated with PS beads at 37°C for 90 min. Cells were labeled with Alexa488-phalloidin. The samples were analyzed by phase contrast and fluorescent confocal microscopy. PS beads are indicated by arrowheads and are shown at high magnification (bottom). F-actin is observed around the bound beads in control cells (left). The actin polymerization in siRNA-treated cells was barely seen (right). Control cells displayed actin polymerization with ∼67.5 ± 10.7% of the associated beads. In contrast, the polymerization occurred with only 10.2 ± 4.0% of associated beads in siRNA-treated cells. (For both treatments, n = 90 cells, from three independent experiments). Bar, 10 μm. (E) Effect of RNAi on amphiphysin 1 in PS-dependent phagocytosis. Control cells and siRNA-treated cells were incubated with PS beads for 6 h at 37°C, and then washed. Control cells were pretreated with Cyt D at 2 μM for 30 min as negative control. Cells were incubated with FITC-annexin at room temperature for 10 min before fixation to determine the level of bead internalization. Bar, 10 μm. (F) Quantitation of levels of phagocytosis in control cells or siRNA-treated cells. Annexin-positive and -negative beads were counted on the cell surface by using phase contrast and fluorescent confocal microscopy. The number of internalized beads was determined in 50 randomly chosen cells from 10 independent fields. Phagocytic index was quantified as the number of annexin-negative beads per cell. The attachment index was determined by counting the number of annexin-positive beads per cell. All results are reported as means ± SEM from three experiments. Statistical significance was determined by Student's t tests (**p < 0.01). (G) Phagocytic activity in the amph 1 (+/+) or amph 1 (−/−) primary cultured Sertoli cells. Cells were processed for phagocytic activity as described in F. Cells from amph 1 (+/+) were pretreated with Cyt D for 30 min at 32°C as negative control. Results are reported as means ± SEM from three experiments. Statistical significance was determined by Student's t tests (**p < 0.01). (H–J) We performed scanning electron microscopy to determine PS-dependent phagocytosis in primary cultured mouse Sertoli cells. Sertoli cells from amph 1 (+/+) or amph 1 (−/−) mice were fixed after 90 min of incubation with PS beads and then imaged. Cells from amph 1 (+/+) were pretreated with Cyt D for 30 min at 32°C as negative control. In amph 1 (+/+) Sertoli cells, phagocytic cups were evident (H, arrowheads). In contrast, the amph 1 (−/−) Sertoli cells displayed incomplete membrane extension and demonstrated low abilities to engulf the beads (I). Similarly, phagocytic cup formation was not observed in Cyt D-treated wild-type Sertoli cells (J). In this image, beads were highlighted using Adobe Photoshop CS2. Bar, 1.5 μm.
To test the possibility that amphiphysin 1 might function in ruffle formation, endogenous amphiphysin 1 was knocked down in Ser-W3 cells by RNAi, and the cells were studied for both PS-dependent membrane changes and phagocytosis. The expression of amphiphysin 1 was decreased by ∼95% by the RNAi. The expression of dynamin 2 was decreased by ∼20%, and expression of dynamin 3 and β-actin was unaffected (Figure 4A). Depletion of amphiphysin 1 in Sertoli cells by RNAi caused smaller cell shape and slight cell detachment. In the siRNA-treated Sertoli cells, both localization of clathrin and transferrin uptake were unchanged, suggesting that depletion of amphiphysin 1 does not affect on clathrin-mediated endocytosis (Supplemental Figure 2). Stimulation of control cells with PS liposomes for 10 min resulted in the formation of apparent ruffles, which accumulated amphiphysin 1 and dynamin 2 (Figure 4B), whereas ruffle formation in the siRNA-treated cells decreased by ∼90% of control (Figure 4C). Transfection of amphiphysin 1 cDNA into siRNA-treated cells restored the ruffle formation (Supplemental Figure 3).
We then examined actin polymerization during uptake of the PS beads. In the control cells, PS beads were associated with the membranes, and they were surrounded by F-actin. In contrast, F-actin was present much less around the beads in the siRNA-treated cells (Figure 4D). As expected, PS-dependent phagocytosis in siRNA-treated cells was reduced to 15% of the control (Figure 4, E and F).
The role of amphiphysin 1 in PS-dependent phagocytosis was also confirmed using primary cultured Sertoli cells from amph 1 (−/−) mice. In the amph 1 (−/−) cells, the number of PS beads attached on the cell surface was unchanged compared with that in the wild type, but uptake of the beads was decreased by 80% in amph 1 (−/−) cells (Figure 4G). Electron scanning microscopy revealed that wild-type Sertoli cells engulfed the PS beads through membrane extension (Figure 4H). In contrast, PS beads attached to amph 1 (−/−) Sertoli cells were rarely engulfed by the plasma membrane (Figure 4I). Similarly phagocytic cup formation was not observed in Cyt D-treated wild-type Sertoli cells (Figure 4J). These results indicate that amphiphysin 1 is required for the actin polymerization that causes membrane deformation at the early stage of PS-dependent phagocytosis.
