Abstract
Supernumerary centrosomes promote the assembly of abnormal mitotic spindles in many human tumors. In human cells, overexpression of the cyclin-dependent kinase (Cdk)2 partner cyclin A during a prolonged S phase produces extra centrosomes, called centrosome reduplication. Cdk2 activity protects the Mps1 protein kinase from proteasome-mediated degradation, and we demonstrate here that Mps1 mediates cyclin A-dependent centrosome reduplication. Overexpression of cyclin A or a brief proteasome inhibition increases the centrosomal levels of Mps1, whereas depletion of Cdk2 leads to the proteasome-dependent loss of Mps1 from centrosomes only. When a Cdk2 phosphorylation site within Mps1 (T468) is mutated to alanine, Mps1 cannot accumulate at centrosomes or participate in centrosome duplication. In contrast, phosphomimetic mutations at T468 or deletion of the region surrounding T468 prevent the proteasome-dependent removal of Mps1 from centrosomes in the absence of Cdk2 activity. Moreover, cyclin A-dependent centrosome reduplication requires Mps1, and these stabilizing Mps1 mutations cause centrosome reduplication, bypassing cyclin A. Together, our data demonstrate that the region surrounding T468 contains a motif that regulates the accumulation of Mps1 at centrosomes. We suggest that phosphorylation of T468 attenuates the degradation of Mps1 at centrosomes and that preventing this degradation is necessary and sufficient to cause centrosome reduplication in human cells.
INTRODUCTION
The number of centrosomes must be tightly controlled because centrosomes orchestrate the assembly of the mitotic spindle (Lingle and Salisbury, 2000). During mitosis, centrosomes reside at the poles of the mitotic spindle, ensuring the transmission of a single centrosome to each daughter cell. In the subsequent cell cycle, this single centrosome is duplicated in a highly regulated manner to generate the two centrosomes necessary to form a bipolar mitotic spindle. Amplification of centrosome number leads to the production of abnormal mitotic spindles that cause chromosome segregation errors and can therefore generate aneuploidy (Lingle and Salisbury, 2000). The appearance of extra centrosomes is an early event in breast and prostate tumor progression that is thought to drive genetic instability (Lingle et al., 1998; Pihan et al., 2001, 2003; Lingle et al., 2002), and errors in the control of centrosome duplication represent one possible mechanism for the increase in centrosome number (Doxsey, 2002; Nigg, 2002). Many cells derived from human tumors, e.g., the U2OS osteosarcoma cell line (Stucke et al., 2002; Fisk et al., 2003) undergo centrosome reduplication, defined as the execution of multiple rounds of centrosome duplication within a single prolonged S phase. Centrosome reduplication in frog (Hinchcliffe et al., 1999; Lacey et al., 1999), rodent (Matsumoto et al., 1999; Fisk and Winey, 2001), and human (Duensing et al., 2004, 2006) systems requires cyclin-dependent kinase (Cdk)2 activity. Interestingly, despite the critical nature of the Cdk2 partner cyclin E in frogs (Hinchcliffe et al., 1999; Lacey et al., 1999) and rodents (Matsumoto et al., 1999; Fisk and Winey, 2001), overexpression of cyclin E is not sufficient to cause centrosome reduplication in human cells (Meraldi et al., 1999; Balczon, 2001). In contrast, overexpression of the other Cdk2 partner cyclin A does cause centrosome reduplication in human cells (Meraldi et al., 1999; Balczon, 2001). This suggests that in human cells cyclin A directs Cdk2 to a specific subset of its substrates whose phosphorylation promotes centrosome reduplication. Cyclin A seems to be limiting in many human cells (Balczon, 2001), thus precluding centrosome reduplication. However, cyclin A is elevated in a variety of human tumors, including breast tumors (Bukholm et al., 2001; Coletta et al., 2004) where there is strong evidence for the involvement of centrosomes in tumorigenesis (Lingle et al., 1998, 2002; Lingle and Salisbury, 1999).
The Mps1 protein kinase is a Cdk2 substrate (Fisk and Winey, 2001) whose function has been implicated in the control of centrosome duplication (Fisk and Winey, 2001; Fisk et al., 2003) and the spindle assembly checkpoint (Abrieu et al., 2001; Stucke et al., 2002; Fisk et al., 2003; Liu et al., 2003). Mps1 has been reported to localize to centrosomes in mouse (Fisk and Winey, 2001) and human (Fisk et al., 2003) cells, and its overexpression in mouse cells causes centrosome reduplication (Fisk and Winey, 2001). Furthermore, overexpression of a catalytically inactive version of Mps1 (Mps1KD) prevents centrosome duplication in mouse (Fisk and Winey, 2001) and human cells (Fisk et al., 2003), although another study did not reveal any evidence for the localization or function of Mps1 at centrosomes in human cells (Stucke et al., 2002). Mps1 also localizes to kinetochores (Abrieu et al., 2001; Fisk and Winey, 2001; Stucke et al., 2002), and its function in the spindle assembly checkpoint has received the most attention. Mps1 is required for the mitotic spindle checkpoint in yeast (Hardwick et al., 1996; Weiss and Winey, 1996; Palframan et al., 2006), frogs (Abrieu et al., 2001; Zhao and Chen, 2006), and human cells (Stucke et al., 2002; Fisk et al., 2003; Liu et al., 2003; Schmidt et al., 2005; Leng et al., 2006), and for the meiotic spindle checkpoint in flies (Gilliland et al., 2005), frogs (Grimison et al., 2006), and zebrafish (Poss et al., 2004). Mps1 is also required for mitotic arrest in response to hypoxia (Fischer et al., 2004), and it may also function in cytokinesis (Fisk et al., 2003).
Centrosome duplication in vertebrate cells occurs around the G1-to-S transition, and initial studies on human Mps1 showed that its levels are 5- to 10-fold lower at the G1/S transition than at the G2/M transition (Hogg et al., 1994). Furthermore, although the global levels of the Mps1 protein are low and rise only gradually during G1 and S phase, there is a sharp peak of Mps1 kinase activity with high specific activity at the G1/S transition (Hogg et al., 1994). We suggest that the peak of Mps1 activity at the G1/S transition, coincident with the timing of centrosome duplication in mammalian cells, is required for centrosome duplication. In support of this suggestion, the depletion of Mps1 with pools of Mps1-specfic siRNAs prevents centrosome duplication in HeLa cells (Fisk et al., 2003), and titrating Mps1 siRNAs shows that centrosome duplication requires significantly less Mps1 than does the spindle checkpoint (Fisk et al., 2003). However, in contrast to our previous observations in mouse cells (Fisk and Winey, 2001), overexpression of Mps1 is not sufficient to cause centrosome reduplication in human cells (Stucke et al., 2002; Fisk et al., 2003).
Although the reason for this difference between mouse and human cells is unclear, it perhaps reflects differences in the regulation of cyclins A and E. Cyclin E is more critical for centrosome reduplication in mouse cells (Saavedra et al., 2003), whereas cyclin A is more critical for centrosome reduplication in human cells (Meraldi et al., 1999; Balczon, 2001). Given the connection between Cdk2 activity and Mps1 stability, we hypothesized that Mps1 does not cause centrosome reduplication in human cells because there is insufficient Cdk2 activity to prevent its degradation at centrosomes and that overexpression of cyclin A might cause centrosome reduplication by preventing the degradation of Mps1. In exploring these hypotheses we have identified both the specific Cdk2 site within Mps1 and a small region surrounding this site that are responsible for regulating the accumulation of Mps1 at centrosomes, and we hypothesize that Cdk2 attenuates a centrosome-specific Mps1 degradation pathway that is required for the proper control of centrosome duplication.
MATERIALS AND METHODS
Cells and Cell Culture
HeLa S3 and U2OS cells were grown in GlutaMAX DMEM supplemented with 10% fetal bovine serum, 50 U/ml penicillin G, and 50 μg/ml streptomycin (all from Invitrogen, Carlsbad CA) at 37°C in a humidified chamber in the presence of 5% CO2. S phase arrest was achieved using a 24-h treatment with 4 mM hydroxyurea (HU) as described previously (Fisk et al., 2003).
Plasmids
The following expression plasmids were used for this study: previously described plasmids, pHF7 (GFP), pHF36 (GFP-hMps1), pHF56 (GFP-hMps1KD), and PHF65 (GST-hMps1KD) (Fisk and Winey, 2001; Fisk et al., 2003); plasmids created for this study, pHF60 (GFP-Mps1Δ12/13), pHF61 (GFP-e12/13STT), pHF63 (GST-e12/13STT), pHF76 (GST-e12/13AAA), pHF81 (GFP-e12/13AAA), pHF87 (GFP-cyclin A1), pHF96 (GST-Mps1KDAAA), pHF97 (GFP-Mps1AAA), pHF98 (GFP-Mps1KDAAA), pHF134 (GFP-Mps1ATT), pHF135 (GFP-Mps1SAT), pHF136 (GFP-Mps1STA), pHF137 (GFP-Mps1AAT), pHF140 (GFP-Mps1STD), pHF141 (GFP-Mps1STE), pHF142 (GFP-Mps1-PACT), pHF145 (GFP-Mps1Δ12/13-PACT), pHF148 (GFP-PACT), pHF149 (GFP-sirMps1), and pHF150 (GFP-sirMps1AAA). Cyclin A1 was isolated by polymerase chain reaction (PCR) from pCDNA3.1-cyclin A1 (the gift from Dr. Heide Forde, University of Colorado Health Sciences Center, Denver, CO). Although we have presented data for cyclin A1, similar results were obtained using cyclin A2 in all experiments. S436, T453, and T468 were mutated singly or in combination to alanine, aspartic acid, or glutamic acid by using either the USE (United States Biological, Swampscott, MA) or GeneTailor (Invitrogen) site-directed mutagenesis kits. The Mps1 and Mps1AAA cDNAs in pHF36 and pHF97 were rendered resistant to an Mps1 Stealth small interfering RNA (siRNA) (see below) by using the GeneTailor kit to introduce multiple silent mutations at the siRNA binding site. Precise cloning details and primer sequences are available upon request. Green fluorescent protein (GFP)-tagged constructs are expressed from the simian virus 40 promoter and glutathione S-transferase (GST)-tagged constructs are expressed from the pGEX-6P-1 vector (GE Healthcare, Piscataway, NJ) as described previously (Fisk and Winey, 2001; Fisk et al., 2003). To modify the Mps1 C terminus with the Pericentrin and AKAP450 centrosomal targeting (PACT) domain, we obtained a cDNA containing the AKAP450 C terminus (Open Biosystems, Huntsville, AL). Briefly, we generated two PCR products: AKAP450 nucleotides (nt) 11,149–11,646 flanked 5′ by Mps1 nt 2561–2571 and 3′ by a stop codon and NotI site; and Mps1 nt 2325–2571 flanked 3′ by AKAP450 nt 11,149–11,161. A fusion of these PCR products was generated in a PCR reaction containing the Mps1 forward and PACT reverse primers, digested with SacI and NotI to cut the unique SacI site at Mps1 nt 2350 and the introduced NotI site, and used to replace the SacI–NotI fragment in pHF36. The resulting plasmid (pHF142) was sequenced to verify the removal of the Mps1 stop codon (nt 2572-2574) and in frame fusion of the PACT domain at the Mps1 C terminus.
