Abstract
Maintenance of intestinal mucosal epithelial integrity requires polyamines that modulate the expression of various genes involved in cell proliferation and apoptosis. Recently, polyamines were shown to regulate the subcellular localization of the RNA-binding protein HuR, which stabilizes its target transcripts such as nucleophosmin and p53 mRNAs. The activating transcription factor-2 (ATF-2) mRNA encodes a member of the ATF/CRE-binding protein family of transcription factors and was computationally predicted to be a target of HuR. Here, we show that polyamines negatively regulate ATF-2 expression posttranscriptionally and that polyamine depletion stabilizes ATF-2 mRNA by enhancing the interaction of the 3′-untranslated region (UTR) of ATF-2 with cytoplasmic HuR. Decreasing cellular polyamines by inhibiting ornithine decarboxylase (ODC) with α-difluoromethylornithine increased the levels of ATF-2 mRNA and protein, whereas increasing polyamines by ectopic ODC overexpression repressed ATF-2 expression. Polyamine depletion did not alter transcription via the ATF-2 gene promoter but increased the stability of ATF-2 mRNA. Increased cytoplasmic HuR in polyamine-deficient cells formed ribonucleoprotein complexes with the endogenous ATF-2 mRNA and specifically bound to 3′-UTR of ATF-2 mRNA on multiple nonoverlapping 3′-UTR segments. Adenovirus-mediated HuR overexpression elevated ATF-2 mRNA and protein levels, whereas HuR silencing rendered the ATF-2 mRNA unstable and prevented increases in ATF-2 mRNA and protein. Furthermore, inhibition of ATF-2 expression prevented the increased resistance of polyamine-deficient cells to apoptosis induced by treatment with tumor necrosis factor-α and cycloheximide. These results indicate that polyamines modulate the stability of ATF-2 mRNA by altering cytoplasmic HuR levels and that polyamine-modulated ATF-2 expression plays a critical role in regulating epithelial apoptosis.
INTRODUCTION
The natural polyamines spermidine and spermine and their diamine precursor putrescine are ubiquitous small basic molecules that are found in all eukaryotic cells and are implicated in many aspects of cellular physiology (Wallace et al., 2003; Casero and Marton, 2007; Wang et al., 1997). Although the concentration of intracellular polyamines is in the millimolar range, free polyamines are considerably less abundant, as they are bound to various cellular anions including DNA, RNA, proteins, and phospholipids (Casero and Marton, 2007; Gerner and Meyskens, 2004). Polyamines are essential for mammalian cell growth and development, and gene disruptions of ornithine decarboxylase (ODC) or S-adenosylmethionine decarboxylase, two key rate-limiting enzymes in polyamine biosynthesis, are lethal at early stages of embryonic development (Pendeville et al., 2001; Nishimura et al., 2002). Our previous studies (Wang et al., 1991a, 1993; Wang and Johnson, 1991; Li et al., 1999, 2001, 2002; Xiao et al., 2007) and others (McCormack and Johnson, 1991; Johnson, 1998; Ray et al., 1999; Seiler and Raul, 2007) showed that normal intestinal mucosal growth depends on the supply of polyamines to the dividing cells in the crypts and that reductions in cellular polyamines inhibit intestinal epithelial cell (IEC) proliferation in vivo as well as in vitro. Polyamines also regulate IEC apoptosis, which occurs in the crypt area to counterbalance the continual proliferation of intestinal epithelial stem cells, and at the luminal surface of the colon and villous tips in the small intestine, where differentiated IECs are lost (Bhattacharya et al., 2004; Zhang et al., 2004; Seiler and Raul, 2005; Zou et al., 2005; Li et al., 2007). Although the specific molecular processes controlled by polyamines are poorly understood, increasing evidence indicates that polyamines regulate intestinal epithelial integrity by modulating the expression of growth-related genes (Patel and Wang, 1997; Gerner and Meyskens, 2004; Liu et al., 2006; Wang, 2007).
Activating transcription factor-2 (ATF-2) belongs to the family of ATF/CREB (CRE-binding protein) transcription factors, which have the b-ZIP-type DNA-binding domain (Shaulian, and Karin, 2001; Papassava et al., 2004). The exact biological role of ATF-2 remains to be identified, but it regulates the transcription of a many genes involved in cytokine synthesis (Tan et al., 2007), cell cycle control (Huguier et al., 1998; Papassava et al., 2004), apoptosis (Fuchs et al., 1998; Bhoumik et al., 2004), and DNA repair (Hayakawa et al., 2003; Bhoumik et al., 2005). The transcriptional activity of ATF-2 requires homo- or heterodimerization with other members of the b-ZIP family of transcription factors such as c-Jun or JunD (Ivashkiv et al., 1990; Livingstone et al., 1995). Both ATF-2/ATF-2 and ATF-2/Jun dimers bind to the cyclic AMP response element (CRE; 5′-TGACGTCA-3′) and direct the transcription of target genes (van Dam et al., 1993; Hai and Curran, 2001). The ATF-2/Jun heterodimers are more potent transcriptional activators for the minimal promoters containing CRE than ATF-2/ATF-2 homodimers (Benbrook et al., 1990; Huguier et al., 1998). Although cellular polyamines have not been shown to regulate ATF-2 expression levels, several studies have found that polyamines modulate the expression of the c-jun, junD, and c-fos genes that encode other members of the b-ZIP family transcription factors (Wang et al., 1993; Wang and Johnson, 1994; Patel and Wang, 1997, 1999; Li et al., 2002; Xiao et al., 2007). Because ATF-2 is ubiquitously expressed in various tissues including the intestinal mucosa and given the fact that ATF-2 critically influences a number of cellular outcomes (Guo et al., 2001; Song et al., 2006), we became interested in studying if ATF-2 expression and ATF-2–elicited regulation of the epithelial integrity were also regulated by polyamines.
Besides regulating transcription, polyamines also potently regulate the stability of mRNAs encoding proteins with critical functions in the intestinal mucosa (Li et al., 2002; Zhang et al., 2007; Zou et al., 2006). For example, depletion of polyamines through specific inhibition of ODC with dl-α-difluoromethylornithine (DFMO) stabilized the mRNAs encoding p53, nucleophosmin, JunD, and transforming growth factor beta (TGF-β; Li et al., 2001a, b, 2002; Liu et al., 2003; Zou et al., 2005) and increased the levels of the corresponding proteins, in turn inhibiting IEC proliferation. mRNA stability is primarily controlled through the association of RNA-binding proteins (RBPs) that specifically bind to U- and AU-rich elements (AREs) located in the 3′-untranslated regions (3′-UTRs) of many labile mRNAs and either increase or decrease their half-life (Brennan and Steitz, 2001; Gorospe, 2003). HuR is a ubiquitously expressed member of the Hu/ELAV (embryonic lethal abnormal vision in Drosophila melanogaster) RBP family and regulates gene expression through binding to mRNAs, which typically bear one or several hits of a recently described ARE-like RNA motif (Brennan and Steitz, 2001; Gorospe, 2003). HuR is predominantly nuclear in unstimulated cells, but translocates to the cytoplasm in response to various stimuli (Mazan-Mamczarz et al., 2003; Heinonen et al., 2005). We recently demonstrated that polyamines modulate the nucleocytoplasmic shuttling of HuR through AMP-activated protein kinase and that depletion of cellular polyamines increases cytoplasmic levels of HuR (Zou et al., 2006).
An en masse search for HuR target mRNAs (Lopez de Silanes et al., 2004) identified the ATF-2 mRNA as a putative HuR target and computationally detected several hits of the HuR signature motif in the ATF-2 3′-UTR. Here, we set out to study if HuR interacts with the ATF-2 mRNA. Our results indicate that cellular polyamines suppress ATF-2 gene expression and that polyamine depletion increases the cytoplasmic levels of HuR, enhance the abundance of [HuR-ATF-2 mRNA] complexes, and elevate ATF-2 mRNA stability and steady-state levels. Furthermore, the increased endogenous ATF-2 was capable of suppressing IEC death, because ATF-2 silencing abrogated the resistance to apoptosis of polyamine-deficient cells.
MATERIALS AND METHODS
Chemicals and Supplies
Tissue culture medium and dialyzed fetal bovine serum were from Invitrogen (Carlsbad, CA), and biochemicals were from Sigma (St. Louis, MO). The antibodies recognizing HuR and ATF-2 were from Santa Cruz Biotechnology (Santa Cruz, CA), and the secondary antibody conjugated to horseradish peroxidase was purchased from Sigma. DFMO (α-difluoromethylornithine) was from Genzyme (Cambridge, MA). l-[1-14C]ornithine (sp. radioactivity 51.6 Ci/mmol) was purchased from NEN (Boston, MA).
Cell Culture and Stable ODC Gene Transfection
The IEC-6 cell line, derived from normal rat intestinal crypt cells (Quaroni et al., 1979), was purchased from the American Type Culture Collection (Manassas, VA) at passage 13 and used at passages 15–20 (Li et al., 2001a, b; Zhang et al., 2004; Zou et al., 2005). Cells were maintained in DMEM supplemented with 5% heat-inactivated fetal bovine serum, 10 μg/ml insulin, and 50 μg/ml gentamicin. ODC-overexpressing IEC-6 (ODC-IEC) cells were developed as described in our previous studies (Liu et al., 2006; Zou et al., 2006) and expressed a more stable ODC variant with full enzyme activity (Ghoda et al., 1989).
Reporter Plasmids and Luciferase Assays
The construct of the ATF-2-promoter luciferase reporter (TransLucent ATF2 gene promoter reporter vector, pATF2-Luc; catalogue no. LR1007) was purchased from Panomics (Fremont, CA), and the CRE-driven luciferase reporter construct (pCRE-Luc, catalogue no. 219076) was from Stratagene (La Jolla, CA). Transient transfections were performed using the Lipofectamine Reagent and performed as recommended by the manufacturer (Invitrogen). The promoter constructs were transfected into cells along with phRL-null, a Renilla luciferase control reporter vector from Promega (Madison, WI), to monitor transfection efficiencies as described previously (Xiao et al., 2007). The transfected cells were lysed for assays of promoter activity using the Dual Luciferase Reporter Assay System (Promega) 48 h after the transfection. The levels of luciferase activity from individual constructs were normalized by Renilla-driven luciferase activity in every experiment.