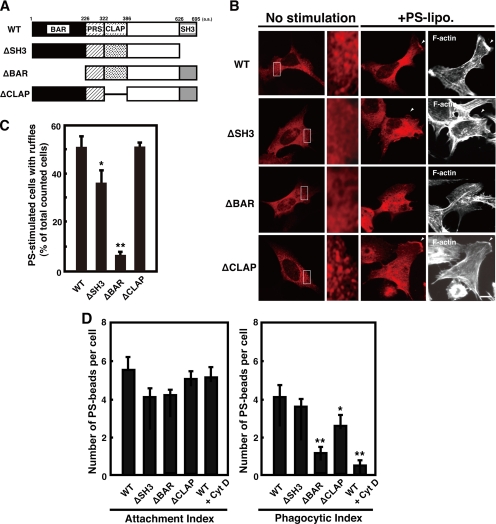
Overexpression of BAR Deletion Mutant Inhibits the Membrane Ruffling and Phagocytosis
We hypothesized that a functional domain of amphiphysin 1 is required for the ruffle formation and phagocytosis. To test this, we used amphiphysin deletion mutants shown in Figure 5A. Wild-type and ΔCLAP amphiphysin 1 transiently expressed in Ser-W3 cells were present as punctate pattern by immunofluorescence, whereas ΔBAR and ΔSH3 were diffusely distributed throughout the cytoplasm (Figure 5B). The expression of ΔBAR in the cells strongly inhibited both PS-dependent ruffle formation and phagocytosis (Figure 5, C and D). ΔBAR-expressing cells were unable to form extensions and tended to be smaller than cells expressing the other mutants (Figure 5B). Expression of ΔSH3 inhibited ruffle formation by ∼35% compared with that of control, but the mutant proteins still localized to the ruffles (Figure 5B). Expression of ΔCLAP did not affect ruffle formation but phagocytosis was inhibited by ∼30% (Figure 5, C and D).
Figure 5.
BAR domain of amphiphysin 1 is required for PS-dependent ruffle formation and phagocytosis. (A) Shown are the domain structures of the amphiphysin 1 constructs used in these assays. The numbers indicate the amino acid residues of full-length amphiphysin 1. (B) Effect of the truncated mutants of amphiphysin 1 on PS-dependent ruffle formation. Ser-W3 cells transiently expressing amphiphysin 1-myc, ΔSH3-myc, ΔBAR-myc, or ΔCLAP-myc were stimulated with 0.25 mM of PS liposomes for 10 min at 37°C. The stimulated or untreated cells were then fixed, permeabilized, and labeled with rabbit anti-myc antibodies to detect the expression of wild type (WT) and mutants. Area enclosed with rectangles in the untreated cells is shown at high magnification. F-actin was visualized by Alexa488-phalloidin. Bar, 10 μm. (C) The efficiency of ruffle formation was determined on 50 transfected cells from 10 independent fields as described in Figure 4C. The mean ± SEM of the three independent experiments is plotted. Statistical significance was determined by Student's t tests (*p < 0.05, **p < 0.01). (D) Effect of the truncation mutant of amphiphysin 1 on PS-dependent phagocytosis. Ser-W3 cells transiently expressing the wild type, ΔSH3, ΔBAR, or ΔCLAP mutants were allowed to phagocytose PS beads for 6 h at 37°C. The number of internalized beads was counted in 50 transfected cells randomly chosen from 10 independent fields. The attachment index, defined as beads bound per cell and phagocytic index, defined as beads internalized per cell, was determined as described in Figure 4E. The mean ± SEM of the three independent experiments is plotted. Statistical significance was determined by Student's t tests (*p < 0.05, **p < 0.01).
These results suggest that the BAR domain of amphiphysin 1 is essential for ruffle formation and phagocytosis, whereas the SH3 domain and the CLAP domain may have regulatory functions at different steps of PS-dependent phagocytosis.
Actin Polymerization Induced by Liposomes Is Decreased in Amph 1 (−/−) Cytosol
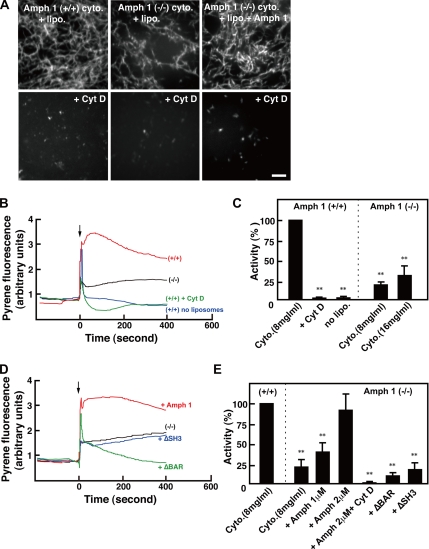
Because amphiphysin 1 RNAi and the expression of the amphiphysin mutants partly inhibited ruffle formation and phagocytosis, we examined the effect of amphiphysin 1 on PI(4,5)P2-dependent actin polymerization using testis cytosol prepared from amph 1 (+/+) or amph 1 (−/−) mice. Actin polymerization in the cytosol can be induced by incubation of the cytosol with liposomes containing PI(4,5)P2 or phosphatidylinositol-3,4,5-trisphosphate (Ma et al., 1998a,b).
Actin polymerization of amph 1 (+/+) and amph 1 (−/−) cytosol was visualized by using rhodamine-actin. When liposomes containing PI(4,5)P2 were added to amph 1 (+/+) cytosol, F-actin started to appear as bright spots within 5 min, and massive actin bundles were visible after 15 min (Figure 6A). The polymerization was abolished by pretreatment of the cytosol with Cyt D or by absence of the liposomes (Figure 6A; data not shown). Not surprisingly, there was less actin polymerization in the amph 1 (−/−) cytosol (Figure 6A). Supplementation of the amph 1 (−/−) cytosol with recombinant amphiphysin 1 recovered actin polymerization to levels comparable with the amph 1 (+/+) cytosol (Figure 6A).