Transfection
GFP and GFP-tagged constructs were expressed by transient transfection using Effectine (QIAGEN, Valencia CA), and the following siRNAs were transfected at 0.2 μM using Oligofectamine (Invitrogen): Mps1 Stealth siRNA (Mps1 nucleotides 1360-1384) and scrambled Mps1 Stealth siRNA (Invitrogen); Cdk2, and cyclin A2 SMARTPool, siGLO Lamin A/C, and siGLO RISC-free control (Dharmacon RNA Technologies, Lafayette, CO). GFP-cyclin A was expressed in Mps1-depleted cells by removing Mps1-siRNA reagents 24 h after transfection and transfecting with GFP-cyclin A for a further 24 h in the presence of 4 mM HU. GFP-Mps1 wild-type and mutant constructs were expressed in Cdk2- and cyclin A-depleted cells in a similar manner. For centrosomal accumulation assays, the correlation between the localization of GFP-Mps1 and centrosomes was analyzed 24 h after completion of the second transfection. For centrosome reduplication assays, cells were examined 48 h after completion of the second transfection. For cDNA rescue of siRNA-mediated Mps1 depletion, we used the same procedure using Mps1 siRNA (1360–1384) and siRNA-resistant GFP-sirMps1 or GFP-sirMps1AAA, but we omitted HU and analyzed centrosome number in asynchronous cells 72 h after siRNA transfection.
Antibodies, Cytology, and Immunoblot Analysis
Indirect immunofluorescence (IIF) was performed as described previously (Fisk and Winey, 2001; Fisk et al., 2003) by using GTU-88 mouse anti-γ-tubulin (Sigma-Aldrich, St. Louis, MO), Ag3 rabbit anti-hMps1 (Fisk et al., 2003; Liu et al., 2003), rabbit H-40 anti-centrin (Santa Cruz Biotechnology, Santa Cruz, CA), and 20H5 mouse anti-centrin (Sanders and Salisbury, 1994). Secondary antibodies were Alexa 488-, 594-, or 750-conjugated donkey anti-rabbit and donkey anti-mouse (Invitrogen), and DNA was stained with Hoechst 33342 (Sigma-Aldrich). All images were acquired at ambient temperature using an Olympus IX-81 microscope, with a 63× Plan Apo oil immersion objective (1.4 numerical aperture) and a QCAM Retiga Exi FAST 1394 camera, and they were analyzed using the Slidebook software package (Intelligent Imaging Innovations, Denver, CO).
For the analysis of centrosomal Mps1 levels, we used a background correction method similar to that described by Howell et al. (2000). HeLa cells transfected with GFP-cyclin A or GFP-cyclin E were arrested in S phase with a 24-h HU treatment, then we analyzed by IIF with antibodies against Mps1 and γ-tubulin. Preimaging visualization was minimized, and the number and timing of exposures were held constant for all images. Background-corrected fluorescence intensity of the Mps1 signal at centrosomes, FM, was determined for centrosomes in pairs of adjacent transfected and untransfected cells that were imaged at the same time as follows: Z-series were deconvoluted using the No Neighbors algorithm, and then they were projected along the z-axis. Using Slidebook we determined the integrated fluorescence intensities of the Mps1 signal in a small 15 × 15 pixel box (FS) surrounding each centrosome and of a large 20 × 20 pixel box (FL) surrounding the first box, and the area of each box (AS and AL). FM was then calculated using the formula described by Howell et al. (2000): FM = (FL − FS) × (AS ÷ (AL − AS)). We then determined FM for centrosomes in 10 cells each for GFP-cyclin A and GFP-cyclin E.
For the analysis of centrosomal accumulation of Mps1, we correlated the localization of GFP-Mps1 with the position of centrosomes as follows. HeLa cells were sequentially transfected with control or Cdk2-specific siRNAs followed by various GFP-Mps1 constructs, arrested in S phase with a 24-h HU treatment, treated with dimethyl sulfoxide DMSO or 5 μM MG115 for 4 h, and then analyzed by IIF with antibodies against γ-tubulin. Preimaging visualization was minimized, and the number and timing of exposures were held constant for all images. Z-series were deconvoluted using the No Neighbors algorithm, and then they were projected along the z-axis. Using Slidebook, we determined the fluorescence intensity of the GFP and γ-tubulin signals along a line drawn through the center of the two centrosomes. The raw fluorescence intensities were then normalized to the maximum intensities along the line. We then examined the normalized intensities to determine the number and position of GFP maxima with respect to γ-tubulin maxima, and the number of cells that satisfied the criteria for centrosome localization described in Results.
For centrosome reduplication assays, centrosome number was determined using γ-tubulin staining as described previously (Fisk et al., 2003). Centrosome number was verified for all experiments using the 20H5 antibody against centrin (Sanders and Salisbury, 1994), which was the generous gift from Dr. Jeffrey Salisbury (Mayo Clinic, Rochester, MN). Values represent the mean ± SD of duplicate samples from three independent experiments. Between 50 and 100 cells were counted for each duplicate sample.
Immunoblot analysis was performed on the Odyssey imaging system (Li-Cor, Lincoln, NE) as described previously (Fisk et al., 2003) by using SCB540 rabbit anti-hMps1 (Santa Cruz Biotechnology), mouse anti-cyclin A, mouse anti-Cdk2, and DM1A mouse anti α-tubulin (Sigma-Aldrich). Secondary antibodies were Alexa 680-conjugated donkey anti-mouse (Invitrogen) and IRDye800-conjugated donkey anti-rabbit (Rockland, Gilbertsville, PA). Efficiency of siRNA knockdown was determined as described previously (Fisk et al., 2003).
Kinase Assays
Kinase assays were performed as described previously (Hartley et al., 1997; Fisk and Winey, 2001) using 1 μg of recombinant protein and 1 unit of human Cdk2/A or Cdk2/E (both from Upstate Biotechnology, Charlottesville, VA). For the mobility shift assay in Figure 1, kinase assays containing 2 mM ATP were subjected to anti-Mps1 immunoblot (using the Ag3 antibody). For the radiographic assays in Figure 4, kinase assays containing 10 μM ATP and 10 μCi of [γ32P]ATP (GE Healthcare) were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) followed by autoradiography of dried gels. The relative phosphorylation levels presented in Figure 4 were estimated as follows. The background corrected pixel intensity of each band from scanned images of autoradiographs was used as a surrogate for the relative incorporation of radioactivity. The relative amount of protein present in each band was determined from Coomassie Blue stained gels using the LI-COR Odyssey scanner. Relative radioactivity was normalized to relative protein. For Figure 4B, the radioactivity-to-protein ratio was normalized both to GST alone and to the difference in molecular weight between GST and GST-e12/13. For Figure 4C, the radioactivity to protein ratio was normalized to the maximal signal.
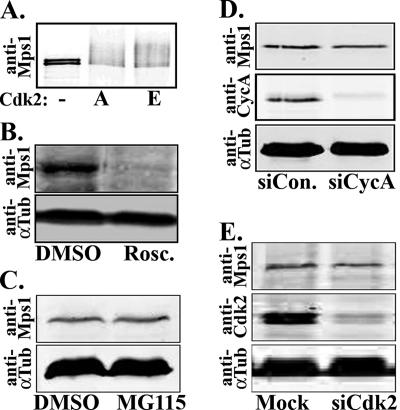
Figure 1.
Depletion of Cdk2 or proteasome activity has little effect on the whole-cell levels of Mps1. (A) Cdk2/A and Cdk2/E phosphorylate GST-Mps1 kinase dead as judged by phosphorylation-dependent mobility shift revealed by immunoblot analysis of kinase assays with the Ag3 anti-Mps1 antibody. (B and C) HeLa cells were arrested in S phase with a 24-h HU treatment, treated with the Cdk2 inhibitor roscovitine (Rosc.), the proteasome inhibitor MG115, or DMSO in the continued presence of HU, and Mps1 levels were analyzed by immunoblot and compared with the α-tubulin loading control. (B) Whole-cell Mps1 levels are depleted by a 24-h treatment with Rosc. (C) Whole-cell Mps1 levels only increased 10% after a 4 h treatment with MG115 (5 μM). (D and E) HeLa cells transfected with control (siCon.), cyclin A- (siCycA), or Cdk2-specific (siCdk2) siRNAs were arrested in S phase with a 24-h HU treatment, and protein levels were analyzed by immunoblot and compared with the α-tubulin loading control. The siRNA transfection efficiency was roughly 90% in all cases. (D) The siRNA-mediated depletion of cyclin A has no effect on the whole-cell levels of Mps1. Cyclin A levels were reduced by 80%. (E) The siRNA-mediated depletion of Cdk2 reduced the whole-cell levels of Mps1 by only 25%. Cdk2 levels were reduced by 85%.
Figure 4.
