Abstract
ER-associated, ubiquitin-proteasome system (UPS)-mediated degradation of the wild-type (WT) gap junction protein connexin32 (Cx32) is inhibited by mild forms of cytosolic stress at a step before its dislocation into the cytosol. We show that the same conditions (a 30-min, 42°C heat shock or oxidative stress induced by arsenite) also reduce the endoplasmic reticulum (ER)-associated turnover of disease-causing mutants of Cx32 and the cystic fibrosis transmembrane conductance regulator (CFTR), as well as that of WT CFTR and unassembled Ig light chain. Stress-stabilized WT Cx32 and CFTR, but not the mutant/unassembled proteins examined, could traverse the secretory pathway. Heat shock also slowed the otherwise rapid UPS-mediated turnover of the cytosolic proteins myoD and GFPu, but not the degradation of an ubiquitination-independent construct (GFP-ODC) closely related to the latter. Analysis of mutant Cx32 from cells exposed to proteasome inhibitors and/or cytosolic stress indicated that stress reduces degradation at the level of substrate polyubiquitination. These findings reveal a new link between the cytosolic stress-induced heat shock response, ER-associated degradation, and polyubiquitination. Stress-denatured proteins may titer a limiting component of the ubiquitination machinery away from pre-existing UPS substrates, thereby sparing the latter from degradation.
INTRODUCTION
Although once thought to be restricted to cytosolic and nuclear proteins, degradation by the ubiquitin/proteasome system (UPS) also plays a major role in regulating the level of proteins synthesized within the endoplasmic reticulum (ER; Meusser et al., 2005). ER-associated degradation by the proteasome (ERAD) can be conceptualized as consisting of the following four tightly linked steps.
Initial recognition, mediated by proteins (e.g., protein chaperones) that bind to the ERAD substrate because of a feature of the latter that destines it to be destroyed, usually incorrect or incomplete folding or the unmasking of a degron.
Polyubiquitination, in which the first ubiquitin moiety becomes linked through an isopeptide bond to (most commonly) the epsilon amino group of a lysine residue within the UPS substrate and is then extended to form a K48-linked polyubiquitinated chain.
Delivery to the proteasome, which requires the extraction (dislocation) of the ERAD substrate from the ER into the cytosol in a process that usually requires the AAA-ATPase p97 (VCP/cdc48; Ye et al., 2001; Kobayashi et al., 2002; Jarosch et al., 2003; Dalal et al., 2004; Huyer et al., 2004; Wojcik et al., 2006). With very few exceptions (e.g., yeast mutant pre-pro α factor; Werner et al., 1996), polyubiquitination is a prerequisite for complete extraction from the ER and association with the 26S proteasome.
Proteolysis, in which the ERAD substrate is degraded to small peptides within the 20S proteasome core. One or more of the 20S peptidase activities are blocked by compounds such as MG132, epoxomicin, lactacystin, ALLN, and the chemotherapeutic bortezimide (Groll and Huber, 2004). In the presence of such proteasome inhibitors, ERAD substrates often accumulate as full-length proteins in both polyubiquitinated and unmodified forms due to repeated cycles of polyubiquitination (performed by E1/E2/E3 enzymes) and deubiquitination (carried out by one of the more than 50 enzymes known or predicted to have protein deubiquitinating activity; Shamu et al., 1999; Nijman et al., 2005). Proteasome inhibitors are also known to slow, but do not completely block, dislocation of ERAD substrates (Yu et al., 1997; Story et al., 1999; Mancini et al., 2000). Although the mechanism of this effect is not known, one possibility is that the ubiquitin-binding sites of proteins that mediate dislocation become occupied by undegradable polyubiquitinated proteins.
With the exception of dislocation, these processes are also required for degradation of UPS substrates located within the cytoplasm or nucleus.