Figure 6.
PI(4,5)P2 induced actin polymerization is reduced with amph (−/−) testis cytosol. (A) Actin polymerization was monitored by a visual assay in which 1 μM rhodamine-actin was used to follow actin polymerization by fluorescent confocal microscopy. The cytosol of amph 1 (+/+) and amph 1 (−/−) testis at a concentration of 20 mg/ml was prepared. Control cytosol (top) or cytosol pretreated with 10 μM (Cyt D) for 5 min (bottom) were mixed with liposomes composed of 30% PS, 46% PC, 20% cholesterol, and 4% PI(4,5)P2. To determine the recovery of activity, 2 μM recombinant amphiphysin 1 was added to the cytosol before mixing. After 15 min of incubation at room temperature, the mixtures were analyzed by fluorescent confocal microscopy. Bar, 10 μm. (B) The liposomes-induced actin polymerization was measured using pyrene fluorescence. Cytosol from amph 1 (+/+) testis (red line) or amph 1 (−/−) testis (black line) were treated with 50 μM PS/PC/Chol liposomes containing 4% of PI(4,5)P2. As controls, the incubation was carried out in presence of 10 μM Cyt D (green line), or in absence of liposomes (blue line). The arrow indicates the time when the liposomes and cytosol were added. (C) Comparison of the actin polymerization activity between amph 1 (+/+) and amph 1 (−/−) cytosol is shown. To avoid effect of the initial peak, activity of F-actin formation at 100 s was measured by pyrene-actin fluorescent intensity. All activities are normalized to that of the amph 1 (+/+) testis cytosol. The mean ± SEM of three independent experiments is plotted. Note the reduced actin polymerization in amph 1 (−/−) cytosol compared with the polymerization in the amph 1 (+/+) cytosol. Statistically significant differences from the value of Amph (+/+) cytosol are indicated by (**p < 0.01) (Student's t test). (D) The liposomes-induced actin polymerization measured by pyrene fluorescence demonstrated the recovery of F-actin formation by recombinant amphiphysin. Amph 1 (−/−) cytosol (black line) was supplemented with 2 μM recombinant full-length amphiphysin 1 (red line), with ΔSH3 mutant (blue line) or with ΔBAR mutant (green line). The incubation and the measurement of activity were carried out as described in B. The arrow indicates the time when the lipids and the cytosol were added. (E) F-actin formation after 100 s induction in amph 1 (−/−) cytosol treated with liposomes containing PI(4,5)P2 alone, with recombinant full-length amphiphysin 1, with ΔSH3 mutant or with ΔBAR mutant was measured by pyrene-actin fluorescence. The recombinant mutant proteins in the mixture were at a concentration of 2 μM. The incubation with 2 μM recombinant full-length amphiphysin 1 was carried out in presence of 10 μM Cyt D as a control. All activities are normalized to that in amph (+/+) cytosol. The mean ± SEM of three independent experiments is plotted. Statistically significant differences from the value of amph (+/+) cytosol are indicated by (**p < 0.01) (Student's t test).
To determine the actin assembly in a quantitative manner, we monitored the activity with pyrene-conjugated actin and polymerization-derived fluorescence. Consistent with the visual polymerization assay described above, rapid actin assembly was induced by the liposomes in amph 1 (+/+) cytosol (Figure 6, B and C). The polymerization did not occur in the absence of the liposomes or with pretreatment of the cytosol with Cyt D (Figure 6, B and C). Under the same conditions, the actin polymerization in the amph 1 (−/−) cytosol was ∼25% of that in amph 1 (+/+) cytosol (Figure 6, B and C). The addition of recombinant amphiphysin 1 to amph 1 (−/−) cytosol was sufficient to recover polymerization activity (Figure 6, D and E). However, the addition of ΔSH3 and ΔBAR proteins could not recover the actin polymerization in amph 1 (−/−) cytosol (Figure 6, D and E). These results suggested that amphiphysin 1 regulates actin polymerization and that the regulation requires for both BAR and SH3. Addition of ΔBAR protein to amph 1 (−/−) cytosol even decreased F-actin formation, suggesting the mutant might depolymerize or destabilize F-actin (Figure 6, D and E).
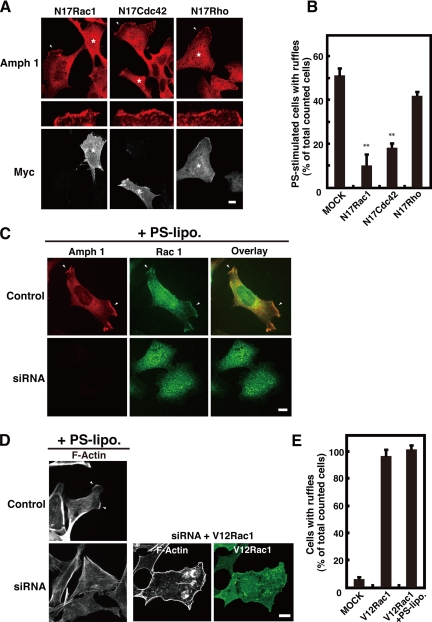
Pathway of Amphiphysin-positive Ruffle Formation May Be Involved in Rac
It is well known that actin polymerization and ruffle formation are stimulated by the activation of the Rho GTPase family (Ridley, 2001; Cox and Greenberg, 2001). We hypothesized that amphiphysin 1 may be involved in the process of Rho GTPase family-dependent ruffle formation. As shown in Figure 7A and B, expression of dominant-negative Rac1 or Cdc42 (N17Rac1 or N17Cdc42) blocked PS-induced ruffle formation by 85 and 65%, respectively. Dominant-negative Rho slightly inhibited PS-dependent ruffle formation. Furthermore, endogenous Rac 1 and amphiphysin 1 colocalized to the ruffles in the PS-stimulated Ser-W3 cells (Figure 7C). Amphiphysin 1 siRNA treatment inhibited both the recruitment of Rac1 to the membrane and ruffle formation in the PS-stimulated cells (Figure 7C). The overexpression of V12Rac1, a constitutively active mutant of Rac1, in the siRNA-treated cells caused the recovery of ruffle formation (Figure 7, D and E). These results strongly suggest that amphiphysin 1 is involved in Rac1-dependent actin polymerization and that it may function upstream of this protein.