Recombinant Viral Construction and Infection
Recombinant adenoviral plasmids containing human HuR were constructed by using the Adeno-X Expression System (Clontech, Mountain View, CA) according to the protocol provided by the manufacturer. Briefly, the full-length cDNA of human wild-type HuR was cloned into the pShuttle by digesting the BamHI/HindIII and ligating the resultant fragments into the XbaI site of the pShuttle vector. pAdeno-HuR (AdHuR) was constructed by digesting the pShuttle construct with PI-SceI/I-CeuI and ligating the resultant fragment into the PI-SceI/I-CeuI sites of the pAdeno-X adenoviral vector. Recombinant adenoviral plasmids were packaged into infectious adenoviral particles by transfecting human embryonic kidney (HEK)-293 cells using LipofectAMINE Plus reagent (Invitrogen-Bethesda Research Laboratory, Gaithersburg, MD). The adenoviral particles were propagated in HEK-293 cells and purified upon cesium chloride ultracentrifugation. Titers of the adenoviral stock were determined by standard plaque assay. Recombinant adenoviruses were screened for the expression of the introduced gene by Western blot analysis using anti-HuR antibody. pAdeno-X, which was the recombinant replication–incompetent adenovirus carrying no HuR cDNA insert (Adnull), was grown and purified as described above and served as a control adenovirus. IEC-6 cells were infected with the AdHuR or Adnull, and expression of HuR was assayed at 48 h after the infection.
RNA Interference
The silencing RNA duplexes that were designed to specifically cleave HuR mRNA were synthesized and transfected into cells as described previously (Zou et al., 2006). The sequence of small interfering RNA (siRNA) that specifically targets the coding region of HuR mRNA (siHuR) was AACACGCTGAACGGCTTGAGG; whereas the sequence of control siRNA (C-siRNA) was AAGTGTAGTAGATCACCAGGC. The ATF-2 siRNA that was designed to specifically cleave ATF-2 mRNA (siATF-2), and the corresponding C-siRNA were synthesized and purchased from Santa Cruz Biotechnology. For each 60-mm cell culture dish, 15 μl of the 20 μM stock duplex siHuR or C-siRNA was mixed with 300 μl of Opti-MEM medium (Invitrogen). This mixture was gently added to a solution containing 15 μl of LipofectAMINE 2000 in 300 μl of Opti-MEM. The solution was incubated for 20 min at room temperature and gently overlaid onto monolayers of cells in 3 ml of medium, and cells were harvested for various assays after 48-h incubation.
Assay for ODC Enzyme Activity
ODC activity was determined by radiometric technique in which the amount of 14CO2 liberated from l-[1-14C]ornithine was estimated (Liu et al., 2005). Sample collection and analysis were carried out as described previously (Patel and Wang, 1997; Li et al., 1999). Enzymatic activity was expressed as picomoles of CO2 per milligram of protein per hour.
Polyamine Analysis
The cellular polyamine content was analyzed by high-performance liquid chromatography (HPLC) analysis as previously described (Li et al., 1999; Liu et al., 2005). Briefly, after 0.5 M perchloric acid was added, the cells were frozen at −80°C until ready for extraction, dansylation, and HPLC analysis. The standard curve encompassed 0.31–10 μM. Values that fell >25% below the curve were considered undetectable. The results are expressed as nanomoles of polyamines per milligram of protein.
Western Blot Analysis
Whole-cell lysates were prepared using 2% SDS, sonicated, and centrifuged (12,000 rpm) at 4°C for 15 min. The supernatants were boiled for 5 min and size-fractionated (Laemmli, 1970) by SDS-PAGE (7.5% acrylamide). After transferring proteins onto nitrocellulose filters, the blots were incubated with primary antibodies recognizing ATF-2, HuR, or TIAR; after incubations with secondary antibodies, immunocomplexes were developed by using chemiluminescence.
RT-PCR and Real-Time PCR Analysis
Total RNA was isolated by using RNeasy mini kit (Qiagen, Valencia, CA) and used in reverse transcription and PCR amplification reactions as described (Liu et al., 2005). PCR primers for rat ATF-2 were 5′-CAGTCAGAAGAGTCTCGTCCACA-3′ (sense) and 5′-GAAGCTGCTGCTCTATTTCGTTC-3′ (antisense), yielding a 187-base pair fragment. The levels of β-actin PCR product were assessed to monitor the even RNA input in RT-PCR samples. Real-time quantitative PCR (Q-PCR) was performed using 7500-Fast Real-Time PCR Systems (Applied Biosystems, Foster City, CA) with specific primers, probes, and software (Applied Biosystems). The levels of HuR and ATF-2 mRNA were quantified by Q-PCR analysis and normalized by glyceraldehyde-3-phosphate dehydrogenase levels.
Preparation of Synthetic RNA Transcripts
cDNA from IEC-6 cells was used as a template for PCR amplification of the coding region (CR) and 3′-UTR of ATF-2. The 5′-primers contained the T7 RNA polymerase promoter sequence (T7): 5′-CCAAGCTTCTAATACGACTCACTATAGGGAGA-3′. To prepare the CR of ATF-2 (spanning position 263-1780), oligonucleotides (T7)5′-CAAGTTACATGTGAATTCTGCCAGGC-3′ and 5′-CGATCTGTGAAAGAGCAGG-CTCTGTACTC-3′ were used. To prepare the ATF-2 3′-UTR template (spanning position 1781–2117), oligonucleotides (T7)5′-CCCAGTCACAGCCCTCAGGAAGTTG-3′ and 5′-TCAGTAACACCCCCATTTATTAAAACACCAGC-3′ were used. To prepare the ATF-2 3′-UTR fragment 1 (F-1) template (spanning position 1781–1875), oligonucleotides (T7)5′-CCCAGTCACAGCCCTCAGGAAGTTG-3′ and 5′-CCACAGATTTCGCATAAATGG-3′ were used. PCR-amplified products were used as templates to transcribe biotinylated RNAs by using T7 RNA polymerase in the presence of biotin-cytidine 5′-triphosphate as described (Zou et al., 2006). Various short RNA probes for ATF-2 3′-UTR fragments, including F-2 (spanning position 1871–1900), F-3 (spanning position 1895–1944), F-4 (spanning position 1941–1990), F-5 (spanning position 1986–2040), and F-6 (spanning position 2038–2117), were synthesized in the Biopolymer Laboratory at the University of Maryland Baltimore.
RNA Protein-binding Assays
For biotin pulldown assays, biotinylated transcripts (6 μg) were incubated with 120 μg of cytoplasmic lysate for 30 min at room temperature. Complexes were isolated with paramagnetic streptavidin-conjugated Dynabeads (Dynal, Oslo, Norway) and analyzed by Western blot analysis. To assess the association of endogenous HuR or TIAR with endogenous ATF-2 mRNAs, immunoprecipitations (IP) of HuR-mRNA or TIAR-mRNA complexes were performed as described (Zou et al., 2006). Twenty million IEC-6 cells were collected per sample, and lysates were used for IP for 4 h at room temperature in the presence of excess (30 μg) IP antibody (IgG, anti-HuR, or anti-TIAR). RNA in IP materials was used in RT followed by PCR analysis to detect the presence of ATF-2 mRNA.
Immunofluorescence Staining
Immunofluorescence was performed as described (Li et al., 2001a, b) with minor changes (Vielkind and Swierenga, 1989). Cells were fixed using 3.7% formaldehyde, and the rehydrated samples were incubated overnight at 4°C with primary antibody anti-ATF-2 diluted 1:300 in blocking buffer and then incubated with secondary antibody conjugated with Alexa Fluor-594 (Molecular Probes, Eugene, OR) for 2 h at room temperature. After rinsing, slides were incubated with 1 μM TO-PRO3 (Molecular Probes) for 10 min to stain nuclei, rinsed again, mounted, and viewed through a Zeiss confocal microscope (model LSM410, Thornwood, NY). Images were processed using PhotoShop software (Adobe, San Jose, CA).
Statistics
Values are means ± SE from three to six samples. Autoradiographic and immunoblotting results were repeated three times. The significance of the difference between means was determined by analysis of variance. The level of significance was determined using Duncan's multiple range test (Harter, 1960).
RESULTS
Polyamine Depletion Increases ATF-2 Expression Levels and Transcriptional Activity
Although polyamines are absolutely required for normal intestinal mucosal growth in vivo (Wang et al., 1991; Wang and Johnson, 1991) as well as in vitro (Wang et al., 1993; Li et al., 2002), the specific functions of polyamines at the molecular level are still far from clear. The focus of the current study was to determine whether polyamines are implicated in regulating ATF-2 expression and if polyamine-modulated ATF-2 plays a role in maintenance of intestinal epithelial integrity. Consistent with our previous studies (Li et al., 1999, 2005), exposure of IEC-6 cells to 5 mM DFMO for 4 and 6 d completely inhibited ODC enzyme activity (the first rate-limiting step for polyamine biosynthesis) and almost totally depleted cellular polyamines. The levels of putrescine and spermidine were undetectable on days 4 and 6 after treatment with DFMO, and spermine had decreased by ∼60% (data not shown). As shown in Figure 1, inhibition of polyamine synthesis by DFMO increased expression of the ATF-2 gene in IEC-6 cells. The steady-state levels of ATF-2 mRNA and protein increased significantly in cells treated with DFMO for 4 and 6 d, but this induction was completely prevented by addition of exogenous putrescine (10 μM) given together with DFMO (Figure 1A). Spermidine (5 μM) had an effect equal to that of putrescine on levels of ATF-2 mRNA and protein when it was added to cultures that contained DFMO (data not shown).
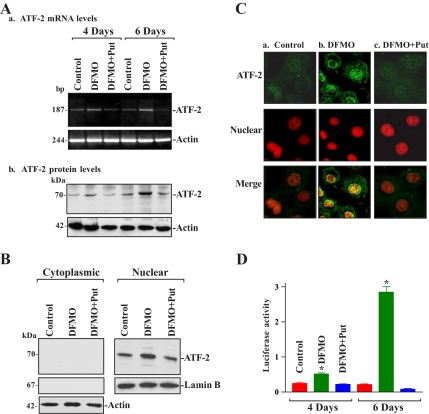
Figure 1.