We have studied the ERAD of connexins, the family of nonglycosylated, four-transmembrane domain proteins that form gap junction intercellular channels in virtually all multicellular tissues in animals ranging from tunicates to man (Goodenough et al., 1996; Laird, 2006). Approximately one-half of newly synthesized wild-type (WT) connexin43 (Cx43) and connexin32 (Cx32) molecules are rapidly degraded even if their exit from the ER is blocked (VanSlyke et al., 2000). It is likely, but has not yet been definitively established, that degradation of newly synthesized connexins at the ER is a consequence of slow and/or inefficient folding. We have reported that fully ER membrane–integrated WT Cx32 and Cx43 can be chased into a soluble cytoplasmic pool in full-length form, if (and only if) the degradation of the dislocated molecules is blocked with proteasome inhibitors. Dislocation of connexins, and therefore their proteasome-mediated degradation, is inhibited by agents (80 μM sodium arsenite or a 10–30-min incubation at 42°C) that induce oxidative or hyperthermic stress, respectively (VanSlyke and Musil, 2002). By causing the thermal or oxidative denaturation of proteins in the cytosol, such treatments (hereafter collectively referred to as H/O stress) trigger the heat shock response. Stress stabilization of connexins does not require expression of heat shock proteins, but is instead closely correlated with the stress-induced accumulation of unfolded protein in the cytosol. Rapid degradation of Cx43 is restored 12–18 h after a 30-min, 42°C heat shock, a time at which the level of unfolded protein in the cytosol would be expected to have returned to normal. Several lines of evidence indicate that H/O stress inhibition of connexin ERAD is a specific and potentially physiologically significant phenomenon (VanSlyke and Musil, 2002). First, the treatments that up-regulate connexin stability do not kill the cells or block their proliferation. Second, agents that increase the level of unfolded protein in the ER instead of in the cytosol (e.g., tunicamycin or dithiothreitol [DTT]) do not reduce connexin ERAD. Third, H/O stress inhibition of ERAD was observed in all cell types examined. Importantly, WT connexin spared from ERAD by H/O stress remains in a full-length, membrane-integrated form capable of folding, multisubunit oligomerization, and transport to the cell surface. This increase in mature connexin was associated with a striking increase in the number of functional gap junctions in cells that would otherwise be gap junction-deficient, most likely because gap junction assembly is an autofacilitated, self-assembly process (Valiunas et al., 1997; Castro et al., 1999). Given that gap junctions mediate the regulatable cell-to-cell transfer of low-molecular-weight substances including ions, antioxidants, and second messengers, their up-regulation by H/O stress could have important consequences for cell signaling and survival under pathophysiological conditions such as ischemia-reperfusion injury or fever.
These studies were the first to demonstrate that ERAD could be inhibited by physiologically relevant forms of stress to increase the functional pools of a protein on the cell surface. An important question raised by our findings is whether the effect of H/O stress is confined to WT connexins. Well over 400 mutations in at least nine different connexin family members have been shown to cause human diseases including the peripheral neuropathy Charcot-Marie-Tooth, X-linked (CMTX; Cx32), oculo-dental-digital dysplasia (ODDD; Cx43), deafness, cataract, and various skin disorders. Several of these mutations have been shown to disrupt transport of the abnormal protein to the cell surface (reviewed by Laird, 2006). It is unknown whether H/O stress also inhibits the degradation of mutant connexins and if this changes their localization within the cell. We have reported that H/O stress reduces the dislocation of unassembled major histocompatibility complex (MHC) I heavy chain (VanSlyke and Musil, 2002). Does stress also slow the degradation of other WT and mutant UPS substrates? Which step in degradation is blocked by H/O stress? We have addressed these issues using a combination of biochemical and morphological techniques. Our findings extend the significance of the effects of H/O stress to well beyond WT connexins and indicate that H/O stress inhibits the proteasome-mediated degradation of both ERAD and cytosolic UPS substrates via a novel mechanism, most likely by interfering with their ubiquitination.
MATERIALS AND METHODS
Cell Culture and Transient Transfection
CHO-K1 (Musil et al., 2000), cystic fibrosis transmembrane conductance regulator (CFTR)-expressing Chinese hamster ovary (CHO) transfectants (Lukacs et al., 1994), and T15L L chain–expressing SP2/0 myeloma cells (Chen et al., 1994) were cultured as previously described. Transient transfections were conducted 1 d after plating using FuGENE 6 (Roche Applied Science, Indianapolis, IN) as specified by the manufacturer. Cells were analyzed 24 h after transfection. Plasmids used and their sources include the following: WT and 142fs Cx32 (in pcDNA3) from Dr. S. Scherer (University of Pennsylvania), 175fs and E208K Cx32 from Dr. R. Bruzzone (Institut Pasteur) (subcloned into pcDNA3), hemagglutinin (HA)-ubiquitin (plasmid pMT123; from Dr. D. Bohmann (University of Rochester Medical Center); Treier et al., 1994), myoD (in pcDNA3) from Dr. M. Thayer (Oregon Health & Science University), GFPu (in pEGFP-C1, purchased from ATCC), and the ornithine decarboxylase (ODC)-green fluorescent protein (GFP)–encoding plasmids peGFP-C1-ODC (from Drs. R. Kopito and E. Bennett, Stanford University) and pd1EGFP-N1 (BD Biosciences, San Jose, CA).