Figure 7.
Rac1 is involved in amphiphysin 1-positive ruffle formation. (A) Effect of dominant-negative mutant of Rac1 or Cdc42 on amphiphysin 1-positive ruffle formation. Ser-W3 cells were transfected with myc-N17Rac, -N17Cdc42, or -N17Rho. After 24 h of transfection, cells were stimulated with 0.25 mM of PS liposomes for 10 min, fixed, permeabilized, and stained with rabbit anti-myc antibodies, anti-amphiphysin 1 antibodies (mab 3). Asterisks show where there was expression of mutant Rho GTPases. The ruffles indicated by arrowheads are enlarged on the middle panels. Note that the expression of N17Rac1 strongly inhibited PS-stimulated membrane ruffling (left). Transfection of N17Cdc42 also caused inhibition on ruffle formation in response to PS-stimulation (middle). N17Rho-transfected cells showed a slight inhibition of membrane ruffling (right). Bar, 10 μm. (B) The number of ruffle-positive cells that expressed mutant small G proteins was counted as described in Figure 4C. Fifty cells expressing each small G protein mutant were analyzed in 10 independent fields. All results represent the mean ± SEM from three experiments. Statistically significant differences are indicated by (**p < 0.01) (Student's t test). (C) RNAi for amphiphysin 1 impaired the recruitment of endogenous Rac1 to the plasma membrane in Ser-W3 cells. SiRNA-treated (bottom) or control Ser-W3 (top) cells were incubated with 0.25 mM PS liposomes at 37°C for 10 min. The cells were stained with both mouse-anti-amphiphysin 1 antibodies (mab 3) (left) and rabbit-anti-Rac1 antibodies (middle). Bar, 10 μm. (D) Constitutively active Rac1 rescued ruffle formation in amphiphysin 1 siRNA-treated Ser-W3 cells. After 48-h transfection with siRNA for amphiphysin 1 or control siRNA, myc-V12Rac1 (middle) or vector only (left) was later transfected into the cells. Cells were stimulated as described in Figure 7A and stained with rabbit-anti-myc antibodies (right) and rhodamine-phalloidin (middle). Bar, 10 μm. (E) The number of ruffle-positive cells expressing myc-V12Rac1 proteins, with or without PS-stimulation, was counted as described in Figure 4C. Fifty cells expressing each small G protein mutant were analyzed in 10 independent fields. All results represent the mean ± SEM from three experiments.
DISCUSSION
In this study, we investigated the role of amphiphysin 1 in Sertoli cell phagocytosis by using knockout animals and cell culture. We demonstrated that amphiphysin 1 is required for PS-dependent phagocytosis in Sertoli cells and that the protein is crucial for actin polymerization during the process. To our knowledge, this is the first indication that amphiphysin 1 regulates actin dynamics.
Amphiphysin 1 Is Involved in PS-dependent Phagocytosis in Sertoli Cells
Phagocytosis in Sertoli cells contributes to removal of the residual bodies, which occur just before sperm release (Clermont et al., 1987). In amph 1 (−/−) testis, the residual bodies were prominent, suggesting that the phagocytic process is decreased. Consistent with this finding, the numbers of unreleased spermatids were increased in amph 1 (−/−) testis (our unpublished data).
On PS-stimulation of cultured Sertoli cells, amphiphysin 1 accumulated at F-actin–rich structures such as phagocytic cups, phagosomes, or ruffles. Formation of these structures was abolished by amphiphysin 1 RNAi, causing a decrease in phagocytic activity. These results clearly show that amphiphysin 1 is essential for phagocytosis and suggest a role of the molecule in actin dynamics. Although amphiphysin 1 functions in clathrin-mediated endocytosis in the neuronal synapse (Takei et al., 1999), both transferrin uptake and clathrin localization in Sertoli cells were unchanged by RNAi (Supplemental Figure 2), indicating that the main cellular function of amphiphysin 1 in the Sertoli cells is phagocytosis.
We demonstrated that the BAR domain of amphiphysin 1 is required for both PS-dependent ruffle formation and for phagocytosis. It has been reported that BAR domain is responsible for homo- or heterodimerization of amphiphysins (Wigge et al., 1997; Friesen et al., 2006), sensing membrane curvature, (Peter et al., 2004; Yoshida et al., 2004), and membrane deformation (Takei et al., 1999). These characteristics of BAR domain might be required for ruffle formation and phagocytosis.