Changes in ATF-2 expression, its subcellular distribution, and ATF-2–dependent transcriptional activity in control IEC-6 cells and cells treated with DFMO (5 mM) alone or DFMO plus putrescine (Put, 10 μM) for 4 and 6 d. Total RNA and whole cell lysates were prepared for various measurements. (A) Changes in ATF-2 expression. Panel a, mRNA levels as measured by RT-PCR analysis. The first-strand cDNAs, synthesized from total cellular RNA, were amplified with the specific sense and antisense primers, and PCR-amplified products displayed in agarose gel for ATF-2 (∼187 base pairs) and β-actin (∼244 base pairs). Panel b, representative immunoblots of Western analysis. Twenty micrograms of total protein were applied to each lane, and immunoblots were hybridized with the antibody specific for ATF-2 (∼70 kDa). After the blot was stripped, actin (∼42 kDa) immunoblotting was performed as an internal control for equal loading. Three experiments were performed that showed similar results. (B) Levels of cytoplasmic (left) and nuclear (right) ATF-2 protein in cells described in A. Cytoplasmic and nuclear proteins were isolated from whole cell lysates, 20 μg of each was subjected to SDS-PAGE (10%), and levels of ATF-2 were measured by Western blot analysis. Equal loading was monitored by β-actin in cytoplasmic proteins and lamin B in nuclear proteins. (C) Cellular distribution of ATF-2: panel a, control; panel b, DFMO treatment for 6 d; and panel c, DFMO plus Put treatment for 6 d. Cells were permeabilized and incubated with the anti-ATF-2 antibody and then with anti-IgG conjugated with Alexa Fluor. Nuclei were stained with the TO-PRO3. ATF-2 is shown as green and nuclei are shown as red. Original magnification, ×1000. (D) Levels of ATF-2–dependent transcriptional activity as measured by ATF-2–dependent promoter luciferase reporter gene assays. After cells were cultured in the medium containing DFMO or DFMO plus Put for 4 d, they were transfected with the ATF-2–dependent promoter construct (pCRE-luc) or control vector using the LipofectAMINE technique. Transfected cells were harvested and assayed for luciferase activity after 48 h of incubation in the presence or absence of DFMO or DFMO plus Put. Data were normalized by Renilla-driven luciferase activity and expressed as means ± SE of data from three separate experiments. *p < 0.05 compared with controls and cells treated with DFMO plus Put.
To determine the subcellular distribution of ATF-2 after polyamine depletion, the levels of cytoplasmic and nuclear ATF-2 protein were measured in control cells and in cells exposed to DFMO alone or DFMO plus putrescine for 6 d. To monitor the quality and abundance of the cytoplasmic and nuclear fractions, we examined the levels of lamin B (a nuclear protein, Figure 1B, right panel) and β-tubulin (a cytoplasmic protein, not shown), respectively. Assessment of these markers revealed that there was no contamination between cytoplasmic and nuclear fractions. Results presented in Figure 1B show that induced levels of ATF-2 in polyamine-deficient cells were only detected in the nuclear fractions but not in the cytoplasmic fractions. Consistent with the Western blotting results, ATF-2 immunostaining was predominantly located in the nucleus after polyamine depletion (Figure 1C). Combined treatment with DFMO and putrescine prevented the increase in ATF-2 signal, rendering the subcellular localization patterns similar to those observed in control cells.
To determine if the elevated nuclear ATF-2 levels after polyamine depletion increased ATF-2–dependent transcriptional transactivation, the correlation of increased ATF-2 with CRE-mediated transcriptional activity was examined using a CRE-driven luciferase reporter construct (pCRE-luc). As shown in Figure 1D, the increased levels of nuclear ATF-2 in polyamine-deficient cells were associated with a significant induction in CRE-mediated transcriptional activity. This increased transcriptional activity correlated well with the levels of induced nuclear ATF-2 protein, showing a maximal increase in both nuclear ATF-2– and CRE-mediated transcriptional activity on day 6 after treatment with DFMO. In the presence of DFMO, exogenous putrescine not only prevented the elevation in nuclear ATF-2, but it also blocked the increase in CRE-mediated transcriptional activity. These results indicate that polyamine depletion stimulates ATF-2 expression and increases its transcriptional activity.
Increasing Cellular Polyamines Represses ATF-2 Expression
To determine the effect of increasing cellular polyamines on ATF-2 expression, two clonal populations of intestinal epithelial cells stably expressing ODC (ODC-IEC) (Liu et al., 2006; Zou et al., 2006) were used in this study. These stable ODC-IEC cells exhibited very high levels of ODC protein (Figure 2Aa) and greater than 50-fold increase in ODC enzyme activity (Figure 2Ab). Accordingly, the levels of putrescine, spermidine, and spermine in ODC-IEC cells were increased by ∼12-fold, ∼2-fold, and ∼25% when compared with cells transfected with the control vector lacking ODC cDNA (data not shown), as previously reported (Liu et al., 2006; Zou et al., 2006). As shown in Figure 2B, ODC-IEC cells displayed a substantial decrease in ATF-2 expression. The levels of ATF-2 protein were decreased by >80% in stable ODC-IEC cells as compared those observed in cells transfected with the control vector (Figure 2Ba). This reduction in ATF-2 levels in stable ODC-IEC cells was associated with a significant inhibition (by ∼60%) in CRE-mediated transcriptional activity (Figure 2Bb). The effects of ODC overexpression on the expression of ATF-2 and its dependent transcriptional activity were not simply due to clonal variation because two stable clones, ODC-IEC-C1 and ODC-IEC-C2, showed similar responses. These results indicate that increasing cellular polyamines represses ATF-2 expression and decreases ATF-2–dependent transcriptional activity in intestinal epithelial cells.
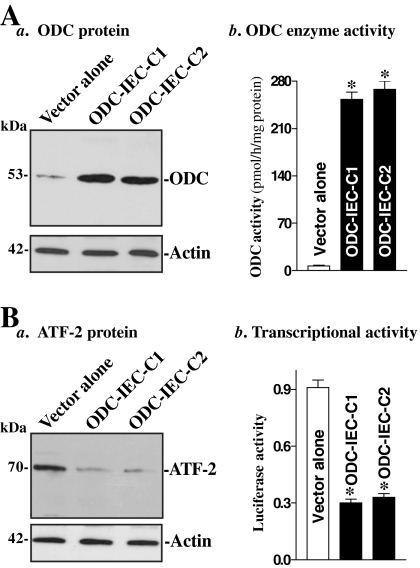
Figure 2.
Changes in ATF-2 expression and ATF-2–dependent transcription activity in stable ODC-IEC cells. (A) Characterization of stable ODC-IEC cells: panel a, representative immunoblots for ODC protein, and panel b, ODC enzyme activity. IEC-6 cells were infected with either the retroviral vector containing the sequence encoding mouse ODC cDNA or control retroviral vector lacking ODC cDNA. The clones resistant to the selection medium containing 0.6 mg/ml G418 were isolated and screened for ODC expression. The levels of ODC protein were measured by Western blot analysis, whereas ODC enzyme activity was determined by using a radiometric technique. Values are means ± SE from six dishes. *p < 0.05 compared with the vector alone. (B) Levels of ATF-2 protein (panel a) and its dependent transcriptional activity (panel b) in cells described in A. ATF-2–dependent transcriptional activity were examined by ATF-2–dependent promoter luciferase reporter gene assays, and data were normalized by Renilla-driven luciferase activity and expressed as means ± SE of data from three separate experiments. *p < 0.05 compared with the vector alone.
Polyamine Depletion Stabilizes ATF-2 mRNA without Affecting Its Gene Promoter Activity
To determine the possibility that the increase in ATF-2 mRNA levels in polyamine-deficient cells results from an increase in ATF-2 gene transcription, we examined changes in the ATF-2 promoter activity after polyamine depletion. Depletion of cellular polyamines by DFMO did not change ATF-2 gene transcription as measured by ATF-2-promoter luciferase reporter gene assays (Figure 3A) in untreated cells compared with cells exposed to DFMO in the presence or absence of putrescine for 4 and 6 d. Analysis of the kinetics of adding exogenous putrescine or spermidine on ATF-2 promoter activity in control cells revealed that exposure of normal IEC-6 cells (without DFMO) to 10 μM putrescine or 5 μM spermidine for 2 and 4 h similarly failed to alter the levels of ATF-2 promoter luciferase reporter activity (data not shown). These results indicate that cellular polyamines do not influence the transcriptional activity of the ATF-2 promoter and that the increase in steady-state levels of ATF-2 mRNA after polyamine depletion is likely related to a mechanism different from ATF-2 gene transcription.
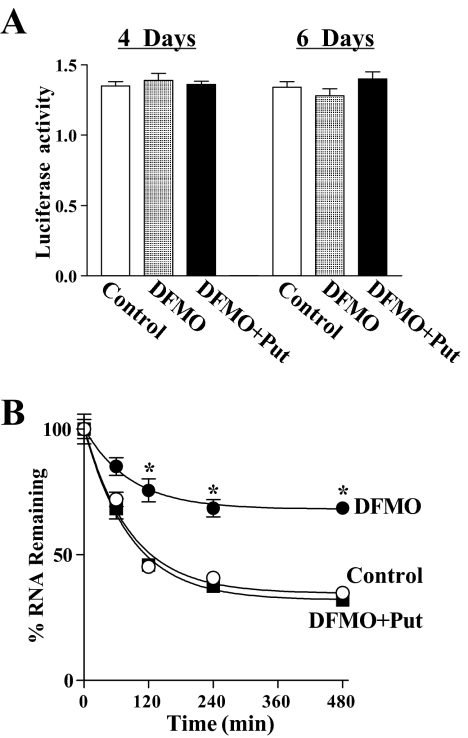
Figure 3.
Effect of polyamine depletion on ATF-2-promoter activity and ATF-2 mRNA stability. (A) Levels of ATF-2-promoter luciferase reporter gene activity. Cells were grown in DMEM containing DFMO alone or DFMO plus Put for 4 d and then transfected with the ATF-2-promoter luciferase reporter plasmid by using the LipofectAMINE technique. Luciferase activity was measured after 48 h of incubation in the presence or absence of DFMO or DFMO plus Put. Data were normalized by Renilla-driven luciferase activity and expressed as means ± SE of data from three separate experiments. (B) Half-life of ATF-2 mRNA, assessed by using 5 μg of actinomycin D/ml. Cells were grown in control media and cultures containing DFMO alone or DFMO plus Put for 6 d and incubated further with actinomycin D for the indicated times. Total cellular RNA was isolated, and the levels of remaining ATF-2 and GAPDH mRNAs were measured by Q-PCR analysis. Values are the means ± SE from triplicate samples. *p < 0.05 compared with control cells and cells treated with DFMO plus Put.