Metabolic Labeling and Treatment with Stressors or Inhibitors
Adherent cells were metabolically labeled with [35S]methionine (EXPRE 35S 35S, Perkin Elmer-Cetus, Norwalk, CY) at 37°C as previously described (VanSlyke and Musil, 2000). Where indicated, cells were then chased in complete tissue culture medium (containing 10% fetal calf serum [FCS]) at 37°C in either the absence (control) or presence of the following compounds: 80 μM sodium arsenite (Sigma-Aldrich, St. Louis, MO), 6 μg/ml brefeldin A (BFA, Epicenter Technologies, Madison, WI), 70 μM cycloheximide (CHX), 2 mM DTT, or 30 μM MG132 (Calbiochem, La Jolla, CA). Heat shock (42°C) and mock shock (37°C) treatments were conducted as previously described (VanSlyke and Musil, 2002), using a thermocouple thermometer to verify that the growing surface of the tissue culture dish was maintained at the desired temperature.
Immunoprecipitation, SDS-PAGE, and Western Transfer
For analysis of all proteins other than CFTR, cells (or cytosolic and membrane fractions prepared as described in VanSlyke and Musil, 2002) were solubilized in 0.6% SDS (VanSlyke and Musil, 2000). Samples were incubated with antibodies, washed, and analyzed by SDS-PAGE as described in VanSlyke and Musil (2002). CFTR-expressing cells were lysed for 30 min at 4°C in RIPA buffer consisting of 0.1 M NaCl, 0.1 M Tris, 1 mM EDTA, 6 mM MgCl2, 1% deoxycholate, 1% Triton X-100, and 0.1% SDS, pH 8.0. The solubilized cells were scraped from the dish, subjected to a 10-min, 15,000 × g centrifugation, and the supernatant was incubated overnight at 4°C with an anti-CFTR antiserum directed against amino acids 45–65, a gift of Dr W. Skach (Oregon Health & Science University) (Xiong et al., 1999). Immune complexes were bound to protein A-Sepharose beads and washed four times in RIPA buffer before incubation at 37°C for 5 min with electrophoresis sample buffer. Other antibodies used for immunoprecipitation were all from rabbit and included the following: anti-Cx32 (Sigma, C3470), anti-Cx43 (AP7298; Musil et al., 1990), anti-light chain (Cappel Laboratories, Malvern, PA, 50236–36252), and anti-myo D (Santa Cruz Biotechnology, Santa Cruz, CA; sc-760). Radiolabeled gels were quantitated on a Bio-Rad Personal FX Imager (Richmond, CA) using Quantity One software. Where indicated, gels were transferred to polyvinylidene fluoride (PVDF) membranes (Immobilon, Millipore, Bedford, MA). Blots were probed with the following primary antibodies: anti-GFP (632381) from Clontech (Palo Alto, CA), anti-Cx32 (M12.13; kind gift of Drs A. Harris, [UMDNJ-New Jersey Medical School] and D. Goodenough [Harvard Medical School]), anti-ubiquitin (sc-8017 from Santa Cruz Biotechnology), or anti-HA (Covance, Madison, WI, MMS-101R). Immunoreactive protein bands were detected using secondary antibodies conjugated to Alexa Fluor680 (Molecular Probes, Eugene, OR) and directly quantified using the LI-COR Biosciences Odyssey infrared imaging system (Lincoln, NE) and associated software. Unless indicated otherwise, all experiments were repeated a minimum of three times and representative experiments are shown.