Mps1 amino acids 420-507 contain a Cdk2-phosphorylated degradation signal. (A) Human Mps1 amino acids 420-507 were compared with Mps1 proteins from a variety of vertebrate species by using the ClustalW program. Purple box/red letter indicates residues identical in all species; blue/yellow indicates residues identical in the majority of species; H.s. Homo sapiens; P.t., Pan troglodytes (chimpanzee); M.f., Macaca fascicularis (Crab eating Macaque); Ma.m., Macaca mulata (rhesus monkey); B.t., Bos taurus (cow); C.f., Canis familiaris (dog); R.n. Rattus norvegicus (rat); Mu.m. Mus musculus (mouse); O.a., Ovis aries (sheep); S.s., Sus scrofa (pig); G.g. Gallus gallus (chicken); X.l., Xenopus laevis (frog); X.l., Xenopus tropicalis (frog); F.r., Fugu rubripes (puffer fish); O.m., Oncorhynchus mykiss (rainbow trout); O.l. Oryzias latipes (rice fish); D.r., Danio rio (zebra fish). (B and C) Kinase assays consisting of purified Cdk2 and GST, GST-e12/13STT (STT), GST-e12/13AAT (AAT), or GST-e12/13AAA (AAA) were analyzed by SDS-PAGE followed by autoradiography. The autoradiographic images (Autorad. and rad) and stained gels (Coomassie and coom) are shown. Numbers below the autoradiographs represent the relative phosphorylation of each band. (B) Cdk2/A phosphorylates the wild-type Mps1 amino acids 420-507 but not the triple alanine mutant. (C) Cdk2/A phosphorylates AAT to the same degree as STT. In contrast Cdk2/E phosphorylates AAT to the same degree as AAA. (D) HeLa cells transfected with GFP-e12/13STT (GFP-STT) or GFP-e12/13AAA (GFP-AAA) were arrested in S phase with a 24-h HU treatment, treated with DMSO or roscovitine (Rosc.) for an additional 24 h in the presence of HU, and then analyzed by immunoblot with antibodies to GFP or α-tubulin as a loading control.
RESULTS
Cdk2 Modulates the Degradation of Mps1 at Centrosomes
The human Mps1 protein is a Cdk2 substrate in vitro (Figure 1A), and the Cdk2 inhibitor roscovitine leads to the proteasome-mediated degradation of the Mps1 protein during S phase arrest in a variety of human cells, including HeLa (Figure 1B), 293, RPE1, MCF7, and 16N cells (data not shown), as expected from our previous data in mouse cells (Fisk and Winey, 2001). Roscovitine does not cause S phase-arrested cells to progress through the cell cycle (Fisk and Winey, 2001), and as in mouse cells (Fisk and Winey, 2001), the decrease in Mps1 in human cells is proteasome dependent (data not shown). However, a brief, 4-h treatment with the proteasome inhibitor MG115 had a comparatively modest effect on the whole cell levels of Mps1, resulting in at most a 10% increase in Mps1 levels on immunoblots (Figure 1C). Similarly, although roscovitine causes the near-complete loss of Mps1, depletion of Cdk2 activity using cyclin A-specific (Figure 1D) or Cdk2-specific (Figure 1E) siRNA molecules had little effect on the whole cell levels of Mps1. After normalization to the α-tubulin loading control, we found that Mps1 levels were unchanged in cells transfected with cyclin A-specific siRNAs and reduced by only 25% in cells transfected with Cdk2-specific siRNAs, despite the 80 and 90% reduction of cyclin A and Cdk2, respectively. Although the reason for this difference between roscovitine and siRNAs is unknown, we speculate that roscovitine may mimic a dominant-negative Cdk2 mutation, whereas siRNA-mediated depletion might allow a second kinase to compensate for Cdk2. Regardless, because it is also possible that roscovitine might inhibit other protein kinases, we limited our studies to the siRNA-mediated depletion of Cdk2 activity.
In addition to having little effect on the whole-cell levels of Mps1, the siRNA-mediated depletion of Cdk2 activity had no obvious effect on the nuclear pore-associated pool of Mps1 reported by Liu et al. (2003) (Figure 2, A and B). However, the centrosomal pool of Mps1 was undetectable in roughly 90% of HeLa cells transfected with either cyclin A-specific (Figure 2A) or Cdk2-specific (Figure 2B) siRNAs, matching well with the roughly 90% transfection efficiency we observed with siRNAs. Therefore, the siRNA-mediated depletion of Cdk2 activity leads to the loss of Mps1 from centrosomes, but not from other locations in the cell. A 4-h MG115 treatment restored the accumulation of Mps1 at centrosomes in Cdk2-siRNA–transfected cells (Figure 2B), consistent with our previous demonstration that Cdk2 activity is not required for the centrosome localization of GFP-Mps1 in mouse cells (Fisk and Winey, 2001). MG115 also increased the levels of Mps1 at centrosomes in mock-transfected cells (Figure 2C), but it had no obvious effect on the nuclear pore-associated pool of Mps1 or the whole-cell level of Mps1 (Figure 1C). Together, these data demonstrate that there is no requirement for Cdk2 in the targeting of Mps1 to centrosomes, that Mps1 is lost from centrosomes but not from other locations in the absence of Cdk2, and that this loss of Mps1 from centrosomes requires proteasome activity. Moreover, the observation that MG115 or Cdk2-specific siRNAs cause dramatic changes in centrosomal Mps1 levels but only modest changes in whole-cell Mps1 suggests that the changes in the whole-cell levels of Mps1 are an indirect consequence of manipulating the level of a minor pool at a specific location. Based on these data, we hypothesize that Cdk2 regulates the levels of Mps1 by modulating a centrosomal degradation event.
Figure 2.
Depletion of Cdk2 or proteasome inhibition greatly affects the centrosomal levels of Mps1. Mock-transfected HeLa cells or HeLa cells transfected with control, cyclin A-, or Cdk2-specific siRNAs were arrested in S phase with (B and C) or without (A) an additional 4-h treatment with MG115 or DMSO and analyzed by IIF. (A) Depletion of cyclin A causes the loss of Mps1 from centrosomes, but not from nuclear pore-associated pools. Arrows indicate centrosomes, carets indicate nuclear pore staining. (B) The loss of Mps1 from centrosomes in cells transfected with Cdk2-specific siRNAs is reversed by a 4-h treatment with 5 μM MG115. (C) Inhibition of the proteasome increases the levels of Mps1 at centrosomes in mock transfected cells. Green, γ-tubulin; red, Mps1; blue, DNA; bar = 5 μm. Insets show fourfold magnified images of centrosomes; bar = 2 μm.
Cyclin A Increases Mps1 Levels at Centrosomes
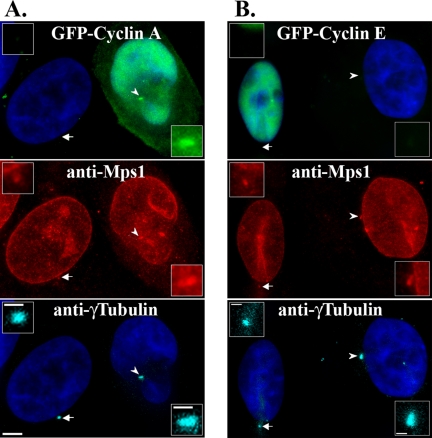
Given that Mps1 is a Cdk2 substrate whose degradation is attenuated by Cdk2 activity, we examined whether overexpressing the Cdk2 partners cyclin A or cyclin E might influence the levels of Mps1 at centrosomes. Using the local background correction method described by Howell et al. (2000), we estimated the centrosomal levels of Mps1 in cells expressing either GFP-cyclin A or GFP-cyclin E by indirect immunofluorescence (IIF). We found that overexpression of cyclin A increased the levels of Mps1 at centrosomes in HeLa cells by ∼2.5-fold (2.4 ± 0.8) (Figure 3A and Supplemental Figure 1) compared with adjacent untransfected cells. Although we cannot rule out the possibility that cyclin A increases Mps1 antibody staining at centrosomes, the antibody we used is unaffected by Cdk2 phosphorylation per se (e.g., Figure 1A). In contrast, overexpression of cyclin E had no effect on the levels of Mps1 (Figure 3B), and the Mps1 signal at centrosomes in cells expressing GFP-cyclin E was not higher (1.1 ± 0.4) than that in adjacent untransfected cells. In our experiments, GFP-cyclin A was found in both the cytoplasm where it localized to centrosomes and in the nucleus (Figure 3A), whereas GFP-cyclin E was almost exclusively nuclear (Figure 3B) and it was found at centrosomes in <5% of cells. However, even in cells where GFP-cyclin E was found at centrosomes, there was no effect on the levels of Mps1 (data not shown).
Figure 3.
Overexpression of cyclin A increases the levels of Mps1 at centrosomes. HeLa cells transfected with GFP-cyclin A (A) or GFP-cyclin E (B) were arrested in S phase with a 24-h HU treatment, and then they were analyzed by IIF. Shown are representative pairs of adjacent transfected and untransfected cells. (A) Overexpression of cyclin A increases Mps1 levels at centrosomes. (B) Overexpression of cyclin E has no effect on Mps1 levels. Red, Mps1; green, GFP; blue, DNA; cyan, γ-tubulin; bar = 5 μm. Insets show twofold magnified images of centrosomes; bar = 2.5 μm.
Mps1 Amino Acids 420-507 Contain a Cdk2-regulated Degradation Signal
The mouse and human Mps1 proteins have no motifs to suggest how they might be degraded, but previous data suggest that Mps1 degradation is regulated by Cdk2 phosphorylation (Fisk and Winey, 2001). We focused our attention on a small region within Mps1 (amino acids 420-507) because we identified a cDNA (Mps1Δ12/13) encoding an Mps1 protein that is not appropriately removed from centrosomes in the absence of Cdk2 activity (discussed below). This region contains five of the nine potential sites of Cdk2 phosphorylation within the Mps1 protein, and it is missing in the Mps1Δ12/13 cDNA from which exons 12 and 13 are deleted. We concentrated on three of these sites (KQS436P, KT453P, and RT468P) because they are close to basic residues and they are highly conserved among vertebrates, two (T453 and T468) being conserved among all vertebrate Mps1 proteins identified to date (Figure 4A). Both cyclin E- and cyclin A-associated Cdk2 complexes (Cdk2/A and Cdk2/E, respectively) phosphorylate a recombinant GST fusion protein containing Mps1 amino acids 410-517 (GST-e12/13STT) in vitro (Figure 4, B and C). Although GST is also phosphorylated, GST-e12/13STT is phosphorylated to a roughly twofold greater extent, suggesting that Cdk2 specifically phosphorylates sites within Mps1 amino acids 420-507. When the predicted Cdk2 phosphorylation sites at S436, T453, and T468 are mutated to alanine, phosphorylation of the resulting fusion protein (GST-e12/13AAA) is reduced to a level similar to that observed for GST alone (Figure 4B). This suggests that S436, T453, and T468 are the predominant sites of Cdk2 phosphorylation within Mps1 amino acids 410-517, at least in vitro. Cdk2/A phosphorylates a mutant version of this fusion protein wherein only T468 is available for phosphorylation (GST-e12/13AAT) at levels comparable with the wild-type fusion protein, whereas the level of Cdk2/E phosphorylation of GST-e12/13AAT is similar to that of GST-e12/13AAA (Figure 4C). This suggests that Cdk2/A predominantly phosphorylates T468, whereas Cdk2/E predominantly phosphorylates S436 and/or T453.