To test if the induction of ATF-2 mRNA levels by polyamine depletion was instead influenced by changes in mRNA turnover, we measured the ATF-2 mRNA half-life under conditions of different polyamine levels. As shown in Figure 3B, depletion of cellular polyamines by DFMO significantly increased the stability of ATF-2 mRNA in IEC-6 cells. In control cells, the mRNA levels declined rapidly after inhibition of gene transcription by addition of actinomycin D, displaying an apparent half-life of ∼100 min. However, the stability of ATF-2 mRNA was dramatically increased by polyamine depletion with a half-life of >480 min, which was prevented by exogenous putrescine. The half-life of ATF-2 mRNA in cells exposed to DFMO plus putrescine was ∼95 min, similar to that of control cells (without DFMO). These findings indicate that polyamines regulate the ATF-2 expression posttranscriptionally and that polyamine depletion induces ATF-2 mRNA levels primarily through mRNA stability.
Cytoplasmic HuR Directly Binds to the ATF-2 3′-UTR
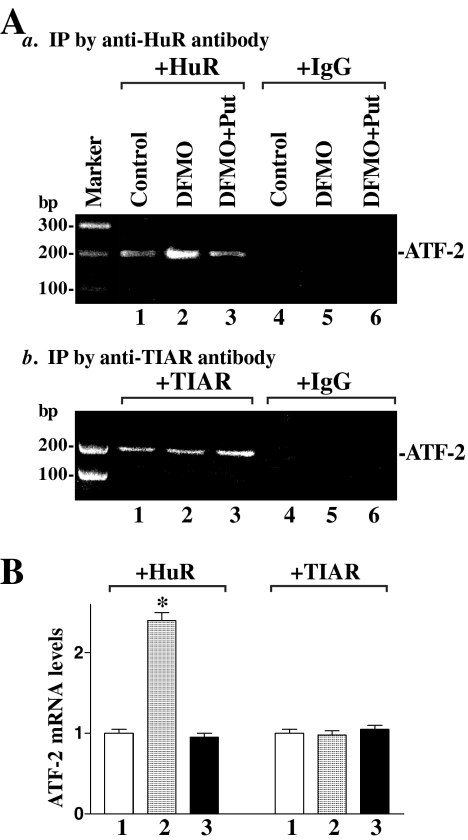
Given that polyamine depletion is shown to increase cytoplasmic levels of HuR and given the predicted affinity of HuR for the 3′-UTR of the ATF-2 mRNA (Lopez de Silanes et al., 2004; Figure 4A), we hypothesized that HuR bound the ATF-2 3′-UTR in IEC-6 cells and further postulated that this association would increase in the cytoplasm after polyamine depletion. To test these possibilities, three experiments were performed. First, we used biotinylated transcripts spanning the ATF-2 3′-UTR in RNA pulldown assays (see Materials and Methods) using cell lysates prepared from either untreated or polyamine-deficient cells. The ATF-2 3′-UTR transcript readily associated with cytoplasmic HuR, as detected by Western blot analysis of the pulldown material (Figure 4Ba); the binding intensity increased significantly when using lysates prepared from cells that were treated with DFMO for 6 d, but was reduced when cells had been treated with DFMO plus putrescine. This increase in the levels of binding of ATF-2 3′-UTR to HuR is specific, because there were no significant changes in binding of ATF-2 3′-UTR to another RNA-binding protein TIAR (T-cell–restricted intracellular antigen-1–related protein). In addition, transcripts corresponding to the coding region (CR) of ATF-2 mRNA did not bind to HuR or TIAR (Figure 4Bb).
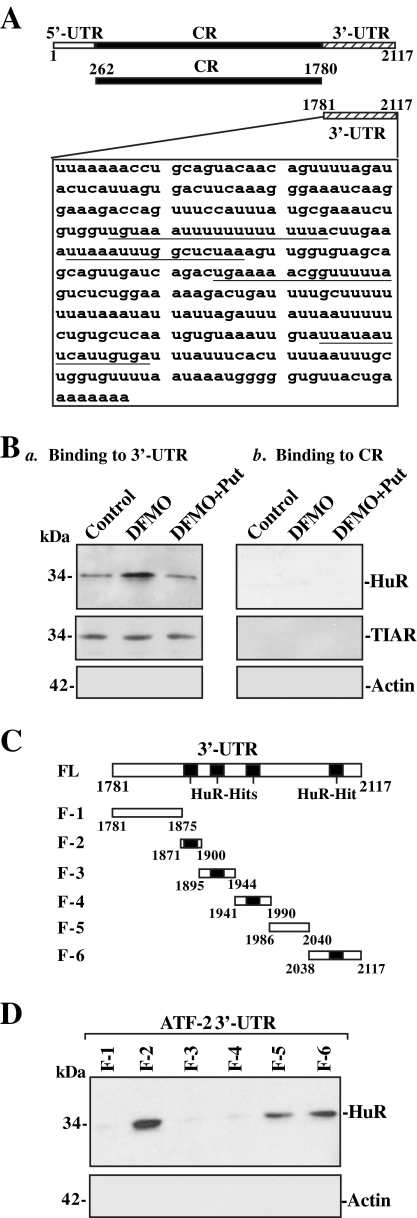
Figure 4.
Binding of cytoplasmic HuR to ATF-2 mRNA. (A) Schematic representation of ATF-2 mRNA and the HuR-hits in its 3′-UTR. (B) Changes in binding of HuR and TIAR to ATF-2 mRNA in control cells and cells treated with DFMO alone or DFMO plus Put for 6 d. Cytoplasmic lysates (120 μg each) prepared from all three groups were incubated with 6 μg of biotinylated ATF-2 3′-UTR or coding region (CR) for 30 min at 25°C, and the resulting RNP complexes were pulled down by using streptavidin-coated beads. Representative immunoblots of HuR and TIAR using the pulldown materials are shown for the 3′-UTR (panel a) or CR (panel b). (C) Schematic representation of various ATF-2 3′-UTR fractions (F) used for mapping ATF-2 3′-UTR for HuR-binding sites. (D) Representative HuR immunoblots using the pulldown materials by different fractions of ATF-2 3′-UTR. Three experiments were performed that showed similar results.
Second, we examined whether HuR binding to the ATF-2 3′UTR is mediated through the specific sites containing predicted hits of the HuR motif (Lopez de Silanes et al., 2004). As shown in Figure 4C, there are four of computationally predicted HuR-binding sites in the ATF-2 3′-UTR. Partial biotinylated transcripts spanning the ATF-2 3′-UTR were prepared and their association with HuR was tested in pulldown assays. HuR was found to bind F-2 and F-6, two transcripts that contained HuR motif hits (Figure 4D). In contrast, there was no detectable binding to F-1 (lacking putative HuR hit) and in the materials pulldown by F-3 and F-4 (containing predicted HuR hits). Interestingly, HuR also slightly bound F-5 (spanning positions 1986–2040) of ATF-2 3′-UTR, although this sequence had no predicted hits of the HuR motif.
Third, we examined the in vivo association of endogenous ATF-2 mRNA with HuR in IEC-6 cells after polyamine depletion through IP of HuR under conditions that preserved its association with target mRNAs in ribonucleoprotein (RNP) complexes. The RNP complexes immunoprecipitated using anti-HuR antibody did contain endogenous ATF-2 mRNA, as measured by RT-PCR analysis (Figure 5). The association of endogenous ATF-2 mRNA with endogenous HuR increased significantly (∼2.5 times) in cells treated with DFMO for 6 d, but was absent when testing lysates from cells treated with putrescine and DFMO. Although ATF-2 mRNA also was detectable in the RNP complexes immunoprecipitated by anti-TIAR antibody, there were no significant differences in the levels of ATF-2 mRNA between control cells and cells exposed to DFMO alone or DFMO plus putrescine for 6 d. Importantly, the ATF-2 mRNA was undetectable in nonspecific IgG1 IPs (Figure 5A, right). Together, these findings support the notion that cytoplasmic HuR specifically binds to the 3′-UTR of ATF-2 mRNA and that this binding increases after polyamine depletion.
Figure 5.
Association of endogenous HuR and TIAR with endogenous ATF-2 mRNA. (A) Whole-cell lysates from control populations and from cells treated with DFMO alone or DFMO plus put for 6 d were used for immunoprecipitation (IP) in the presence of anti-HuR antibody (panel a), anti-TIAR-antibody (panel b), or nonspecific IgG1 (right). RNA in the IP material was used in RT-PCR reactions to detect the presence of ATF-2 mRNA, and the resulting PCR products (∼187 base pairs) were visualized in agarose gels. (B) Quantitative analysis of RT-PCR results of ATF-2 mRNA by densitometry from cells described in A. Values are the means ± SE from three separate experiments. *p < 0.05 compared with controls and cells treated with DFMO plus Put.
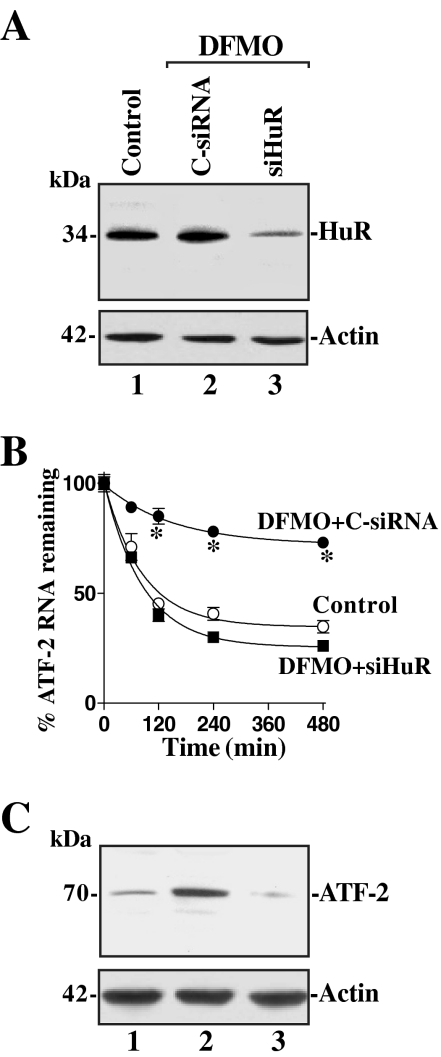
HuR Silencing Abolishes the Increased Stability of ATF-2 mRNA in Polyamine-deficient Cells
siRNA targeting the siHuR was used to reduce HuR levels and thus directly examine its putative role in the increased ATF-2 mRNA stability after polyamine depletion. With >95% cells transfected (data not shown), siHuR potently and specifically silenced HuR expression in polyamine-deficient cells (Figure 6A). As shown in Figure 6B, silencing HuR completely prevented the increase stability of ATF-2 mRNA in polyamine-deficient cells, because the half-life of ATF-2 mRNA in DFMO-treated siRNA-transfected cells was similar to that measured in control cells (without DFMO). Furthermore, in HuR-silenced populations, the increase in ATF-2 protein levels after polyamine depletion was also prevented (Figure 6C) and was reduced to the levels observed in control populations. On the other hand, transfection with C-siRNA had no effect on the stability of ATF-2 mRNA or the levels of ATF-2 protein in polyamine-deficient cells. These findings strongly suggest that the increased ATF-2 mRNA stability after polyamine depletion results from the enhanced interaction of HuR with ATF-2 3′-UTR in intestinal epithelial cells.