Microscopy
CHO cells grown on glass coverslips were fixed in 2% paraformaldehyde in phosphate-buffered saline and processed for immunocytochemical detection of Cx32 or T15L light chain as previously described (Musil et al., 2000). Antibodies used included M12.13 (anti-Cx32), anti-Ig light chain (Cappel, 50236–36252), anti-Sec61β (kind gift of Dr. V. Lingappa, University of California, San Francisco), and anti-calnexin (BD Transduction Laboratories, Lexington, KY, 45520). Immunofluorescence images were captured using a Leica DM LD photomicrography system (Deerfield, IL) and Scion Image 1.60 software (Frederick, MD).
RESULTS
Effect of H/O Stress on ERAD and Intracellular Transport of Cx32 Mutants
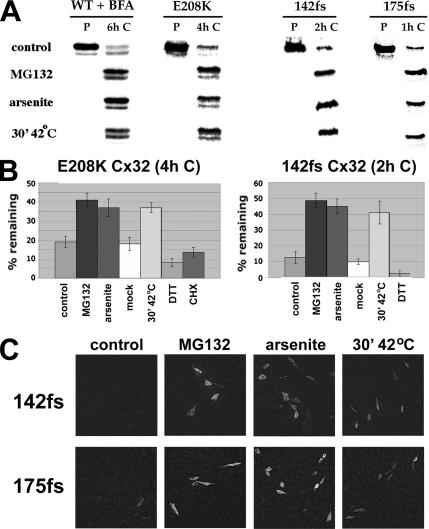
To investigate the effect of H/O stress on disease-associated mutant forms of connexins, we transiently expressed various species of Cx32 in otherwise Cx32-null CHO cells. Cells were metabolically labeled for 20 min with [35S]methionine and then chased under various conditions before cell lysis and immunoprecipitation of Cx32 under denaturing conditions. Initial experiments were conducted with WT Cx32 in the continuous presence of the intracellular transport inhibitor BFA to block exit of the newly synthesized connexin from the ER. Most of the pulse-labeled [35S]met-WT Cx32 was lost within 6 h in a process slowed by the addition of proteasome inhibitors such as MG132, ALLN, or epoxomicin to the chase medium (Figure 1A), as expected for an ERAD substrate (VanSlyke et al., 2000). Turnover of WT Cx32 was reduced to a comparable extent when the cells were subjected to H/O stress (either a 30-min incubation at 42°C immediately after the pulse before return to 37°C, or a 6-h chase in the continuous presence of 80 μM sodium arsenite). These results are similar to those obtained for the WT Cx43 endogenously expressed in CHO cells (VanSlyke and Musil, 2002). E208K Cx32 is a point mutant of Cx32 that causes the peripheral neuropathy CMTX (Fairweather et al., 1994). Under control conditions, E208K Cx32 is completely confined to the ER and rapidly degraded by ERAD even in the absence of an intracellular transport blocker (VanSlyke et al., 2000). Both oxidative and hyperthermic stress, but not the ER stressor DTT under conditions in which it blocks disulfide bond formation (Musil et al., 2000), increased the amount of pulse-labeled [35S]met-E208K Cx32 recovered after a 4-h chase to an extent comparable to that obtained with the proteasome inhibitor MG132 (Figure 1, A and B).
Figure 1.
Induction of H/O stress decreases ERAD of WT and mutant forms of Cx32. (A) CHO cells transiently transfected with plasmids encoding wild-type (WT), E208K, 142fs, or 175fs Cx32 were metabolically labeled with [35S]methionine at 37°C for 20 min and lysed either immediately (P) or after being chased for the indicated period with no additions (control), 30 μM MG132, 80 μM sodium arsenite, or for 30 min at 42°C immediately after the pulse and returned to 37°C for the remainder of the chase period. For WT Cx32 only, BFA was included in the pulse and chase media. Total cellular Cx32 was immunoprecipitated and analyzed by SDS-PAGE and PhosphorImaging. (B) The amount of [35S]methionine-Cx32 recovered by immunoprecipitation after a 4-h (E208K Cx32) or 2-h (142fs Cx32) chase under the indicated conditions was expressed as a percentage of [35S]methionine-Cx32 immunoprecipitable immediately after the pulse. Mock-shocked cells were treated identically to heat-shocked samples, except that the temperature was maintained at 37°C throughout the experiment. For each experimental condition, n = 3. (C) CHO cells transiently transfected to express 142fs or 175fs Cx32 were incubated for 4 h at 37°C with no additions (control), MG132, or arsenite as indicated. Heat-shocked cells were incubated at 42°C for 30 min and then returned to 37°C for an additional 3.5 h. All cells were then fixed and immunostained with anti-Cx32 antibodies.