The level of a GFP fusion protein containing Mps1 amino acids 410-517 (GFP-e12/13STT) is greatly reduced by roscovitine treatment in S phase-arrested HeLa cells (Figure 4D). In contrast, the level of the triple alanine mutant version of this fusion protein (GFP-e12/13AAA) is low, and it does not change in response to roscovitine (Figure 4D), suggesting that this region of Mps1 targets GFP for degradation in the absence of Cdk2 activity or when it cannot be phosphorylated at these Cdk2 sites. However, we cannot directly compare the levels of these constructs because their transfection efficiencies vary greatly (roughly 10% for GFP-e12/13STT vs. 50% for GFP-e12/13AAA). Furthermore, as described above, roscovitine affects the whole-cell levels of Mps1, unlike cyclin A- and Cdk2-specific siRNAs that cause Mps1 degradation only at centrosomes. Regardless, these data suggest that Mps1 amino acids 410–517 contain a transferable degradation signal whose function is regulated by Cdk2 phosphorylation. However, because these degradation signal constructs do not localize to centrosomes, we have further explored the function of the putative Mps1 centrosomal degradation signal in the context of the full-length Mps1 protein by fluorescence microscopy in siRNA-transfected cells.
Cdk2 Modulates the Accumulation of Mps1 at Centrosomes via the Mps1 Degradation Signal
To test the hypothesis that Mps1 amino acids 420-507 contain a Cdk2-regulated degradation signal, we analyzed the ability of various GFP-Mps1 proteins to accumulate at centrosomes in S phase-arrested HeLa cells in the presence or absence of Cdk2 and/or proteasome activity. We were unable to use the local background correction method to compare the levels of GFP-Mps1 at centrosomes under various conditions, because expression levels were heterogeneous and because it was impossible to perform comparisons between adjacent cells expressing different constructs under different conditions. Therefore, rather than attempting to estimate the levels of GFP-Mps1 at centrosomes, we analyzed the correlation between the localization of GFP-Mps1 and the position of centrosomes in S phase-arrested HeLa cells. Using the standardized imaging protocol described in Materials and Methods, we determined the normalized GFP and γ-tubulin signals along a line drawn through the center of the centrosomes from 20 cells. We limited our analysis to cells with two centrosomes to minimize any effects due to cell cycle position, and we established the following criteria for centrosome localization: the maximum GFP signal must fall within 2 pixels of one of the two γ-tubulin maxima, and all GFP maxima with a value >0.8 must fall within the boundary of one of the two γ-tubulin peaks. These criteria proved rigorous; only 40% of GFP-Mps1–expressing cells transfected with control siRNAs satisfied our criteria, despite apparent centrosomal localization in virtually all cells expressing wild-type GFP-Mps1 (Table 1). Figure 5A shows a representative projection image from a cell expressing GFP-Mps1 and the normalized GFP and γ-tubulin signals along a line drawn through the center of the centrosomes of the same cell (a).
Table 1.
Correlation of GFP-Mps1 and degradation signal mutants with the position of centrosomes
| % of Cells satisfying centrosome localization criteria |
||||
|---|---|---|---|---|
| DMSO |
MG115 |
|||
| Control | Cdk2 | Control | Cdk2 | |
| GFP-Mps1 | 40 | 10 | N.D. | 38 |
| GFP-Mps1Δ12/13 | 35 | 35 | N.D. | N.D. |
| GFP-Mps1AAA | 10 | 10 | 36 | N.D. |
| GFP-Mps1STD | 43 | 40 | N.D. | N.D. |
| GFP-Mps1STA | 17 | N.D | N.D. | N.D. |
N.D., not determined.
Cells sequentially transfected with either control or Cdk2-specific (Cdk2) siRNAs and the indicated GFP-Mps1 expression construct were analyzed as described in the text and in Figure 5. Twenty cells were examined for each condition, with the exception of STA and STD, for which only 17 and 14 cells were analyzed, respectively. Numbers represent the percentage of cells in which the criteria for centrosome localization of GFP-Mps1 described in the text were met.
Figure 5.
The removal of Mps1 from centrosomes in the absence of Cdk2 requires proteasome activity. HeLa cells were sequentially transfected with control or Cdk2-specific siRNAs (siCdk2) and GFP-Mps1, arrested in S phase with a 24-h HU treatment, treated for 4 h with DMSO or MG115, and then they were analyzed by IIF as described in Materials and Methods. Shown are projection images and the normalized γ-tubulin and GFP signals on a line drawn through the center of the centrosomes in representative cells from each condition. Red, γ-tubulin; green, GFP-Mps1; blue, DNA; bars = 5 μm. Insets show fourfold magnified images of centrosomes; bars = 2 μm. (A) GFP-Mps1 localizes to centrosomes in cells transfected with control siRNAs. (a) Normalized centrosomal γ-tubulin (red line) and GFP (green line) signals. Red brackets indicate the boundaries of the γ-tubulin peaks. Green arrowheads indicate the position of the maximal GFP signal (largest arrowhead) and all GFP maxima within 80% of the maximal GFP signal (small arrowheads). (B) GFP-Mps1 is lost from centrosomes in cells transfected with Cdk2-specific siRNAs. (b) Normalized GFP and γ-tubulin signals; lines, brackets, and arrowheads are as described in A. (C) Proteasome inhibition restores centrosome localization of GFP-Mps1 in cells transfected with Cdk2-specific siRNAs (siCdk2/MG115). (c) Normalized GFP and γ-tubulin signals; lines, brackets, and arrowheads are as described in A.
We then analyzed the ability of GFP-Mps1 to localize to centrosomes in the absence of Cdk2 and proteasome activity. Only 10% of GFP-Mps1–expressing cells transfected with Cdk2-siRNAs satisfied our criteria for centrosomal localization (Table 1 and Figure 5B). Therefore, like endogenous Mps1, GFP-Mps1 cannot efficiently accumulate at centrosomes in the absence of Cdk2 activity. However, a 4-h treatment with MG115 restored the ability of GFP-Mps1 to accumulate at centrosomes in cells transfected with Cdk2-specific siRNAs (Table 1 and Figure 5C). This demonstrates both that there is no requirement for Cdk2 in the binding of GFP-Mps1 to centrosomes and that the absence of GFP-Mps1 from centrosomes in cells transfected with Cdk2-specific siRNAs is due to proteasome activity. Because GFP-Mps1 is readily detected in the cytoplasm in Cdk2-siRNA–transfected cells, we suggest that the proteasome requirement is most likely to reflect a centrosomal degradation event.
To determine whether Mps1 amino acids 420-507 are responsible for the accumulation of Mps1 at centrosomes, we repeated this analysis in cells overexpressing GFP-Mps1Δ12/13, a mutant that lacks the two exons (12 and 13) that encode this putative degradation signal. Similar to wild-type GFP-Mps1, GFP-Mps1Δ12/13 localized to centrosomes in 35% of cells transfected with control siRNAs (Table 1 and Figure 6A). However, unlike wild-type GFP-Mps1, transfection with Cdk2-siRNAs had no effect on the localization of GFP-Mps1Δ12/13 to centrosomes (Table 1 and Figure 6B). This suggests that Mps1 amino acids 420-507 are required for the removal of Mps1 from centrosomes in the absence of Cdk2 activity. To determine whether phosphorylation within this degradation signal regulates the accumulation of Mps1 at centrosomes, we repeated this analysis for GFP-Mps1AAA that cannot be phosphorylated at S436, T453, or T468. GFP-Mps1AAA was readily detectable in the cytoplasm, but unlike wild-type GFP-Mps1, GFP-Mps1AAA only localized to centrosomes in 10% of cells transfected with either control or Cdk2-specific siRNAs (Table 1, Figure 7, A and B). However, after a 4-h treatment with MG115 GFP-Mps1AAA localized to centrosomes in 36% of cells transfected with control siRNAs (Table 1 and Figure 7C). Together, these observations suggest that a centrosome-specific degradation event removes Mps1 from centrosomes in the absence of Cdk2 or when S436, T453, and/or T468 cannot be phosphorylated. We specifically hypothesize that GFP-Mps1AAA is constitutively degraded at centrosomes.
Figure 6.
Mps1 amino acids 420-507 (encoded by exons 12 and 13) are required for the removal of Mps1 from centrosomes in the absence of Cdk2. HeLa cells were sequentially transfected with control or Cdk2-specific siRNAs (siCdk2) and GFP-Mps1Δ12/13 and analyzed as described in Figure 5. Red, γ-tubulin; green, GFP-Mps1Δ12/13; blue, DNA; bars = 5 μm. Insets show fourfold magnified images of centrosomes; bars = 2 μm. (A) GFP-Mps1Δ12/13 localizes to centrosomes in HeLa cells. (a) Normalized γ-tubulin and GFP signals; lines, brackets, and arrowheads are as described in Figure 5. (B) GFP-Mps1Δ12/13 accumulates at centrosomes in the absence of Cdk2. (b) Normalized GFP and γ-tubulin signals.
Figure 7.
Cdk2 phosphorylation sites within Mps1 amino acids 420-507 modulate the accumulation of Mps1 at centrosomes. HeLa cells were sequentially transfected with control or Cdk2-specific siRNAs (siCdk2) and GFP-Mps1AAA and analyzed as described in Figure 5. Red, γ-tubulin; green, GFP-Mps1; blue, DNA; bars = 5 μm. Insets show fourfold magnified images of centrosomes; bars = 2 μm. (A and B) GFP-Mps1AAA does not localize to centrosomes in HeLa cells transfected with either control (A) or Cdk2-specfic (B) siRNAs. (a and b) Normalized GFP and γ-tubulin signals; lines, brackets, and arrowheads are as in Figure 5. (C) Proteasome inhibition allows GFP-Mps1AAA to accumulate at centrosomes in HeLa cells transfected with control siRNAs (siCon./MG115). (c) Normalized GFP and γ-tubulin signals.