Figure 6.
Effect of HuR silencing on the expression and stability of ATF-2 mRNA. (A) Representative HuR immunoblots. After cells were grown in the cultures containing DFMO for 4 d, they were transfected with either short interfering (si)RNA targeting the HuR mRNA coding region (siHuR) or control siRNA (C-siRNA), and whole cell lysates were harvested 48 h thereafter. The levels of HuR protein were measured by Western blot analysis, and equal loading was monitored by β-actin immunoblotting. (B) Half-life of the ATF-2 mRNA in cells described in A. Total cellular RNA was isolated at indicated times after administration of actinomycin D (5 μg/ml), and the remaining levels of ATF-2 and GAPDH mRNAs were measured by Q-PCR analysis. Values are the means ± SE from triplicate samples. *p < 0.05 compared with control cells and DFMO-treated cells transfected with siHuR. (C) Changes in expression of ATF-2 protein as measured by Western blot analysis in cells described in A. Data are representative from three independent experiments showing similar results.
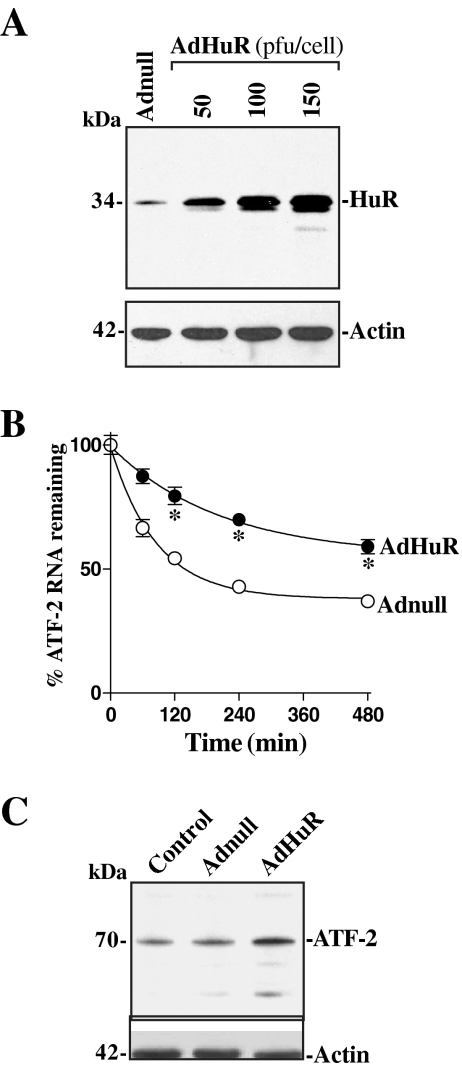
Ectopic Overexpression of HuR Stabilizes ATF-2 mRNA
To further define the role of HuR in regulating ATF-2 posttranscriptionally, we examined the effect of overexpressing the wild-type HuR upon the ATF-2 mRNA half-life in control IEC-6 cells (without DFMO). The adenoviral vector containing the corresponding human HuR cDNA under the control of the human cytomegalovirus immediate early gene promoter (AdHuR) was previously described (Liu et al., 2006). The adenoviral vectors used in this study infect intestinal epithelial cells with near 100% efficiency (Liu et al., 2006); >95% of IEC-6 cells were positive when they were infected for 24 h with the adenoviral vector encoding green fluorescent protein (GFP; data not shown). As noted in Figure 7A, the levels of HuR protein increased with viral load and reached ∼3-, ∼10-, and ∼14-fold higher levels than control cells when AdHuR was used at 50, 100, and 150 pfu/cell, respectively. A control adenovirus that lacked exogenous HuR cDNA (Adnull) failed to induce HuR. Transient infection with the AdHuR (100 pfu/cell) for 24 h stabilized ATF-2 mRNA, as indicated by a significant increase in its half-life in IEC-6 cells (Figure 7B). Consistently, the increased ATF-2 mRNA stability was associated with an increase in the levels of HuR protein after the infection with AdHuR compared with those observed in control cells and cells infected with Adnull (Figure 7C). These results indicate that HuR overexpression enhances ATF-2 expression by stabilizing its mRNA in intestinal epithelial cells.
Figure 7.
Effect of ectopic expression of the HuR gene on ATF-2 mRNA stability and expression. (A) Representative immunoblots of HuR protein. The complete open reading frame of the human HuR cDNA was amplified by PCR, sequenced, and then cloned to pShuttle of the Adeno-X Expression system. Cells were infected with the recombinant adenoviral vector encoding HuR cDNA (AdHuR) or adenoviral vector lacking HuR cDNA (Adnull) at a multiplicity of infection of 50–150 plaque-forming units (pfu)/cell, and expression of HuR protein was analyzed 24 h after the infection. (B) Half-life of the ATF-2 mRNA after HuR overexpression. After cells were infected with either the AdHuR or Adnull at the dose of 100 pfu/cell for 24 h, actinomycin D (5 μg/ml) was added to the medium. Total cellular RNA was isolated at indicated times after exposure to actinomycin, and the remaining levels of ATF-2 and GAPDH mRNAs were measured by Q-PCR analysis. Values are the means ± SE from triplicate samples. *p < 0.05 compared with cells infected with Adnull. (C) Changes in expression of ATF-2 protein as measured by Western blot analysis in cells described in A. Data are representative from three independent experiments showing similar results.
ATF-2 Is Crucial for Induced Resistance of Polyamine-deficient Cells to Apoptosis
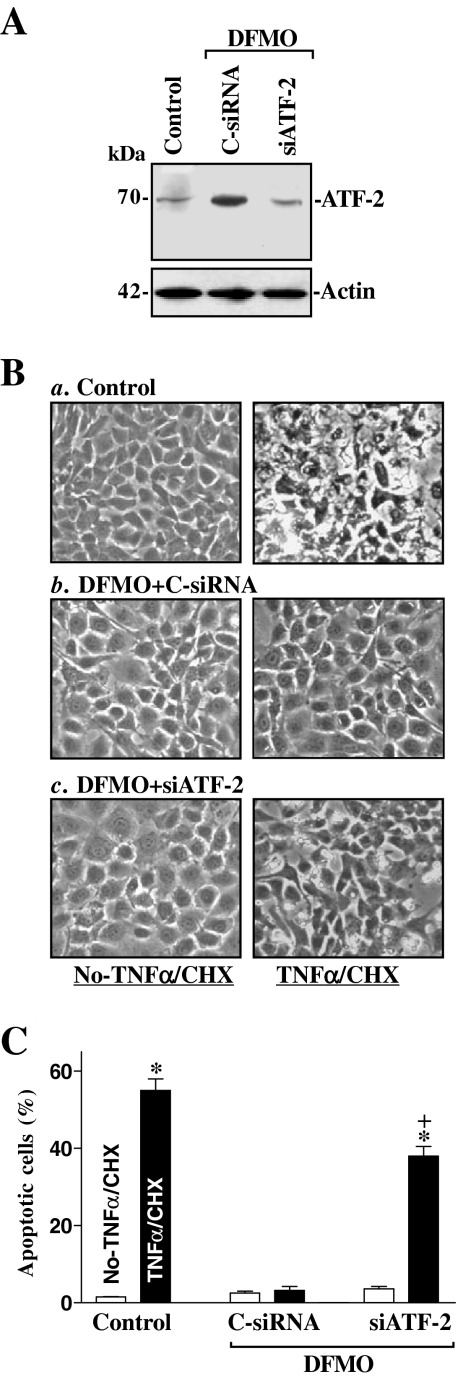
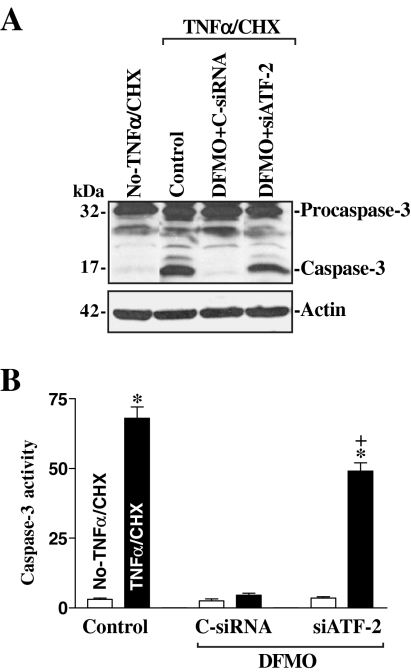
To investigate the biological consequences of inducing endogenous ATF-2 levels after polyamine depletion, we examined its possible involvement in regulating IEC apoptosis. Our previous studies (Li et al., 2001a, b; Zhang et al., 2004) and others (Bhattacharya et al., 2004; Seiler and Raul, 2005) have shown that polyamines regulate apoptosis through multiple signaling pathways and that depletion of cellular polyamines promotes the resistance to apoptosis in normal IECs. Here, we first examined the spontaneous apoptotic cell death without any challenge of apoptotic stimulators after inhibition of ATF-2 expression by using siRNA targeting ATF-2 mRNA (siATF-2) in the absence of cellular polyamines. Transfection with siATF-2 for 48 h completely prevented the increased expression of ATF-2 in polyamine-deficient cells (Figure 8A) but failed to directly induce apoptosis (Figure 8, B and C). There were no apparent differences in cell viability in DFMO-treated cells transfected with C-siRNA compared with DFMO-treated cells transfected with siATF-2, as measured by trypan blue staining assay. No morphological features of apoptosis (Figure 8B, left) or detectable levels of active caspase-3 protein (Figure 9) were obtained in polyamine-deficient cells after ATF-2 silencing.