It is possible that inhibition of ruffle formation by ΔBAR mutant is indirect. Because ΔSH3 mutant, which contains BAR domain partially inhibited the ruffle formation, suggesting that binding molecules via SH3 domain may be involved. In addition, because ΔSH3 mutant still localized at ruffles, the mutant may form partially functional dimer with endogenous amphiphysin 1. ΔBAR mutant contains proline-rich stretch (PRS), CLAP and SH3, which can bind to functional proteins required for ruffle formation. In particular, the SH3 domain of amphiphysin 1 interacts with dynamin 2, which has been suggested to be involved in formation of membrane ruffling (McNiven et al., 2000; Schafer, 2004). In this study, expression of dynamin 2 was decreased by 20% in amphiphysin 1 knocked down cells. Because amphiphysin 1 binds to dynamin 2, some of free dynamin 2 may be degraded in the absence of amphiphysin 1. It is unlikely, however, that the decrease of dynamin 2 contributes to the inhibition of ruffle formation. Because exogenous amphiphysin 1 rescued ruffle formation in amphiphysin 1 depleted Sertoli cells. Furthermore, clathrin-mediated endocytosis was not changed in siRNA-treated cells, suggesting that dynamin 2 is still functional in the cell (Supplemental Figure 3).
ΔCLAP inhibited by ∼30% of PS-dependent phagocytosis. It has been reported that clathrin accumulates at nascent phagosomes to assist in the recycle of membranes and receptors (Aggeler and Werb, 1982), and the CLAP domain may contribute to this process. Amphiphysin 2m, an isoform lacking a CLAP domain, has known functions in phagocytosis in macrophages (Gold et al., 2000), suggesting the presence of different phagocytic pathway in this cell type.
Amphiphysin 1 Regulates Actin Polymerization
Phagocytosis involves actin dynamics regulated by the Rho GTPase family (Cox et al., 1997; Tolias et al., 2000; Bokoch, 2005). In the ruffling area, an increased production of PI(4,5)P2 activates the Rho GTPase family proteins, such as Cdc42 or Rac (Czech, 2000). These in turn stimulate actin polymerization by the Arp 2/3 complex and the WASP family (Chimini and Chavrier, 2000). As shown in Figure 6, PI(4,5)P2-induced F-actin formation was significantly reduced in the amph 1 (−/−) cytosol. This effect was restored by the addition of recombinant full-length amphiphysin 1, but not by ΔBAR or ΔSH3, suggesting that BAR and SH3 domain are necessary for recruitment of amphiphysin 1 to membrane, and for recruitment of its binding proteins required for F-actin formation, respectively.
It has been reported that dynamin 2 is involved in actin dynamics as well as ruffle formation (Kruchten and McNiven, 2006). Dynamin 2 binds to cortactin, which specifically binds to F-actin (McNiven et al., 2000; Schafer, 2004). Furthermore, dynamin 2 interacts with Rac1 and has been implicated both in the formation of ruffles (Krueger et al., 2003) and in the regulation of the localization of intracellular Rac1 (Schlunck et al., 2004). In addition, Drosophila amphiphysin interacts with neural Wiskott-Aldrich syndrome protein, and this interaction is necessary for the morphogenesis of rhabdomere microvilli (Zelhof and Hardy, 2004). Thus, amphiphysin 1 may indirectly function on actin dynamics.
The dominant-negative mutant of Rac1 or Cdc42 abolished the formation of amphiphysin 1-positive ruffles. In addition, amphiphysin 1 RNAi inhibited ruffle formation, but constitutively active Rac1 expression in the siRNA-treated cells rescued it, indicating that amphiphysin 1 functions upstream of Rac1. Consistently, amphiphysin 1 is known to bind to the proline-rich domains of RICH-1, a RhoGAP protein (Richnau et al., 2004). Additionally, direct binding of the yeast amphiphysin homologue RVS to actin has been shown in a yeast two-hybrid system (Amberg et al., 1995).