The data from this analysis demonstrate that Mps1 amino acids 420-507 are responsible for the loss of Mps1 from centrosomes in the absence of Cdk2 activity and that phosphorylation sites within this region regulate the accumulation of Mps1 at centrosomes. However, Mps1 can accumulate at centrosomes in the absence of Cdk2 activity if this region is removed or if the proteasome is inhibited. Therefore, neither Mps1 amino acids 420-507 nor Cdk2 activity is required for the targeting of Mps1 to centrosomes per se. Because the loss of Mps1 from centrosomes in the absence of Cdk2 activity requires both amino acids 420-507 and proteasome activity, we conclude that this region of Mps1 contains a degradation signal. Based on the observations that the siRNA-mediated depletion of Cdk2 activity and the inhibition of the proteasome influence centrosomal Mps1 but not other pools of Mps1, we favor the hypothesis that Mps1 amino acids 420-507 contain a degradation signal that regulates the degradation of Mps1 at centrosomes.
Mps1 Is Degraded at Centrosomes
To verify that Mps1 can be degraded at centrosomes, we examined an exclusively centrosomal version of Mps1 in the presence and absence of Cdk2 and proteasome activity. The PACT domain (Gillingham and Munro, 2000) was recently used to tether checkpoint kinase (Chk)1 exclusively to centrosomes and demonstrate that Chk1 regulates Cdk1 specifically at centrosomes (Kramer et al., 2004). We inserted the PACT domain at the C terminus of GFP-Mps1, and to the extent we could determine by fluorescence microscopy GFP-Mps1-PACT was exclusively centrosomal (Supplemental Figure 2A). Therefore, if GFP-Mps1-PACT were degraded at centrosomes, there would be no cytoplasmic pool and no way to identify transfected cells for the centrosomal accumulation assay described above. Accordingly, to determine whether GFP-Mps1-PACT was removed from centrosomes in the absence of Cdk2, we estimated the apparent GFP-Mps1-PACT transfection efficiency under various conditions. Treatment with MG115 caused a roughly fivefold increase in the percentage of HeLa cells transfected with Cdk2-specific siRNAs that expressed GFP-Mps1-PACT, compared with identical samples from the same transfection treated with DMSO (Supplemental Figure 2B). MG115 treatment of cells transfected with only GFP-Mps1-PACT also increased the percentage of GFP-positive cells. These data demonstrate that an exclusively centrosomal version of Mps1 is removed from centrosomes by proteasome activity in the absence of Cdk2 activity. Because the PACT domain presumably tethers proteins to centrosomes independently of the Mps1 centrosomal binding partners, it is likely that it is GFP-Mps1-PACT itself rather than the centrosomal Mps1 binding site that is the direct target of proteasome-mediated degradation.
Accumulation of Mps1 at Centrosomes Is Essential for the Function of Mps1 in Centrosome Duplication
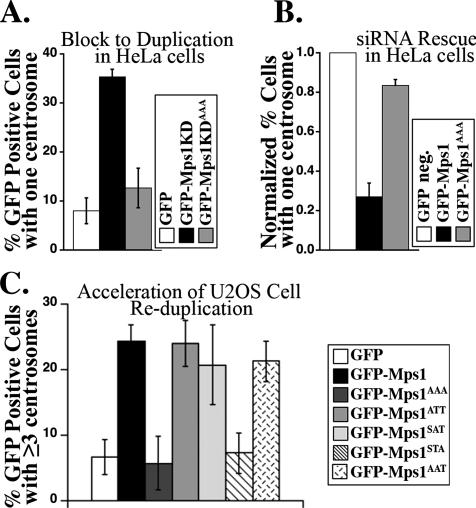
To determine the importance of Mps1 degradation control for the normal centrosome duplication cycle, we examined the ability of Mps1KDAAA to prevent centrosome duplication, and the ability of Mps1AAA to substitute for Mps1 in the normal centrosome duplication cycle. We previously demonstrated that Mps1KD prevents centrosome duplication in mouse (Fisk and Winey, 2001) and human cells (Fisk et al., 2003) and that Mps1 is required for the normal centrosome duplication cycle in human cells (Fisk et al., 2003). Although roughly 35% of HU-arrested HeLa cells expressing GFP-Mps1KD possess only a single centrosome only 13% of HU-arrested HeLa cells expressing GFP-Mps1KDAAA have a single centrosome (Figure 8A). Because mutating S436, T453, and T468 to alanine prevents the accumulation of Mps1 at centrosomes, this suggests that the ability of Mps1KD to efficiently prevent the normal centrosome duplication cycle requires its presence at centrosomes.
Figure 8.
Accumulation of Mps1 at centrosomes is critical for the function of Mps1 in centrosome duplication. (A) Bar graph showing the percentage of HeLa cells expressing GFP (white), GFP-Mps1KD (black), or GFP-Mps1KDAAA (gray) that have a single centrosome after 24 h of S phase arrest. (B) Bar graph showing the ability of Mps1 and Mps1AAA to rescue the Mps1-siRNA phenotype. The percentage of asynchronous HeLa cells with a single centrosome was determined 72 h after transfection with an Mps1-specific siRNA in cells expressing siRNA-resistant (sir) Mps1 (GFP-sirMps1, black) and Mps1AAA (GFP-sirMps1AAA, gray) and normalized to the percentage of GFP-negative cells (GFP neg., white) with a single centrosome from the same experiment. (C) Bar graph showing the percentage of U2OS cells expressing GFP (white), GFP-Mps1 (black), GFP-Mps1AAA (dark gray), GFP-Mps1ATT (gray), GFP-Mps1SAT (light gray), GFP-Mps1STA (striped), or GFP-Mps1AAT (stippled) that have three or more centrosomes after the first 24 h of S phase arrest. Values represent the mean ± SD of triplicate samples, with 50–100 cells per sample.
Although the observation that Mps1KD prevents centrosome duplication does not demonstrate that Mps1 is required for centrosome duplication, we have also shown that pools of Mps1-specific siRNAs prevent centrosome duplication in HeLa cells (Fisk et al., 2003). We have subsequently identified a Stealth siRNA (Invitrogen) specific to Mps1 nucleotides 1360–1384 that recapitulates the phenotype observed using siRNA pools; whereas only roughly 5% of cells in asynchronous populations transfected with control siRNA have a single centrosome, roughly 25% of cells transfected with this Stealth siRNA have a single centrosome. This is actually an underestimate of centrosome duplication failures, because like our siRNA pools (Fisk et al., 2003), this Stealth siRNA also causes mitotic catastrophe and cytokinesis failures that mask centrosome duplication failures by generating cells that enter the subsequent cell cycle with two centrosomes. A version of GFP-Mps1 engineered to be resistant to this stealth siRNA (GFP-sirMps1) reduces the number of cells with a single centrosome in Stealth siRNA-transfected populations by nearly fivefold (Figure 8B). In contrast, GFP-sirMps1AAA reduced the number of Stealth siRNA-transfected cells with a single centrosome by only 1.2-fold (Figure 8B). These data suggest that although GFP-sirMps1 can substitute for the function of endogenous Mps1 in centrosome duplication, GFP-sirMps1AAA cannot. Therefore, the ability of Mps1 to accumulate at centrosomes is important for the normal centrosome duplication cycle.
T468 Phosphorylation Is Essential for Mps1 Function in the U2OS Centrosome Reduplication Assay
Although overexpression of wild-type Mps1 is not sufficient to cause centrosome reduplication in human cells (Stucke et al., 2002; Fisk et al., 2003), it does modulate the centrosome reduplication that occurs during prolonged S phase arrest in some human tumor-derived cells, such as the U2OS osteosarcoma cell line; although overexpression of Mps1 has no effect on the overall extent of reduplication in U2OS cells after 48 h of S phase arrest (Stucke et al., 2002; Fisk et al., 2003) the catalytically inactive Mps1KD prevents centrosome reduplication in U2OS cells (Fisk et al., 2003). Furthermore, overexpression of Mps1 accelerates the onset of centrosome reduplication in U2OS cells (Fisk et al., 2003; Kanai et al., 2007). After only 24 h of S phase arrest, U2OS cells have not yet initiated centrosome reduplication, but at this early time point overexpression of GFP-Mps1 increases the number of U2OS cells with excess centrosomes by roughly fivefold compared with cells overexpressing GFP alone (Figure 8C) (Fisk et al., 2003). Although we have previously documented that γ-tubulin staining accurately reports the production of extra centrioles upon the overexpression of Mps1 (Fisk and Winey, 2001; Fisk et al., 2003), we have verified that overexpression of Mps1 accelerates the production of extra centrioles in this assay by using an antibody against centrin (Supplemental Figure 3A). However, GFP-Mps1AAA had no effect in the U2OS centrosome reduplication assay, and after 24 h of S phase arrest centrosome number in U2OS cells overexpressing GFP-Mps1AAA was similar to that in cells overexpressing GFP alone (Figure 8C). These data suggest that the ability of Mps1 to accumulate at centrosomes is critical for its function in the U2OS centrosome reduplication assay.
We exploited the U2OS assay to determine which of the three Cdk2 sites within the Mps1 degradation signal is responsible for regulating the accumulation of Mps1 at centrosomes. Like wild-type GFP-Mps1, both the S436A and T453A single mutants (GFP-Mps1ATT, GFP-Mps1SAT) accelerated the onset of centrosome reduplication in U2OS cells (Figure 8). In contrast, the T468A single mutant (GFP-Mps1STA) failed to accelerate the onset of centrosome reduplication in U2OS cells (Figure 8C). This suggests that the ability of Mps1 to accelerate the onset of centrosome reduplication in U2OS cells requires phosphorylation of T468, but not that of S436 or T453. In support of this suggestion, the double S436A/T453A mutant version of Mps1 (GFP-Mps1AAT) wherein only T468 could be phosphorylated also accelerated the onset of centrosome reduplication in U2OS cells (Figure 8). Furthermore, GFP-Mps1STA recapitulated the behavior of GFP-Mps1AAA in the centrosome accumulation assay described above (Table 1 and Supplemental Figure 4). Together, these data suggest that phosphorylation at T468 can attenuate the degradation of Mps1 at centrosomes, but phosphorylation at S436 or T453 cannot.