Figure 8.
Effect of specific inhibition of ATF-2 expression by siRNA targeting ATF-2 mRNA coding region (siATF-2) on apoptotic sensitivity in polyamine-deficient IEC-6 cells. (A) Representative immunoblots for ATF-2 protein. Cells were grown in the presence of DFMO for 4 d and then transfected with either siATF-2 or control siRNA (C-siRNA). The levels of ATF-2 protein were measured by Western immunoblotting analysis 48 h after the transfection. Actin immunoblotting on the stripped blots was performed as an internal loading control. Three separate experiments were performed that showed similar results. (B) Images of apoptotic cell death after treatment with (right) or without (left) TNF-α/CHX for 4 h. Panel a, control cells; panel b, DFMO-treated cells transfected with C-siRNA; and panel c, DFMO-treated cells transfected with siATF-2. Original magnification, ×150. (C) Percentage of apoptotic cells as described in B. Values are means ± SE of data from three experiments. *p < 0.05 compared with No-TNF-α/CHX. + p < 0.05 compared with DFMO-treated cells that were transfected with C-siRNA and then treated with TNF-α/CHX for 4 h.
Figure 9.
Effect of specific inhibition of ATF-2 expression on caspase-3 activity after exposure to TNF-α/CHX. (A) Representative immunoblots for procaspase-3 and caspase-3. Cells were treated DFMO for 4 d and then transfected with either siATF-2 or C-siRNA for 48 h. Whole cell lysates were harvested 4 h after treatment with TNF-α/CHX, and the levels of caspase-3 protein were examined by Western blot analysis. Three experiments were performed that showed similar results. (B) Changes in caspase-3 activity in cells described in A. Values are means ± SE from six samples. *p < 0.05 compared with No-TNF-α/CHX. + p < 0.05 compared with DFMO-treated cells that were transfected with C-siRNA and then treated with TNF-α/CHX for 4 h.
Second, we determined whether ATF-2 silencing altered the polyamine depletion–mediated resistance to apoptosis after exposure to tumor necrosis factor-α (TNF-α) plus cycloheximide (CHX). This apoptotic model was chosen because TNF-α/CHX–induced apoptosis is widely accepted as a form of programmed cell death induced by a biological apoptotic inducer (Cardone et al., 1998; Zhang et al., 2006). As shown in Figure 8Ba, when control cells were exposed to TNF-α/CHX for 4 h, morphological features characteristic of programmed cell death were observed. The assessments of apoptosis was confirmed by an increase in levels of active caspase-3 protein (Figure 9A, left) and its enzyme activity (Figure 9B, left) after treatment with TNF-α/CHX. Consistent with our previous studies, exposure of polyamine-deficient cells to the same doses of TNF-α/CHX caused no apoptosis. In keeping with our earlier findings (Li et al., 2001a, b; Zhang et al., 2004), there were no differences in the morphological features and percentages of apoptotic cells when comparing cells treated with DFMO alone and DFMO-treated cells exposed to TNF-α/CHX for 4 h (data not shown). This increased resistance to TNF-α/CHX–induced apoptosis was not altered when polyamine-deficient cells were transfected with C-siRNA (Figure 8Bb), but it was lost when ATF-2 expression was silenced by siATF-2 (Figure 8Bc). The percentages of apoptotic cells (Figure 8C) and levels of active caspase-3 protein (Figure 9A) and its enzyme activity (Figure 9B) in DFMO-treated cells transfected with siATF-2 were significantly increased compared with those observed in DFMO-treated cells transfected with C-siRNA after exposure to TNF-α/CHX. These results indicate that the elevation in ATF-2 levels promotes an increase in resistance to apoptosis after polyamine depletion.
DISCUSSION
The mammalian intestinal epithelium is a rapidly self-renewing tissue in the body, and its homeostasis is preserved through strict regulation of epithelial cell proliferation, growth arrest, and apoptosis (Johnson, 1998). A significant body of literature has indicated that polyamines are absolutely required for maintaining intestinal epithelial integrity through distinct mechanisms (McCormack and Johnson, 1991; Wang et al., 1991; Liu et al., 2005). In this regard, polyamines have shown to stimulate expression of the growth-promoting genes such as c-fos, c-jun, and c-myc through enhancement of their gene transcription (Wang et al., 1993; Patel and Wang, 1997) but suppress expression of growth-inhibiting genes, including p53, p21, TGFβ, nucleophosmin, and junD, by controlling their mRNA stability (Li et al., 2001a, b, 2002; Wang, 2007), leading to the induction of cell proliferation. On the other hand, polyamines also regulate apoptosis in intestinal epithelial cells by regulating nuclear factor κB (NF-κB) nuclear translocation (Li et al., 2001a, b; Zou et al., 2004), Akt phosphorylation (Zhang et al., 2004), and ERK activation (Bhattacharya et al., 2004). Here, we provide new evidence showing that polyamines are involved in regulating the ATF-2 gene posttranscriptionally, thereby advancing our understanding of the functions of cellular polyamines in intestinal epithelial cells. Among our most salient findings is the discovery that polyamines modulate ATF-2 mRNA stability by altering the abundance of HuR in the cytoplasm, and hence HuR's interaction with specific ATF-2 3′-UTR regions.
Expression of the ATF-2 gene is constitutive in a range of tissues (Herdegen and Leah, 1998; Huguier at al., 1998) and the basal level of the ATF-2 gene transcription in intestinal epithelial cells is generally elevated. The data gathered in this study demonstrate that polyamines negatively regulate expression of the ATF-2 gene posttranscriptionally. Decreasing cellular polyamines by inhibiting ODC with DFMO led to increases in the levels of ATF-2 mRNA and protein (Figure 1), whereas increasing cellular polyamines by ectopic ODC overexpression repressed the expression of the ATF-2 in IEC-6 cells (Figure 2). Our results further indicate that polyamines influence ATF-2 expression posttranscriptionally rather through changes in gene transcription through ATF-2 promoter. Instead, depletion of cellular polyamines significantly increased the half-life of ATF-2 mRNA (Figure 3). The increase in ATF-2 mRNA stability and the corresponding elevation in ATF-2 protein levels in DFMO-treated cells were completely prevented by the addition of exogenous putrescine, indicating that the observed posttranscriptional changes in ATF-2 gene expression are due to the depletion of polyamines rather than to nonspecific effects of DFMO. The prolonged half-life of ATF-2 mRNA after polyamine depletion leads to mRNA accumulation, which is paralleled by an increase in the levels of nuclear ATF-2 protein, and increased ATF-2–dependent promoter activity.
Our results also indicate that increased cytoplasmic HuR specifically bound to the 3′-UTR of ATF-2 mRNA after polyamine depletion. Increasing evidence shows that HuR-mediated transcript stabilization and translational control are closely linked to its cytoplasmic presence (Mazan-Mamczarz et al., 2003; Heinonen et al., 2005). We recently demonstrated that depletion of cellular polyamines dramatically enhances the cytoplasmic abundance of HuR, whereas increased levels of polyamines decrease cytoplasmic HuR, although neither intervention alters whole cell HuR levels (Zou et al., 2006). Although it remains to be formally shown that the role of HuR in the stabilization of ATF-2 mRNA in polyamine-deficient cells is linked to the cytoplasmic localization of HuR RNP complexes, our current studies show that the cytoplasmic HuR bound to 3′-UTR of ATF-2 mRNA primarily through two specific regions containing hits of the HuR signature motif after polyamine depletion (Figure 4). These findings are consistent with studies demonstrating that HuR binds to AREs commonly found in the 3′-UTRs of labile mRNAs (Lal et al., 2004) and in particular mRNAs bearing a recently identified RNA motif in HuR target transcripts (Lopez de Silanes et al., 2004). Computational analysis of the ATF-2 mRNA revealed that its 3′-UTR has four HuR motif hits, thus making it a putative HuR target, as confirmed here. These findings are supported by evidence that the endogenous ATF-2 mRNA associated with the endogenous HuR in the materials immunoprecipitated by anti-HuR antibody (Figure 5). However, the cytoplasmic HuR also bound a region spanning positions 1986–2040 of ATF-2 3′-UTR, although this sequence had no predicted hits of the HuR motif (Figure 4D), suggesting that additional HuR recognition/binding motifs remain to be identified. Moreover, the sequence spanning positions 1895–1990 of ATF-2 3′-UTR (F-3 and F-4), with two predicted HuR-hits, failed to bind to HuR in polyamine-deficient cells, suggesting that perhaps these sequences were inaccessible to HuR, possibly because they were targeted by other RBPs which had greater affinity as measured by the biotin pulldown assays.
The transcript stability data presented in Figure 6 show that increases in cytoplasmic HuR levels after polyamine depletion critically influence ATF-2 mRNA stability. Depletion of cellular polyamines by DFMO increased the half-life of ATF-2 mRNA, but this effect was abrogated in cells in which HuR expression was silenced by transfection with an siRNA targeting HuR, which in turn caused a marked reduction of ATF-2 protein. On the other hand, ectopic HuR overexpression increased the stability of ATF-2 mRNA, which was associated with increased production of ATF-2 protein (Figure 7). Although the exact mechanisms whereby cytoplasmic HuR regulates ATF-2 mRNA stability after changes in levels of cellular polyamines are still unclear, several studies suggest that HuR acts by protecting the body of the mRNAs from degradation, rather than slowing the rate of deadenylation (Peng et al., 1998).