In conclusion, we have demonstrated that amphiphysin 1 participates in phagocytosis by changing actin polymerization activity in Sertoli cells. Amphiphysin 1 is implicated in the signaling pathway that involves Rac1 and Cdc42. The precise role of amphiphysin 1 in the actin–regulatory signal cascade should to be elucidated with further studies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Pietro De Camilli (Yale University) for providing amphiphysin 1 knockout mice and Toshiki Ito (Kobe University) for providing the plasmids pEF-BOS-myc-N17Rac1, V12Rac1, N17Cdc42, and N17Rho. This work was supported in part by grants from the Ministry of Education, Science, Sports, and Culture of Japan, the Nissan Science Foundation, the Asahi Glass Foundation, and the Wesco Scientific Promotion Foundation.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-04-0296) on September 12, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Abe K., Shen L. S., Takano H. The cycle of the seminiferous epithelium and stages in spermatogenesis in dd-mice. Hokkaido Igaku Zasshi. 1991;66:286–299. [PubMed] [Google Scholar]
- Aggeler J., Werb Z. Initial events during phagocytosis by macrophages viewed from outside and inside the cell: membrane-particle interactions and clathrin. J. Cell Biol. 1982;94:613–623. doi: 10.1083/jcb.94.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg D. C., Basart E., Botstein D. Defining protein interactions with yeast actin in vivo. Nat. Struct. Biol. 1995;2:28–35. doi: 10.1038/nsb0195-28. [DOI] [PubMed] [Google Scholar]
- Anderem A., Underhill D. M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Bauerfeind R., Takei K., DeCamilli P. Amphiphysin I is associated with coated endocytic intermediates and undergoes stimulation-dependent dephosphorylation in nerve terminals. J. Biol. Chem. 1997;272:30984–30992. doi: 10.1074/jbc.272.49.30984. [DOI] [PubMed] [Google Scholar]
- Beardsley A., O'Donnell L. Characterization of normal spermiation and spermiation failure induced by hormone suppression in adult rats. Biol. Reprod. 2003;68:1299–1307. doi: 10.1095/biolreprod.102.009811. [DOI] [PubMed] [Google Scholar]
- Blanco-Rodriguez J., Martinez-Garcia C. Apoptosis is physiologically restricted to a specialized cytoplasmic compartment in rat spermatids. Biol. Reprod. 1999;61:1541–1547. doi: 10.1095/biolreprod61.6.1541. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M. Regulation of innate immunity by Rho GTPases. Trends. Cell Biol. 2005;15:163–171. doi: 10.1016/j.tcb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Caron E., Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- Chemes H. The phagocytic function of Sertoli cells: a morphological, biochemical, and endocrinological study of lysosomes and acid phosphatase localization in the rat testis. Endocrinology. 1986;119:1673–1681. doi: 10.1210/endo-119-4-1673. [DOI] [PubMed] [Google Scholar]
- Chimini G., Chavrier P. Function of Rho family proteins in actin dynamics during phagocytosis and engulfment. Nat. Cell Biol. 2000;2:E191–E196. doi: 10.1038/35036454. [DOI] [PubMed] [Google Scholar]
- Clermont Y., Morales C., Hermo L. Endocytic activities of Sertoli cells in the rat. Ann. N Y Acad. Sci. 1987;513:1–15. doi: 10.1111/j.1749-6632.1987.tb24994.x. [DOI] [PubMed] [Google Scholar]
- Cox D., Chang P., Zhang Q., Reddy P. G., Bokoch G. M., Greenberg S. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J. Exp. Med. 1997;186:1487–1494. doi: 10.1084/jem.186.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D., Greenberg S. Phagocytic signaling strategies: Fc (gamma) receptor-mediated phagocytosis as a model system. Semin. Immunol. 2001;13:339–345. doi: 10.1006/smim.2001.0330. [DOI] [PubMed] [Google Scholar]
- Czech M. P. PIP2 and PIP3: complex roles at the cell surface. Cell. 2000;100:603–606. doi: 10.1016/s0092-8674(00)80696-0. [DOI] [PubMed] [Google Scholar]
- David C., McPherson P. S., Mundigl O., DeCamilli P. A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc. Natl. Acad. Sci. USA. 1996;93:331–335. doi: 10.1073/pnas.93.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J. C., Legg J. A., Machesky L. M. Bar domain proteins: a role in tubulation, scission and actin assembly in clathrin-mediated endocytosis. Trends Cell Biol. 2006;16:493–498. doi: 10.1016/j.tcb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Diakonova M., Bokoch G., Swanson J. A. Dynamics of cytoskeletal proteins during Fcgamma receptor-mediated phagocytosis in macrophages. Mol. Biol. Cell. 2002;13:402–411. doi: 10.1091/mbc.01-05-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G., et al. Decreased synaptic vesicle recycling efficiency and cognitive deficits in amphiphysin 1 knockout mice. Neuron. 2002;33:789–804. doi: 10.1016/s0896-6273(02)00601-3. [DOI] [PubMed] [Google Scholar]
- Filippini A., Russo M. A., Palombi F., Bertalot G., De Cesaris P., Stefanini M., Ziparo E. Modulation of phagocytic activity in cultured Sertoli cells. Gamate Res. 1989;23:367–375. doi: 10.1002/mrd.1120230402. [DOI] [PubMed] [Google Scholar]
- Friesen H., Humphrries C., Ho Y., Schub O., Colwill K., Andrews B. Characterization of the yeast amphiphysin Rvs161p and Rvs167p reveals roles for the Rvs heterodimer in vivo. Mol. Biol. Cell. 2006;17:1306–1321. doi: 10.1091/mbc.E05-06-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusaro G., Dasgupta P., Rastogi S., Joshi B., Chellappan S. Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling. J. Biol. Chem. 2003;278:47853–47861. doi: 10.1074/jbc.M305171200. [DOI] [PubMed] [Google Scholar]