Cyclin A-dependent Centrosome Reduplication Requires Mps1
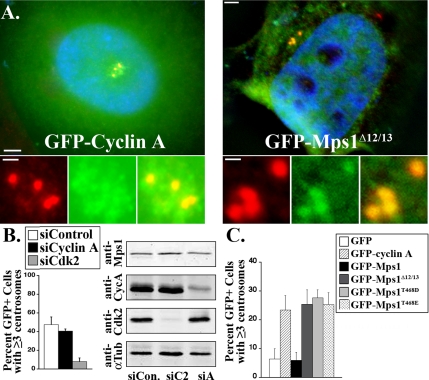
Unlike U2OS cells, HeLa cells do not normally undergo centrosome reduplication during a prolonged S phase arrest. Overexpression of cyclin A has been reported to cause centrosome reduplication in human cells, whereas overexpression of cyclin E does not (Meraldi et al., 1999; Balczon, 2001). The experiments described above demonstrate that Cdk2 activity regulates the accumulation of Mps1 at centrosomes through T468 and that only cyclin A phosphorylates T468 in vitro. Together, these observations suggest that perhaps cyclin A causes centrosome reduplication by attenuating the degradation of Mps1 and increasing its levels at centrosomes. To test this suggestion, we have examined the ability of cyclin A to cause centrosome reduplication in S phase–arrested HeLa cells transfected with the Stealth siRNA specific to Mps1 nucleotides 1360–1384 described above. Consistent with the previous reports, transient transfection of HeLa cells with GFP-cyclin A caused centrosome reduplication in HeLa cells transfected with control siRNAs (Figures 9 and 10), whereas overexpression of GFP-cyclin E did not (data not shown). Whereas the data in Figure 9 were collected using an antibody against γ-tubulin, we have verified that overexpression of cyclin A causes the production of extra centrioles using an antibody against centrin (Supplemental Figure 3, B and C). We observed a roughly 90% transfection efficiency with our Mps1-siRNAs and an 84% reduction in Mps1 levels (Figure 9). Depletion of Mps1 had no effect on the levels of cyclin A in these cells, but abrogated cyclin A-dependent centrosome reduplication (Figure 9). This demonstrates that Mps1 is required for cyclin A-dependent centrosome reduplication.
Figure 9.
Cyclin A-dependent centrosome reduplication requires Mps1. (A) Bar graph showing the percentage of S phase-arrested HeLa cells transfected with control (white) or Mps1-specific (black) siRNAs expressing GFP-cyclin A (GFP+) with three or more centrosomes after 48 h of S phase arrest. Values represent mean ± SD of triplicate samples, with 50–100 cells per sample. (B) Protein levels were analyzed by immunoblot. The siRNA transfection efficiency was roughly 90%, Mps1 levels were reduced by 84%, and levels of endogenous cyclin A were unaffected.
Figure 10.
Preventing the degradation of Mps1 at centrosomes is sufficient to cause centrosome reduplication in human cells. (A) GFP-Mps1Δ12/13 causes centrosome reduplication that is indistinguishable from that caused by GFP-cyclin A. Red, γ-tubulin; green, GFP; blue, DNA; bar = 5 μm. Insets show fourfold magnified images of centrosomes; bar = 2 μm. (B) Bar graph showing the percentage of HeLa cells transfected with control (white), cyclin A- (black), or Cdk2-specific (gray) siRNAs expressing GFP-Mps1Δ12/13 (GFP+) with three or more centrosomes (as judged by γ-tubulin staining) after 48 h of S phase arrest. The siRNA transfection efficiency was roughly 90%. Cdk2 was reduced by 94% and cyclin A by 80%. Cyclin A-specific siRNAs did not affect Mps1, whereas Cdk2-specific siRNAs reduced Mps1 by roughly 25%. (C) Bar graph showing the percentage of S-phase arrested HeLa cells with a given centrosome number (as judged by γ-tubulin staining) after 48 h of S-phase arrest expressing GFP (white), GFP-cyclin A (striped), GFP-Mps1 (black), GFP-Mps1Δ12/13, (dark gray), GFP-Mps1T468D (light gray), or GFP-Mps1T468E (stippled). Values represent the mean ± SD of triplicate samples, with 50–100 cells per sample.
Preventing Mps1 Degradation Bypasses the Requirement for Cyclin A in Centrosome Reduplication
Together, the observations that cyclin A overexpression increases the levels of Mps1 at centrosomes and that cyclin A-dependent centrosome reduplication requires Mps1 suggest that cyclin A might cause centrosome reduplication by preventing the degradation of Mps1 at centrosomes. This makes the prediction that preventing the degradation of Mps1 at centrosomes independently of cyclin A overexpression would also cause centrosome reduplication. To test this prediction, we have examined centrosome reduplication in HeLa cells overexpressing GFP-Mps1Δ12/13 that lacks the Mps1 degradation signal and is not removed from centrosomes in Cdk2-siRNA transfected cells. We have found that unlike GFP-Mps1, GFP-Mps1Δ12/13 causes centrosome reduplication that is indistinguishable from that caused by the overexpression of cyclin A in a variety of cell types, including HeLa (Figure 10A), 293, MCF7, and 16N cells (data not shown). Moreover, GFP-Mps1Δ12/13 can also cause centrosome reduplication in HeLa cells transfected with cyclin A-specific siRNAs, despite an 80% reduction of cyclin A levels (Figure 10B). This is not a neomorphic effect of Mps1Δ12/13, because Mps1Δ12/13-dependent centrosome reduplication still requires Cdk2 and is abrogated by both Cdk2-specific siRNAs (Figure 10B) and roscovitine. Although the data in Figure 10B were collected using an antibody against γ-tubulin, we have verified that like the overexpression of cyclin A, overexpression of Mps1Δ12/13 causes the production of extra centrioles by using an antibody against centrin (Supplemental Figure 3, B and C). These data suggest that the essential function of cyclin A in centrosome reduplication is to prevent the degradation of Mps1 at centrosomes.
Similar to the extent of reduplication caused by Mps1 in mouse cells (Fisk and Winey, 2001), GFP-Mps1Δ12/13-PACT causes centrosome reduplication in roughly 80% of cells (Supplemental Figure 5), a much greater extent than caused by GFP-Mps1Δ12/13 lacking the PACT domain. To a lesser extent GFP-Mps1-PACT also causes centrosome reduplication (Supplemental Figure 5). These data suggest that the centrosomal pool of Mps1 is responsible for centrosome reduplication and that artificially tethering Mps1 to centrosomes raises its levels above a threshold required for centrosome reduplication. However, GFP-PACT also caused modest reduplication (Supplemental Figure 5) and the PACT domain can perturb centrosome structure and function by displacing endogenous pericentrin and AKAP450 (Gillingham and Munro, 2000; Keryer et al., 2003; Mikule et al., 2007). Therefore, these data should be interpreted conservatively.
Given that our in vitro kinase and U2OS centrosome reduplication assays both implicate T468 as the relevant phosphorylation site, we also examined the consequences of mimicking phosphorylation at T468. We found that mutation of T468 to aspartic acid (T468D) or glutamic acid (T468E) mimicked the effect of Mps1Δ12/13. Both GFP-Mps1T468D and GFP-Mps1T468E cause centrosome reduplication that is indistinguishable from that caused by cyclin A or Mps1Δ12/13 (Figure 10C), and GFP-Mps1T468D was not removed from centrosomes in Cdk2-siRNA–transfected cells (Table 1 and Supplemental Figure 6). These data demonstrate that removing the Mps1 degradation signal or mimicking constitutive phosphorylation within the Mps1 degradation signal is sufficient to cause centrosome reduplication in human cells. Taken together, our data suggest that the levels of Mps1 at centrosomes are critical for the temporal restriction of centrosome duplication and that they are regulated by competing phosphorylation and degradation.
DISCUSSION
We previously reported that Cdk2 activity prevents the proteasome-mediated degradation of its substrate Mps1 during S phase (Fisk and Winey, 2001). In this study, we localize the site of CDK-regulated degradation to centrosomes, identify a single Cdk2 phosphorylation site and a degradation signal within Mps1 that regulate the accumulation of Mps1 at centrosomes, and we describe three mutant Mps1 alleles that cause centrosome reduplication. Our data also suggest that Mps1 is the essential Cdk2 substrate in cyclin A-dependent centrosome reduplication and that preventing the degradation of Mps1 at centrosomes is sufficient to cause centrosome reduplication in human cells. Although it has been controversial (Stucke et al., 2002), these new data are most easily interpreted if one assumes a centrosomal localization and centrosome duplication function for Mps1.
Centrosome-specific proteolysis has been previously implicated in centrosome duplication and function. The anaphase promoting complex/cyclosome (APC/C)-dependent, centrosome-specific degradation of Nek2 is part of a mechanism that regulates centrosome separation (Hames et al., 2005). In addition, recent evidence suggests that activation of the cysteine protease separase during mitosis leads to the disengagement of centrioles and licenses them for replication in the subsequent cell cycle (Tsou and Stearns, 2006). Skp1/Cdc53/F-box–dependent proteolysis has also been implicated in the control of centrosome duplication in flies (Wojcik et al., 2000; Murphy, 2003), frogs (Freed et al., 1999), and mammals (Nakayama et al., 2000). Furthermore, active proteasome complexes are found at centrosomes (Fabunmi et al., 2000), and several proteasome subunits were found in the recent proteomic analysis of the human centrosome (Andersen et al., 2003). Our hypothesis that Mps1 is degraded at centrosomes is supported by the proteasome dependence of the centrosomal accumulation of GFP-Mps1-PACT that is presumably tethered to centrosomes independently of Mps1 centrosomal binding partners.
Our results place Mps1 in a growing list of proteins including c-jun (Musti et al., 1997), c-myc (Yeh et al., 2004), FUS (Perrotti et al., 2000), and Cdc6 (Mailand and Diffley, 2005) for which phosphorylation attenuates degradation. In two cases, Cdk2 is responsible for attenuating degradation. We concluded previously that Cdk2 attenuates Mps1 degradation as a mechanism restricting when centrosome duplication can occur (Fisk and Winey, 2001) that is apparently conserved from yeast to mammals (Jaspersen et al., 2004). Mailand and Diffley recently demonstrated that Cdk2 also attenuates the degradation of Cdc6 to restrict when DNA can replicate (Mailand and Diffley, 2005). Therefore, Cdk2 seems to regulate two distinct cell cycle processes that involve precise duplication mechanisms by attenuating degradation of the critical regulators.