Increased accumulation of ATF-2 through posttranscriptional regulation plays an important role in the process of increased resistance to apoptosis after polyamine depletion. The exact roles of polyamines in apoptotic pathways has been rather controversial, depending on the cell type and death stimuli (Li et al., 2001a, b), but our previous studies (Li et al., 2001a, b; Zhang et al., 2004) and others (Bhattacharya et al., 2004; Seiler and Raul, 2005) have demonstrated that polyamine depletion promotes the resistance of intestinal epithelial cells to apoptosis, which is mediated through multiple signaling pathways. For example, polyamines are shown to down-regulate NF-κB activity in intestinal epithelial cells and depletion of cellular polyamines increases the basal levels of NF-κB proteins, induces NF-κB nuclear translocation, and activates its transcriptional activity (Li et al., 2001a, b). The induced NF-κB stimulates the expression of inhibitor of apoptosis proteins (IAPs), leading to the inhibition of apoptosis in polyamine-deficient cells (Zou et al., 2004). Polyamines also are needed for the inhibition of Akt signaling, as polyamine depletion induces Akt phosphorylation and increases its kinase activity (Zhang et al., 2004). The data presented in Figures 8 and 9 further show that increased levels of endogenous ATF-2 after polyamine depletion also contribute to the increased resistance to apoptosis induced by treatment with TNF-α and CHX, because this tolerance was significantly blocked by ATF-2 silencing with RNA interference. Consistent with our results, ATF-2 is shown to enhance cell resistance to UV radiation-induced apoptosis in human late-stage melanoma cells (Fuchs et al., 1998). In regard, HuR has been shown to have an anti-apoptotic influence (Lal et al., 2005). This effect is implemented via the positive influence of HuR upon the expression of target mRNAs encoding anti-apoptotic factors such as prothymosin α, Bcl-2, Mcl-1, and p21 (recently reviewed in Abdelmohsen et al., 2007). In light of our results reported here, ATF-2 may constitute another critical downstream effector of the prosurvival program elicited by HuR.
In summary, these results indicate that polyamines negatively regulate ATF-2 expression posttranscriptionally in intestinal epithelial cells and that depletion of cellular polyamines increases the half-life of the ATF-2 mRNA without affecting its gene transcription. A search for mechanisms by which polyamines modulate ATF-2 mRNA levels revealed that increases in the cytoplasmic levels of the mRNA-stabilizing protein HuR after polyamine depletion were linked to increased HuR binding to the 3′-UTR of ATF-2 through specific RNA regions containing hits of the HuR motif and to increased stability of the ATF-2 mRNA. Silencing of HuR prevented the stabilization of ATF-2 mRNA and reduced the levels of ATF-2 protein in polyamine-deficient cells, whereas ectopic HuR overexpression increased the half-life of ATF-2 mRNA and induced ATF-2 protein abundance. The present study also shows that ATF-2 promotes the survival of intestinal epithelial cells and that it elevates their resistance to apoptosis triggered by treatment with TNF-α and CHX after polyamine depletion. Because polyamines are required for maintaining intestinal epithelial integrity and their cellular levels are highly regulated, these findings suggest that polyamine-modulated ATF-2 expression through HuR plays an important role in regulating intestinal mucosal homeostasis under physiological and pathological conditions.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a Merit Review Grant (J.-Y.W.) from the Department of Veterans Affairs and by National Institutes of Health (NIH) Grants DK-57819, DK-61972, and DK-68491 (J.-Y.W.), and AI-68432 and AT-4148 (H.Z.). J.-Y.W. is a Research Career Scientist, Medical Research Service, US Department of Veterans Affairs. D.J.T. is supported by Research Career Development award from the US Department of Veterans Affairs. M.G. is supported by the National Insitute on Aging (NIA)-Intramural Research Program, NIH.
Abbreviations used:
- AREs
AU-rich elements
- ATF-2
activating transcription factor-2
- CHX
cycloheximide
- Con
control
- pfu
plaque-forming units
- CR
coding region
- CRE
cyclic AMP response element
- CREB
CRE-binding protein
- DFMO
dl-α-difluoromethylornithine
- HEK
human embryonic kidney
- IECs
intestinal epithelial cells
- IP
immunoprecipitation
- Luc
luciferase
- NPM
nucleophosmin
- ODC
ornithine decarboxylase
- Put
putrescine
- RBPs
RNA-binding proteins
- RNP
ribonucleoprotein
- si
small interfering
- siATF-2
siRNA targeting ATF-2 mRNA
- siHuR
siRNA targeting HuR mRNA
- TNF-α
tumor necrosis factor-α
- UTRs
untranslated regions.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-07-0675) on September 5, 2007.
REFERENCES
- Abdelmohsen K., Lal A., Kim H. H., Gorospe M. Posttranscriptional orchestration of an anti-apoptotic program by HuR. Cell Cycle. 2007;6:1288–1292. doi: 10.4161/cc.6.11.4299. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Ray R. M., Johnson L. R. Prevention of TNF-α-induced apoptosis in polyamine-depleted IEC-6 cells is mediated through the activation of ERK1/2. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:479–490. doi: 10.1152/ajpgi.00342.2003. [DOI] [PubMed] [Google Scholar]
- Bhoumik A., Jones N., Ronai Z. Transcriptional switch by activating transcription factor 2-derived peptide sensitizes melanoma cells to apoptosis and inhibits their tumorigenicity. Proc. Natl. Acad. Sci. USA. 2004;101:4222–4227. doi: 10.1073/pnas.0400195101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhoumik A., Takahashi S., Breitweiser W., Shiloh Y., Jones N., Ronai Z. ATM-dependent phosphorylation of ATF2 is required for the DNA damage response. Mol. Cell. 2005;18:577–587. doi: 10.1016/j.molcel.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan C. M., Steitz J. A. HuR and mRNA stability. Cell Mol. Life Sci. 2001;58:266–277. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone M. H., Roy N., Stennicke H. R., Salvesen G. S., Franke T. F., Stanbridge E., Frisch S., Reed J. C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Casero R. A., Jr, Marton L. J. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. 2007;6:373–390. doi: 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- Fuchs S. Y., Fried V. A., Ronai Z. Stress-activated kinases regulate protein stability. Oncogene. 1998;17:1483–1490. doi: 10.1038/sj.onc.1202184. [DOI] [PubMed] [Google Scholar]
- Gerner E. W., Meyskens F. L. Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- Ghoda L., van Daalen Wetters T., Macrae M., Ascherman D., Coffino P. Prevention of rapid intracellular degradation of ODC by a carboxyl-terminal truncation. Science. 1989;243:1493–1495. doi: 10.1126/science.2928784. [DOI] [PubMed] [Google Scholar]
- Gorospe M. HuR in the mammalian genotoxic response: post-transcriptional multitasking. Cell Cycle. 2003;2:412–414. [PubMed] [Google Scholar]
- Guo Y. S., Hellmich M. R., Wen X. D., Townsend C. M. Activator protein-1 transcription factor mediates bombesin-stimulated cyclooxygenase-2 expression in intestinal epithelial cells. J. Biol. Chem. 2001;27:22941–22947. doi: 10.1074/jbc.M101801200. [DOI] [PubMed] [Google Scholar]
- Hai T., Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc. Natl. Acad. Sci. USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter J. L. Critical values for Duncan's new multiple range test. Biometrics. 1960;16:671–685. [Google Scholar]
- Hayakawa J., Depatie C., Ohmichi M., Mercola D. The activation of c-Jun NH2-terminal kinase (JNK) by DNA-damaging agents serves to promote drug resistance via activating transcription factor 2 (ATF2)-dependent enhanced DNA repair. J. Biol. Chem. 2003;278:20582–20592. doi: 10.1074/jbc.M210992200. [DOI] [PubMed] [Google Scholar]
- Heinonen M., Bono P., Narko K., Chang S. H., Lundin J., Joensuu H., Furneaux H., Hla T., Haglund C., Ristimäki A. Cytoplasmic HuR expression is a prognostic factor in invasive ductal breast carcinoma. Cancer Res. 2005;65:2157–2161. doi: 10.1158/0008-5472.CAN-04-3765. [DOI] [PubMed] [Google Scholar]
- Herdegen T., Leah J. D. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res. Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Huguier S., Baguet J., Perez S., van Dam H., Castellazzi M. Transcription factor ATF2 cooperates with v-Jun to promote growth factor-independent proliferation in vitro and tumor formation in vivo. Mol. Cell. Biol. 1998;18:7020–7029. doi: 10.1128/mcb.18.12.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashkiv L. B., Liou H. C., Kara C. J., Lamph W. W., Verma I. M., Glimcher L. H. mXBP/CRE-BP2 and c-Jun form a complex which binds to the cyclic AMP, but not to the 12-O-tetradecanoylphorbol-13-acetate, response element. Mol. Cell. Biol. 1990;10:1609–1621. doi: 10.1128/mcb.10.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. R. Regulation of gastrointestinal mucosal growth. Physiol. Rev. 1988;68:456–502. doi: 10.1152/physrev.1988.68.2.456. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lal A., Mazan-Mamczarz K., Kawai T., Yang X., Martindale J. L., Gorospe M. Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J. 2004;23:3092–3102. doi: 10.1038/sj.emboj.7600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A., Kawai T., Yang X., Mazan-Mamczarz K., Gorospe M. Antiapoptotic function of RNA-binding protein HuR effected through prothymosin alpha. EMBO J. 2005;24:1852–1862. doi: 10.1038/sj.emboj.7600661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Li J., Rao J. N., Li M., Bass B. L., Wang J. Y. Inhibition of polyamine synthesis induces p53 gene expression but not apoptosis. Am. J. Physiol. Cell Physiol. 1999;276:946–954. doi: 10.1152/ajpcell.1999.276.4.C946. [DOI] [PubMed] [Google Scholar]
- Li L., Liu L., Rao J. N., Esmaili A., Strauch E. D., Bass B. L., Wang J. Y. JunD stabilization results in inhibition of normal intestinal epithelial cell growth through p21 after polyamine depletion. Gastroenterology. 2002;123:764–779. doi: 10.1053/gast.2002.35386. [DOI] [PubMed] [Google Scholar]