- Gillot I., Jehl-Pietri C., Gounon P., Luquet S., Rassoulzadegan M., Grimaldi P., Vidal F. Germ cells and fatty acids induce translocation of CD36 scavenger receptor to the plasma membrane of Sertoli cells. J. Cell Sci. 2005;118:3027–3035. doi: 10.1242/jcs.02430. [DOI] [PubMed] [Google Scholar]
- Gold E. S., Morrissette N. S., Underhill D. M., Guo J., Bassetti M., Anderem A. Amphiphysin IIm, a novel amphiphysin II isoform, is required for macrophage phagocytosis. Immunity. 2000;12:285–292. doi: 10.1016/s1074-7613(00)80181-8. [DOI] [PubMed] [Google Scholar]
- Greenberg S., Grinstein S. Phagocytosis and innate immunity. Curr. Opin. Immunol. 2002;14:136–145. doi: 10.1016/s0952-7915(01)00309-0. [DOI] [PubMed] [Google Scholar]
- Gu X., Kozarsky K., Krieger M. Scavenger receptor class B, type I-mediated [3H]cholesterol efflux to high and low density lipoproteins is dependent on lipoprotein binding to the receptor. J. Biol. Chem. 2000;275:29993–30001. doi: 10.1074/jbc.275.39.29993. [DOI] [PubMed] [Google Scholar]
- Kaksonen M., Toret C. P., Drubin D. G. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123:305–320. doi: 10.1016/j.cell.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Kamitani A., Yamada H., Kinuta M., Watanabe M., Li S. A., Matsukawa T., McNiven M. A., Kumon H., Takei K. Distribution of dynamins in testis and their possible relation to spermatogenesis. Biochem. Biophys. Res. Commun. 2002;294:261–267. doi: 10.1016/S0006-291X(02)00470-9. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y., Nakagawa A., Nagaosa K., Shiratsuchi A., Nakanishi Y. Phosphatidylserine binding of class B scavenger receptor type I, a phagocytosis receptor of testicular Sertoli cells. J. Biol. Chem. 2002;277:27559–27566. doi: 10.1074/jbc.M202879200. [DOI] [PubMed] [Google Scholar]
- Krauss M., Kinuta M., Wenk M. R., DeCamilli P., Takei K., Haucke V. ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Iγ. J. Cell Biol. 2003;162:113–124. doi: 10.1083/jcb.200301006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruchten A. E., McNiven M. A. Dynamin as a mover and pincher during cell migration and invasion. J. Cell Sci. 2006;119:1683–1690. doi: 10.1242/jcs.02963. [DOI] [PubMed] [Google Scholar]
- Krueger E. W., Orth J. D., Cao H., McNiven M. A. A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol. Biol. Cell. 2003;14:1085–1096. doi: 10.1091/mbc.E02-08-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L. E., Rohatgi R., Kirschner M. W. The Arp2/3 complex mediates actin polymerization induced by the small GTP-binding protein Cdc42. Proc. Natl. Acad. Sci. USA. 1998a;95:15362–15367. doi: 10.1073/pnas.95.26.15362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L. E., Cantley L. C., Janmey P. A., Kirschner M. W. Corequirement of specific phosphoinositides and small GTP-binding protein Cdc42 in inducing actin assembly in Xenopus egg extracts. J. Cell Biol. 1998b;140:1125–1136. doi: 10.1083/jcb.140.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiven M. A., Kim L., Krueger E. W., Orth J. D., Cao H., Wong T. W. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J. Cell Biol. 2000;151:187–198. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales C. R., Clermont Y. Phagocytosis and endocytosis in Sertoli cells of the rat. Bull. Assoc. Anat. 1991;75:157–162. [PubMed] [Google Scholar]
- Mundigl O., Ochoa G. C., David C., Slepnev V. I., Kabanov A., DeCamilli P. Amphiphysin I antisense oligonucleotides inhibit neurite outgrowth in cultured hippocampal neurons. J. Neurosci. 1998;18:93–103. doi: 10.1523/JNEUROSCI.18-01-00093.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn A. L., Stevenson B. J., Geli M. I., Riezman H. end5, end6, and end 7, mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell. 1995;6:1721–1742. doi: 10.1091/mbc.6.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedergang F., Chavrier P. Signaling and membrane dynamics during phagocytosis: many roads lead to the phagos(R)ome. Curr. Opin. Cell Biol. 2004;16:422–428. doi: 10.1016/j.ceb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Oakberg E. F. A description of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am. J. Anat. 1956;99:391–413. doi: 10.1002/aja.1000990303. [DOI] [PubMed] [Google Scholar]
- Peter B. J., Kent H. M., Mills I. G., Vallis Y., Butler P. J., Evans P. R., McMahon H. T. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- Prognan F., Masson M. T., Lagelle F., Charuel C. Establishment of a rat Sertoli cell line that displays the morphological and some of the functional characteristics of the native cell. Cell Biol. Toxicol. 1997;13:453–463. doi: 10.1023/a:1007475928452. [DOI] [PubMed] [Google Scholar]
- Richnau N., Fransson A., Farsad K., Aspenstrom P. RICH-1 has a BIN/Amphiphysin/Rvsp domain responsible for binding to membrane lipids and tubulation of liposomes. Biochem. Biophys. Res. Commun. 2004;320:1034–1042. doi: 10.1016/j.bbrc.2004.05.221. [DOI] [PubMed] [Google Scholar]