Cdc6 has both D- and KEN-type destruction boxes, and phosphorylation at three Cdk2 sites adjacent to these motifs attenuates its APC/C-dependent degradation (Mailand and Diffley, 2005). Like Cdc6, the yeast Mps1p has both D-and KEN-boxes, and it is degraded in an APC/C-dependent manner (Palframan et al., 2006). In contrast, the mechanism of human Mps1 degradation is unknown, although our data implicate a single Cdk2 site in its control. Amino acids 420-507 of the human Mps1 protein are sufficient to recapitulate regulated degradation but lack all known APC/C recognition motifs, and the degradation of GFP-e12/13AAA is not enhanced by roscovitine, a treatment that activates the APC/C and stimulates the degradation of Cdc6AAA (Mailand and Diffley, 2005). Human Mps1 degradation is also distinguished from that of Cdc6, because it occurs at centrosomes. The precise motif responsible for degradation of the human Mps1 and the proteins it binds to remain to be determined, suggesting that additional centrosomal degradation machinery remains to be discovered.
We initially implicated Cdk2 in Mps1 degradation by using the Cdk2 inhibitor roscovitine (Fisk and Winey, 2001). However, siRNAs do not mimic the effect of roscovitine on whole-cell Mps1 levels. This might reflect multiple targets for roscovitine, or compensation by a second kinase in siRNA-transfected cells. In support of the later suggestion, centrosomes duplicate normally in Cdk2 null mice (Berthet et al., 2003) and in human cells transfected with Cdk2-specific siRNAs (Tetsu and McCormick, 2003), whereas dominant-negative Cdk2 mutations and other Cdk2 perturbations cause cell cycle arrest (e.g., Berthet et al., 2003) and prevent centrosome duplication (Meraldi and Nigg, 2001). We limited this study to siRNAs, allowing us to specifically examine the centrosomal accumulation of Mps1. Together, our data suggest that Cdk2 regulates the accumulation of Mps1 at centrosomes, but that only cyclin A can direct Cdk2 to phosphorylate T468 within the Mps1 degradation signal. This fits well with the previous observations that only cyclin A can cause centrosome reduplication in human cells (Meraldi et al., 1999; Balczon, 2001). Our data provide likely explanations for the failure of cyclin E and wild-type Mps1 to cause centrosome reduplication in human cells. We hypothesize that cyclin E cannot cause centrosome reduplication in human cells, because it lacks the subcellular distribution and substrate specificity required to regulate Mps1 at centrosomes. Similarly, we hypothesize that wild-type Mps1 does not cause centrosome reduplication, because cyclin A is limiting, as has been suggested (Balczon, 2001), and because T468 cannot be efficiently phosphorylated the overexpressed Mps1 protein is rapidly degraded at centrosomes. Mimicking T468 phosphorylation or removing the Mps1 degradation signal bypasses the requirement for cyclin A in centrosome reduplication, but not the requirement for Cdk2.
The observations that proteasome inhibition or Cdk2-depletion cause only modest changes in whole-cell Mps1 levels but have dramatic effects on centrosomal Mps1 pools suggest that a minor pool of Mps1 is degraded at a specific site. Our hypothesis that the site of Mps1 degradation is the centrosome is also supported by three mutations that allow Mps1 to accumulate at centrosomes in the absence of Cdk2 and cause centrosome reduplication in the absence of cyclin A. Together, these data demonstrate that the essential function of cyclin A in centrosome reduplication is to increase the levels of Mps1 at centrosomes and that preventing the degradation of Mps1 at centrosomes is sufficient to cause centrosome reduplication in human cells. We propose that the level of Mps1 at centrosomes is critical for the temporal regulation of centrosome duplication and that it is controlled by a balance between mutually exclusive proteasome-mediated degradation and phosphorylation at T468. This proposition is supported by our observation that Mps1AAA cannot accumulate at centrosomes and cannot substitute for the function of endogenous Mps1 in centrosome duplication. Our proposed model represents a mechanism for exerting tight control over the local concentration of Mps1 at centrosomes, and it is supported by the cell cycle profile of Mps1. Hogg et al. (1994) found that there is a sharp peak of Mps1 kinase activity at the G1/S transition, whereas the whole-cell levels of Mps1 are very low at the G1/S transition and increase only gradually through S phase. Based on these data, Hogg et al. (1994) hypothesized the existence of multiple pools of Mps1 that are differentially regulated (Hogg et al., 1994). Our data suggest that the peak of Mps1 kinase activity at the G1/S transition results from the attenuation of Mps1 degradation at centrosomes, which allows the accumulation of a centrosomal Mps1 pool that controls the normal centrosome duplication cycle. Our data also support the hypothesis that it is the centrosomal pool of Mps1 that drives the events of centrosome duplication and that increasing the level of this pool and/or extending the duration for which it persists is sufficient to cause centrosome reduplication in human cells.
Supplementary Material
ACKNOWLEDGMENTS
We thank Michelle Jones, Berl Oakley, Steve Osmani, Susan Cole, Richard Fishel, and Michael Ostrowski for critical reading of this manuscript. We are extremely grateful to Emma Lees (DNAX Research, Palo Alto CA) for providing the hMps1Ag3 antibody. This work was supported by National Institutes of Health grant GM-51312 (to M.W.), aided by a Special Fellow Award from the Leukemia and Lymphoma Society (to H.A.F.), and by seed grants from The Ohio State University Comprehensive Cancer Center Institutional American Cancer Society (ACS) fund and the ACS, Ohio Division (to H.A.F.).
Abbreviations used:
- Cdk2/A
cyclin A-associated Cdk2
- Cdk2/E
cyclin E-associated Cdk2
- HU
hydroxyurea
- IIF
indirect immunofluorescence
- siRNA
small interfering RNA.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-03-0283) on September 5, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Abrieu A., Magnaghi-Jaulin L., Kahana J. A., Peter M., Castro A., Vigneron S., Lorca T., Cleveland D. W., Labbe J. C. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell. 2001;106:83–93. doi: 10.1016/s0092-8674(01)00410-x. [DOI] [PubMed] [Google Scholar]
- Andersen J. S., Wilkinson C. J., Mayor T., Mortensen P., Nigg E. A., Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–574. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- Balczon R. C. Overexpression of cyclin A in human HeLa cells induces detachment of kinetochores and spindle pole/centrosome overproduction. Chromosoma. 2001;110:381–392. doi: 10.1007/s004120100157. [DOI] [PubMed] [Google Scholar]
- Berthet C., Aleem E., Coppola V., Tessarollo L., Kaldis P. Cdk2 knockout mice are viable. Curr. Biol. 2003;13:1775–1785. doi: 10.1016/j.cub.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Bukholm I. R., Bukholm G., Nesland J. M. Over-expression of cyclin A is highly associated with early relapse and reduced survival in patients with primary breast carcinomas. Int. J. Cancer. 2001;93:283–287. doi: 10.1002/ijc.1311. [DOI] [PubMed] [Google Scholar]
- Coletta R. D., et al. The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1. Proc. Natl. Acad. Sci. USA. 2004;101:6478–6483. doi: 10.1073/pnas.0401139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey S. Duplicating dangerously: linking centrosome duplication and aneuploidy. Mol. Cell. 2002;10:439–440. doi: 10.1016/s1097-2765(02)00654-8. [DOI] [PubMed] [Google Scholar]
- Duensing A., Liu Y., Tseng M., Malumbres M., Barbacid M., Duensing S. Cyclin-dependent kinase 2 is dispensable for normal centrosome duplication but required for oncogene-induced centrosome overduplication. Oncogene. 2006;25:2943–2949. doi: 10.1038/sj.onc.1209310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing S., Duensing A., Lee D. C., Edwards K. M., Piboonniyom S. O., Manuel E., Skaltsounis L., Meijer L., Munger K. Cyclin-dependent kinase inhibitor indirubin-3′-oxime selectively inhibits human papillomavirus type 16 E7-induced numerical centrosome anomalies. Oncogene. 2004;23:8206–8215. doi: 10.1038/sj.onc.1208012. [DOI] [PubMed] [Google Scholar]
- Fabunmi R. P., Wigley W. C., Thomas P. J., DeMartino G. N. Activity and regulation of the centrosome-associated proteasome. J. Biol. Chem. 2000;275:409–413. doi: 10.1074/jbc.275.1.409. [DOI] [PubMed] [Google Scholar]
- Fischer M. G., Heeger S., Hacker U., Lehner C. F. The mitotic arrest in response to hypoxia and of polar bodies during early embryogenesis requires Drosophila Mps1. Curr. Biol. 2004;14:2019–2024. doi: 10.1016/j.cub.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Fisk H. A., Mattison C. P., Winey M. Human Mps1 protein kinase is required for centrosome duplication and normal mitotic progression. Proc. Natl. Acad. Sci. USA. 2003;100:14875–14880. doi: 10.1073/pnas.2434156100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk H. A., Winey M. The mouse mps1p-like kinase regulates centrosome duplication. Cell. 2001;106:95–104. doi: 10.1016/s0092-8674(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Freed E., Lacey K. R., Huie P., Lyapina S. A., Deshaies R. J., Stearns T., Jackson P. K. Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle. Genes Dev. 1999;13:2242–2257. doi: 10.1101/gad.13.17.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland W. D., Wayson S. M., Hawley R. S. The meiotic defects of mutants in the Drosophila mps1 gene reveal a critical role of Mps1 in the segregation of achiasmate homologs. Curr. Biol. 2005;15:672–677. doi: 10.1016/j.cub.2005.02.062. [DOI] [PubMed] [Google Scholar]