- Li L., Rao J. N., Bass B. L., Wang J. Y. NF-κB activation and susceptibility to apoptosis after polyamine depletion in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2001a;280:992–1004. doi: 10.1152/ajpgi.2001.280.5.G992. [DOI] [PubMed] [Google Scholar]
- Li L., Rao J. N., Guo X., Liu L., Santora R., Bass B. L., Wang J. Y. Polyamine depletion stabilizes p53 resulting in inhibition of normal intestinal epithelial cell proliferation. Am. J. Physiol. Cell Physiol. 2001b;281:941–953. doi: 10.1152/ajpcell.2001.281.3.C941. [DOI] [PubMed] [Google Scholar]
- Liu L., Guo X., Rao J. N., Zou T., Marasa B. S., Chen J., Greenspon J., Casero R. A., Jr, Wang J. Y. Polyamine-modulated c-myc expression in normal intestinal epithelial cells regulates p21Cip1 transcription through a proximal promoter region. Biochem. J. 2006;398:257–267. doi: 10.1042/BJ20060217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Li L., Rao J. N., Zou T., Zhang H. M., Boneva D., Bernard M. S., Wang J. Y. Polyamine-modulated expression of c-myc plays a critical role in stimulation of normal intestinal epithelial cell proliferation. Am. J. Physiol. Cell Physiol. 2005;288:89–99. doi: 10.1152/ajpcell.00326.2004. [DOI] [PubMed] [Google Scholar]
- Liu L., Santora R., Rao J. N., Guo X., Zou T., Zhang H. M., Turner D. J., Wang J. Y. Activation of TGF-β-Smad signaling pathway following polyamine depletion in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:1056–1067. doi: 10.1152/ajpgi.00151.2003. [DOI] [PubMed] [Google Scholar]
- Livingstone C., Patel G., Jones N. ATF-2 contains a phosphorylation-dependent transcriptional activation domain. EMBO J. 1995;14:1785–1797. doi: 10.1002/j.1460-2075.1995.tb07167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Silanes I. L., Zhan M., Lal A., Yang X., Gorospe M. Identification of a target RNA motif for RNA-binding protein HuR. Proc. Natl. Acad. Sci. USA. 2004;101:2987–2992. doi: 10.1073/pnas.0306453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazan-Mamczarz K., Galban S., de Silanes I. L., Martindale J. L., Atasoy U., Keene J. D., Gorospe M. RNA-binding protein HuR enhances p53 translation in response to ultraviolet light irradiation. Proc. Natl. Acad. Sci. USA. 2003;100:8354–8359. doi: 10.1073/pnas.1432104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack S. A., Johnson L. R. Role of polyamines in gastrointestinal mucosal growth. Am. J. Physiol. Gastrointest. Liver Physiol. 1991;260:795–806. doi: 10.1152/ajpgi.1991.260.6.G795. [DOI] [PubMed] [Google Scholar]
- Nishimura K., Nakatsu F., Kashiwagi K., Ohno H., Saito T., Igarashi K. Essential role of S-adenosylmethionine decarboxylase in mouse embryonic development. Genes Cells. 2002;7:41–47. doi: 10.1046/j.1356-9597.2001.00494.x. [DOI] [PubMed] [Google Scholar]
- Papassava P., Gorgoulis V. G., Papaevangeliou D., Vlahopoulos S., van Dam H., Zoumpourlis V. Overexpression of activating transcription factor-2 is required for tumor growth and progression in mouse skin tumors. Cancer Res. 2004;64:8573–8584. doi: 10.1158/0008-5472.CAN-03-0955. [DOI] [PubMed] [Google Scholar]
- Patel A. R., Wang J. Y. Polyamine depletion is associated with an increase in JunD/AP-1 activity in small intestinal crypt cells. Am. J. Physiol. Gastrointest. Liver Physiol. 1999;276:441–450. doi: 10.1152/ajpgi.1999.276.2.G441. [DOI] [PubMed] [Google Scholar]
- Patel A. R., Wang J. Y. Polyamines modulate transcription but not posttranscription of c-myc and c-jun in IEC-6 cells. Am. J. Physiol. Cell Physiol. 1997;273:1020–1029. doi: 10.1152/ajpcell.1997.273.3.C1020. [DOI] [PubMed] [Google Scholar]
- Pendeville H., Carpino N., Marine J. C., Takahashi Y., Muller M., Martial J. A., Cleveland J. L. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol. Cell. Biol. 2001;21:6549–6558. doi: 10.1128/MCB.21.19.6549-6558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S. S., Chen C. Y., Xu N., Shyu A. B. RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J. 1998;17:3461–3470. doi: 10.1093/emboj/17.12.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A., Wands J., Trelstad R. L., Isselbacher K. J. Epithelioid cell cultures from rat small intestine: characterization by morphologic and immunologic criteria. J. Cell Biol. 1979;80:248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R. M., Zimmerman B. J., McCormack S. A., Patel T. B., Johnson L. R. Polyamine depletion arrests cell cycle and induces inhibitors p21(Waf1/Cip1), p27(Kip1), and p53 in IEC-6 cells. Am. J. Physiol. Cell Physiol. 1999;276:684–691. doi: 10.1152/ajpcell.1999.276.3.C684. [DOI] [PubMed] [Google Scholar]
- Seiler N., Raul F. Polyamines and apoptosis. J. Cell Mol. Med. 2005;9:623–642. doi: 10.1111/j.1582-4934.2005.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., Raul F. Polyamines and the intestinal tract. Crit. Rev. Clin. Lab. Sci. 2007;44:365–411. doi: 10.1080/10408360701250016. [DOI] [PubMed] [Google Scholar]
- Shaulian E., Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- Song H., Ki S. H., Kim S. G., Moon A. Activating transcription factor 2 mediates matrix metalloproteinase-2 transcriptional activation induced by p38 in breast epithelial cells. Cancer Res. 2006;66:10487–10496. doi: 10.1158/0008-5472.CAN-06-1461. [DOI] [PubMed] [Google Scholar]
- Tan K. S., Nackley A. G., Satterfield K., Maixner W., Diatchenko L., Flood P. M. β2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-κB-independent mechanisms. Cell Signal. 2007;19:251–260. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- van Dam H., Duyndam M., Rottier R., Bosch A., de Vries-Smits L., Herrlich P., Zantema A., Angel P., van der Eb A. J. Heterodimer formation of c-Jun and ATF-2 is responsible for induction of c-jun by the 243 amino acid adenovirus E1A protein. EMBO J. 1993;12:479–487. doi: 10.1002/j.1460-2075.1993.tb05680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielkind U., Swierenga S. H. A simple fixation procedure for immunofluorescent detection of different cytoskeletal components within the same cell. Histochem. 1989;91:81–88. doi: 10.1007/BF00501916. [DOI] [PubMed] [Google Scholar]
- Wallace H. M., Fraser A. V., Hughes A. A perspective of polyamine metabolism. Biochem. J. 2003;376:1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., McCormack S. A., Viar M. J., Johnson L. R. Stimulation of proximal small intestinal mucosal growth by luminal polyamines. Am. J. Physiol. Gastrointest. Liver Physiol. 1991;261:504–511. doi: 10.1152/ajpgi.1991.261.3.G504. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., McCormack S. A., Viar M. J., Wang H., Tzen C. Y., Scott R. E., Johnson L. R. Decreased expression of protooncogenes c-fos, c-myc, and c-jun following polyamine depletion in IEC-6 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 1993;265:331–338. doi: 10.1152/ajpgi.1993.265.2.G331. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Johnson L. R. Expression of protooncogenes c-fos and c-myc in healing of gastric mucosal stress ulcers. Am. J. Physiol. Gastrointest. Liver Physiol. 1994;266:878–886. doi: 10.1152/ajpgi.1994.266.5.G878. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Johnson L. R. Polyamines and ornithine decarboxylase during repair of duodenal mucosa after stress in rats. Gastroenterology. 1991;100:333–343. doi: 10.1016/0016-5085(91)90200-5. [DOI] [PubMed] [Google Scholar]
- Wang J. Y. Polyamines and mRNA stability in regulation of intestinal mucosal growth. Amino Acids. 2007;33:241–252. doi: 10.1007/s00726-007-0518-z. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Viar M. J., Li J., Shi H. J., McCormack S. A., Johnson L. R. Polyamines are necessary for normal expression of the transforming growth factor-β gene during cell migration. Am. J. Physiol. Gastrointest. Liver Physiol. 1997;272:713–720. doi: 10.1152/ajpgi.1997.272.4.G713. [DOI] [PubMed] [Google Scholar]
- Xiao L., Rao J. N., Zou T., Liu L., Marasa B. S., Chen J., Turner D. J., Passaniti A., Wang J. Y. Induced JunD in intestinal epithelial cells represses CDK4 transcription through its proximal promoter region following polyamine depletion. Biochem. J. 2007;403:573–581. doi: 10.1042/BJ20061436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A. H., Rao J. N., Zou T., Liu L., Marasa B. S., Xiao L., Chen J., Turner D. J., Wang J. Y. p53-dependent NDRG1 expression induces inhibition of intestinal epithelial cell proliferation but not apoptosis after polyamine depletion. Am. J. Physiol. Cell Physiol. 2007;293:C379–C389. doi: 10.1152/ajpcell.00547.2006. [DOI] [PubMed] [Google Scholar]
- Zhang H. M., Keledjian K. M., Rao J. N., Zou T., Liu L., Marasa B. S., Wang S. R., Ru L., Strauch E. D., Wang J. Y. Induced focal adhesion kinase expression suppresses apoptosis by activating NF-κB signaling in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2006;290:C1310–C1320. doi: 10.1152/ajpcell.00450.2005. [DOI] [PubMed] [Google Scholar]
- Zhang H. M., Rao J. N., Guo X., Liu L., Zou T., Turner D. J., Wang J. Y. Akt kinase activation blocks apoptosis in intestinal epithelial cells by inhibiting caspase-3 after polyamine depletion. J. Biol. Chem. 2004;279:22539–22547. doi: 10.1074/jbc.M314337200. [DOI] [PubMed] [Google Scholar]
- Zou T., Mazan-Mamczarz K., Rao J. N., Liu L., Marasa B. S., Zhang A. H., Xiao L., Pullmann R., Gorospe M., Wang J. Y. Polyamine depletion increases cytoplasmic levels of RNA-binding protein HuR leading to stabilization of nucleophosmin and p53 mRNAs. J. Biol. Chem. 2006;281:19387–19394. doi: 10.1074/jbc.M602344200. [DOI] [PubMed] [Google Scholar]
- Zou T., Rao J. N., Guo X., Liu L., Zhang H. M., Strauch E. D., Bass B. L., Wang J. Y. NF-κB-mediated IAP expression induces resistance of intestinal epithelial cells to apoptosis after polyamine depletion. Am. J. Physiol. Cell Physiol. 2004;286:1009–1018. doi: 10.1152/ajpcell.00480.2003. [DOI] [PubMed] [Google Scholar]
- Zou T., Rao J. N., Liu L., Marasa B. S., Keledjian K. M., Zhang A. H., Xiao L., Bass B. L., Wang J. Y. Polyamine depletion induces nucleophosmin modulating stability and transcriptional activity of p53 in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2005;289:686–696. doi: 10.1152/ajpcell.00085.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.