- Ridley A. J. Rho family proteins: coordinating cell responses. Trends Cell Biol. 2001;11:471–477. doi: 10.1016/s0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- Russell L. D., Ren H. P., Sinha Hikim I., Schulze W., Sinha Hikim A. P. A comparative study in twelve mammalian species of volume densities, volumes, and numerical densities of selected testis components, emphasizing those related to the Sertoli cell. Am. J. Anat. 1990;188:21–30. doi: 10.1002/aja.1001880104. [DOI] [PubMed] [Google Scholar]
- Schafer D. A. Regulating actin dynamics at membranes: a focus on dynamin. Traffic. 2004;5:463–469. doi: 10.1111/j.1600-0854.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- Schlunck G., Damke H., Kiosses W. B., Rusk N., Symons M. H., Waterman-Storer C. M., Schmidt S. L., Schwartz M. A. Modulation of Rac localization and function by dynamin. Mol. Biol. Cell. 2004;15:256–267. doi: 10.1091/mbc.E03-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S. L., McNiven M. A., DeCamilli P. Dynamin and its partners: a progress report. Curr. Opin. Cell Biol. 1998;10:504–512. doi: 10.1016/s0955-0674(98)80066-5. [DOI] [PubMed] [Google Scholar]
- Sivadon P., Bauer F., Aigle M., Crouzet M. Actin cytoskeleton and budding pattern are altered in the yeast rvs161 mutant: the Rvs161 protein shares common domains with the brain protein amphiphysin. Mol. Gen. Genet. 1995;246:485–495. doi: 10.1007/BF00290452. [DOI] [PubMed] [Google Scholar]
- Shiratsuchi A., Umeda M., Ohba Y., Nakanishi Y. Recognition of phosphatidylserine on the surface of apoptotic spermatogenic cells and subsequent phagocytosis by Sertoli cells of the rat. J. Biol. Chem. 1997;272:2354–2358. doi: 10.1074/jbc.272.4.2354. [DOI] [PubMed] [Google Scholar]
- Shiratsuchi A., Kawasaki Y., Ikemoto M., Arai H., Nakanishi Y. Role of class B scavenger receptor type I in phagocytosis of apoptotic rat spermatogenic cells by Sertoli cells. J. Biol. Chem. 1999;274:5901–5908. doi: 10.1074/jbc.274.9.5901. [DOI] [PubMed] [Google Scholar]
- Slepnev V. I., Ochoa G. C., Butler M. H., DeCamilli P. Tandem arrangement of the clathrin and AP-2 binding domains in amphiphysin 1 and disruption of clathrin coat function by amphiphysin fragments comprising these sites. J. Biol. Chem. 2000;275:17583–17589. doi: 10.1074/jbc.M910430199. [DOI] [PubMed] [Google Scholar]
- Small J. V., Stradal T., Vignal E., Rotter K. The lamellipodium: where motility begins. Trends Cell Biol. 2002;12:112–120. doi: 10.1016/s0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- Smythe E., Ayscough K. R. Actin regulation in endocytosis. J. Cell Sci. 2006;119:4589–4598. doi: 10.1242/jcs.03247. [DOI] [PubMed] [Google Scholar]
- Suetsugu S., Yamazaki D., Kurisu S., Takenawa T. Differential roles of WAVE1 and WAVE2 in dorsal and peripheral ruffle formation for fibroblast cell migration. Dev. Cell. 2003;5:595–609. doi: 10.1016/s1534-5807(03)00297-1. [DOI] [PubMed] [Google Scholar]
- Tadakuma H., Yamaguchi J., Ishihama Y., Funatsu T. Imaging of single fluorescent molecules using video-rate confocal microscopy. Biochem. Biophys. Res. Commun. 2001;287:323–327. doi: 10.1006/bbrc.2001.5574. [DOI] [PubMed] [Google Scholar]
- Takei K., Slepnev V. I., Haucke V., DeCamilli P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat. Cell Biol. 1999;1:33–39. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]
- Tolias K. F., Hartwig J. H., Ishihara H., Shibasaki Y., Cantley L. C., Carpenter C. L. Type Ialpha phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr. Biol. 2000;10:153–156. doi: 10.1016/s0960-9822(00)00315-8. [DOI] [PubMed] [Google Scholar]
- Tran D., Picard J. Y., Campargue J., Josso N. Immunocytochemical detection of anti-Mullerian hormone in Sertoli cells of various mammalian species including human. J. Histochem. Cytochem. 1987;35:733–743. doi: 10.1177/35.7.3295030. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Tsutsui K., Hosoya O., Tsutsui K., Kumon H., Tokunaga A. Expression of amphiphysin I in Sertoli cells and its implication in spermatogenesis. Biochem. Biophys. Res. Commun. 2001;287:739–745. doi: 10.1006/bbrc.2001.5650. [DOI] [PubMed] [Google Scholar]
- Wigge P., Kohler K., Vallis Y., Doyle C. A., Owen D., Hunt S. P., McMahon H. T. Amphiphysin heterodimers: potential role in clathrin-mediated endocytosis. Mol. Biol. Cell. 1997;8:2003–2015. doi: 10.1091/mbc.8.10.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge P., McMahon H. T. The amphiphysin family of proteins and their role in endocytosis at the synapse. Trends Neurosci. 1998;21:339–344. doi: 10.1016/s0166-2236(98)01264-8. [DOI] [PubMed] [Google Scholar]
- Yoo J., Jeong M. J., Kwon B. M., Hur M. W., Park Y. M., Han M. Y. Activation of dynamin I gene expression by Sp1 and Sp3 is required for neuronal differentiation of N1E-115 cells. J. Biol. Chem. 2002;277:11904–11909. doi: 10.1074/jbc.M111788200. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., et al. The stimulatory action of amphiphysin on dynamin function is dependent on lipid bilayer curvature. EMBO J. 2004;23:3483–3491. doi: 10.1038/sj.emboj.7600355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelhof A. C., Hardy R. W. WASp is required for the correct temporal morphogenesis of rhabdomere microvilli. J. Cell Biol. 2004;164:417–426. doi: 10.1083/jcb.200307048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Liu X., Scherer D. C., van Kaer L., Wang X., Xu M. The 40-kDa subunit of DNA fragmentation factor induces DNA fragmentation and chromatin condensation during apoptosis. Proc. Natl. Acad. Sci. USA. 1998;95:8461–8466. doi: 10.1073/pnas.95.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.