- Gillingham A. K., Munro S. The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep. 2000;1:524–529. doi: 10.1093/embo-reports/kvd105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimison B., Liu J., Lewellyn A. L., Maller J. L. Metaphase arrest by cyclin E-Cdk2 requires the spindle-checkpoint kinase Mps1. Curr. Biol. 2006;16:1968–1973. doi: 10.1016/j.cub.2006.08.055. [DOI] [PubMed] [Google Scholar]
- Hames R. S., Crookes R. E., Straatman K. R., Merdes A., Hayes M. J., Faragher A. J., Fry A. M. Dynamic recruitment of Nek2 kinase to the centrosome involves microtubules, PCM-1, and localized proteasomal degradation. Mol. Biol. Cell. 2005;16:1711–1724. doi: 10.1091/mbc.E04-08-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick K., Weiss E., Luca F. C., Winey M., Murray A. Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science. 1996;273:953–956. doi: 10.1126/science.273.5277.953. [DOI] [PubMed] [Google Scholar]
- Hartley R. S., Sible J. C., Lewellyn A. L., Maller J. L. A role for cyclin E/Cdk2 in the timing of the midblastula transition in Xenopus embryos. Dev. Biol. 1997;188:312–321. doi: 10.1006/dbio.1997.8647. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe E. H., Li C., Thompson E. A., Maller J. L., Sluder G. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extract. Science. 1999;283:851–854. doi: 10.1126/science.283.5403.851. [DOI] [PubMed] [Google Scholar]
- Hogg D., Guidos C., Bailey D., Amendola A., Groves T., Davidson J., Schmandt R., Mills G. Cell cycle dependent regulation of the protein kinase TTK. Oncogene. 1994;9:89–96. [PubMed] [Google Scholar]
- Howell B. J., Hoffman D. B., Fang G., Murray A. W., Salmon E. D. Visualization of Mad2 dynamics at kinetochores, along spindle fibers, and at spindle poles in living cells. J. Cell Biol. 2000;150:1233–1250. doi: 10.1083/jcb.150.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspersen S. L., Huneycutt B. J., Giddings T. H., Jr, Resing K. A., Ahn N. G., Winey M. Cdc28/Cdk1 regulates spindle pole body duplication through phosphorylation of Spc42 and Mps1. Dev. Cell. 2004;7:263–274. doi: 10.1016/j.devcel.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kanai M., Ma Z., Izumi H., Kim S. H., Mattison C. P., Winey M., Fukasawa K. Physical and functional interaction between mortalin and Mps1 kinase. Genes Cells. 2007;12:797–810. doi: 10.1111/j.1365-2443.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- Keryer G., Witczak O., Delouvee A., Kemmner W. A., Rouillard D., Tasken K., Bornens M. Dissociating the centrosomal matrix protein AKAP450 from centrioles impairs centriole duplication and cell cycle progression. Mol. Biol. Cell. 2003;14:2436–2446. doi: 10.1091/mbc.E02-09-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A., Mailand N., Lukas C., Syljuasen R. G., Wilkinson C. J., Nigg E. A., Bartek J., Lukas J. Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase. Nat. Cell Biol. 2004;6:884–891. doi: 10.1038/ncb1165. [DOI] [PubMed] [Google Scholar]
- Lacey K., Jackson P., Stearns T. Cyclin-dependent kinase control of centrosome duplication. Proc. Natl. Acad. Sci. USA. 1999;96:2817–2822. doi: 10.1073/pnas.96.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng M., Chan D. W., Luo H., Zhu C., Qin J., Wang Y. MPS1-dependent mitotic BLM phosphorylation is important for chromosome stability. Proc. Natl. Acad. Sci. USA. 2006;103:11485–11490. doi: 10.1073/pnas.0601828103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle W. L., Barrett S. L., Negron V. C., D'Assoro A. B., Boeneman K., Liu W., Whitehead C. M., Reynolds C., Salisbury J. L. Centrosome amplification drives chromosomal instability in breast tumor development. Proc. Natl. Acad. Sci. USA. 2002;99:1978–1983. doi: 10.1073/pnas.032479999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle W. L., Lutz W. H., Ingle J. N., Maihle N. J., Salisbury J. L. Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc. Natl. Acad. Sci. USA. 1998;95:2950–2955. doi: 10.1073/pnas.95.6.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle W. L., Salisbury J. L. Altered centrosome structure is associated with abnormal mitoses in human breast tumors. Am. J. Pathol. 1999;155:1941–1951. doi: 10.1016/S0002-9440(10)65513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle W. L., Salisbury J. L. The role of the centrosome in the development of malignant tumors. Curr. Top. Dev. Biol. 2000;49:313–329. doi: 10.1016/s0070-2153(99)49015-5. [DOI] [PubMed] [Google Scholar]
- Liu S. T., Chan G. K., Hittle J. C., Fujii G., Lees E., Yen T. J. Human MPS1 kinase is required for mitotic arrest induced by the loss of CENP-E from kinetochores. Mol. Biol. Cell. 2003;14:1638–1651. doi: 10.1091/mbc.02-05-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailand N., Diffley J. F. CDKs promote DNA replication origin licensing in human cells by protecting Cdc6 from APC/C-dependent proteolysis. Cell. 2005;122:915–926. doi: 10.1016/j.cell.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Hayashi K., Nishida E. Cyclin-dependent kinase 2 (Cdk2) is required for centrosome duplication in mammalian cells. Curr. Biol. 1999;9:429–432. doi: 10.1016/s0960-9822(99)80191-2. [DOI] [PubMed] [Google Scholar]
- Meraldi P., Lukas J., Fry A. M., Bartek J., Nigg E. A. Centrosome duplication in mammalian somatic cells requires E2F and Cdk2-cyclin A. Nat. Cell Biol. 1999;1:88–93. doi: 10.1038/10054. [DOI] [PubMed] [Google Scholar]
- Meraldi P., Nigg E. A. Centrosome cohesion is regulated by a balance of kinase and phosphatase activities. J. Cell Sci. 2001;114:3749–3757. doi: 10.1242/jcs.114.20.3749. [DOI] [PubMed] [Google Scholar]
- Mikule K., Delaval B., Kaldis P., Jurcyzk A., Hergert P., Doxsey S. Loss of centrosome integrity induces p38–p53-p21-dependent G1-S arrest. Nat. Cell Biol. 2007;9:160–170. doi: 10.1038/ncb1529. [DOI] [PubMed] [Google Scholar]
- Murphy T. D. Drosophila skpA, a component of SCF ubiquitin ligases, regulates centrosome duplication independently of cyclin E accumulation. J. Cell Sci. 2003;116:2321–2332. doi: 10.1242/jcs.00463. [DOI] [PubMed] [Google Scholar]
- Musti A. M., Treier M., Bohmann D. Reduced ubiquitin-dependent degradation of c-Jun after phosphorylation by MAP kinases. Science. 1997;275:400–402. doi: 10.1126/science.275.5298.400. [DOI] [PubMed] [Google Scholar]
- Nakayama K., et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A. Centrosome aberrations: cause or consequence of cancer progression? Nat. Rev. Cancer. 2002;2:815–825. doi: 10.1038/nrc924. [DOI] [PubMed] [Google Scholar]
- Palframan W. J., Meehl J. B., Jaspersen S. L., Winey M., Murray A. W. Anaphase inactivation of the spindle checkpoint. Science. 2006;313:680–684. doi: 10.1126/science.1127205. [DOI] [PubMed] [Google Scholar]
- Perrotti D., Iervolino A., Cesi V., Cirinna M., Lombardini S., Grassilli E., Bonatti S., Claudio P. P., Calabretta B. BCR-ABL prevents c-jun-mediated and proteasome-dependent FUS (TLS) proteolysis through a protein kinase CbetaII-dependent pathway. Mol. Cell. Biol. 2000;20:6159–6169. doi: 10.1128/mcb.20.16.6159-6169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihan G. A., Purohit A., Wallace J., Malhotra R., Liotta L., Doxsey S. J. Centrosome defects can account for cellular and genetic changes that characterize prostate cancer progression. Cancer Res. 2001;61:2212–2219. [PubMed] [Google Scholar]
- Pihan G. A., Wallace J., Zhou Y., Doxsey S. J. Centrosome abnormalities and chromosome instability occur together in pre-invasive carcinomas. Cancer Res. 2003;63:1398–1404. [PubMed] [Google Scholar]
- Poss K. D., Nechiporuk A., Stringer K. F., Lee C., Keating M. T. Germ cell aneuploidy in zebrafish with mutations in the mitotic checkpoint gene mps1. Genes Dev. 2004;18:1527–1532. doi: 10.1101/gad.1182604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra H. I., Maiti B., Timmers C., Altura R., Tokuyama Y., Fukasawa K., Leone G. Inactivation of E2F3 results in centrosome amplification. Cancer Cell. 2003;3:333–346. doi: 10.1016/s1535-6108(03)00083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M., Salisbury J. Centrin plays an essential role in microtubule severing during flagellar excision in Chlamydomonas reinhardtii. J. Cell Biol. 1994;124:795–805. doi: 10.1083/jcb.124.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M., Budirahardja Y., Klompmaker R., Medema R. H. Ablation of the spindle assembly checkpoint by a compound targeting Mps1. EMBO Rep. 2005;6:866–872. doi: 10.1038/sj.embor.7400483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucke V. M., Sillje H. H., Arnaud L., Nigg E. A. Human Mps1 kinase is required for the spindle assembly checkpoint but not for centrosome duplication. EMBO J. 2002;21:1723–1732. doi: 10.1093/emboj/21.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu O., McCormick F. Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell. 2003;3:233–245. doi: 10.1016/s1535-6108(03)00053-9. [DOI] [PubMed] [Google Scholar]
- Tsou M. F., Stearns T. Mechanism limiting centrosome duplication to once per cell cycle. Nature. 2006;442:947–951. doi: 10.1038/nature04985. [DOI] [PubMed] [Google Scholar]
- Weiss E., Winey M. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J. Cell Biol. 1996;132:111–123. doi: 10.1083/jcb.132.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik E. J., Glover D. M., Hays T. S. The SCF ubiquitin ligase protein slimb regulates centrosome duplication in Drosophila. Curr. Biol. 2000;10:1131–1134. doi: 10.1016/s0960-9822(00)00703-x. [DOI] [PubMed] [Google Scholar]
- Yeh E., et al. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat. Cell Biol. 2004;6:308–318. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Chen R. H. Mps1 phosphorylation by MAP kinase is required for kinetochore localization of spindle-checkpoint proteins. Curr. Biol. 2006;16:1764–1769. doi: 10.1016/j.cub.2006.07.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.