Scherer and colleagues have described a subset of Cx32 mutants (hereafter referred to as class 3 mutants) that when stably expressed in tissue culture cells or transgenic mice produce high levels of mutant-encoding mRNA, but no protein product as assessed by Western blot (Deschenes et al., 1997; Abel et al., 1999). We hypothesized that class 3 mutants are translated but are then very rapidly degraded because of their severely abnormal structure, rendering them undetectable when expressed at physiologically relevant levels. This was confirmed after transient overexpression of two such mutants from a CMV promoter-based plasmid in CHO cells. Proteasome inhibitors, as well as H/O stress (but not DTT or the lysosomal inhibitor chloroquine; not shown) significantly slowed the degradation of both the 175fs and 142fs Cx32 mutants (Figure 1, A and B). Although H/O stress did not completely block the degradation of any Cx32 species, it stabilized both frameshift mutants to an extent sufficient to markedly increased the number of transiently transfected cells that accumulated 142fs and 175fs Cx32 to levels above the threshold for detection by anti-Cx32 immunofluorescence microscopy (Figure 1C). Control experiments verified that H/O stress did not enhance the synthesis of any of the constructs under the conditions used; instead, hyperthermia caused a transient decrease in total protein translation, as expected (Brostrom and Brostrom, 1998).
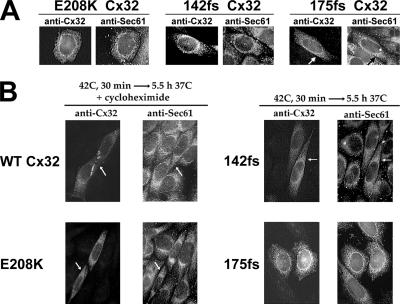
Under control conditions, transiently expressed E208K Cx32 largely colocalized with a specific marker for the ER (Sec61β; Figure 2A), in keeping with its distribution in stable transfectants (VanSlyke et al., 2000). A similar result was obtained for the small fraction of cells that synthesized detectable levels of the class 3 mutants 142fs and 175fs Cx32 under basal conditions. Incubation at 20°C did not alter the staining pattern of the mutants, indicating that their localization in the ER was due to retention instead of retrieval from post-ER compartments (not shown; VanSlyke et al., 2000). Control experiments demonstrated the expected redistribution of a fraction of the intracellular pool of WT Cx32 upon incubation of cells with chloroquine or BFA, indicating the presence of WT connexin in the endolysosomal system or Golgi/trans-Golgi network (TGN), respectively (Musil and Goodenough, 1993; VanSlyke et al., 2000). Neither compound affected the subcellular localization of any of the Cx32 mutants (data not shown). A 30-min, 42°C heat shock followed by a 5.5-h chase in the presence of cycloheximide resulted in the accumulation of presynthesized WT Cx32 in gap junctional plaques and other post-ER compartments (Figure 2B), as was previously observed for endogenous Cx43 (VanSlyke and Musil, 2002). In contrast, E208K Cx32 spared from ERAD by the same treatment (Figure 2B), or with either arsenite or MG132 (not shown), never formed gap junctions and instead remained colocalized with Sec61β. H/O stress also did not detectably change the localization of the class 3 frameshift mutants (Figure 2B). These findings show for the first time that mutant forms of connexins are spared from degradation by H/O stress before their dislocation into the cytosol. Unlike WT connexins, they remain confined to the ER where their accumulation could contribute to disease by trapping coassembled WT connexins.
Figure 2.
H/O stress does not restore trafficking of mutant Cx32. CHO cells transiently transfected with plasmids encoding the indicated Cx32 species were incubated for 6 h at 37°C with no additions (A), or at 42°C for 30 min and then returned to 37°C for an additional 5.5-h incubation in either the presence (WT and E208K Cx32) or absence (175fs and 142fs Cx32) of cycloheximide (B). All cells were then fixed and doubly immunostained for Cx32 and the ER marker Sec61β.
Effect of H/O Stress on Non-connexin ERAD Substrates
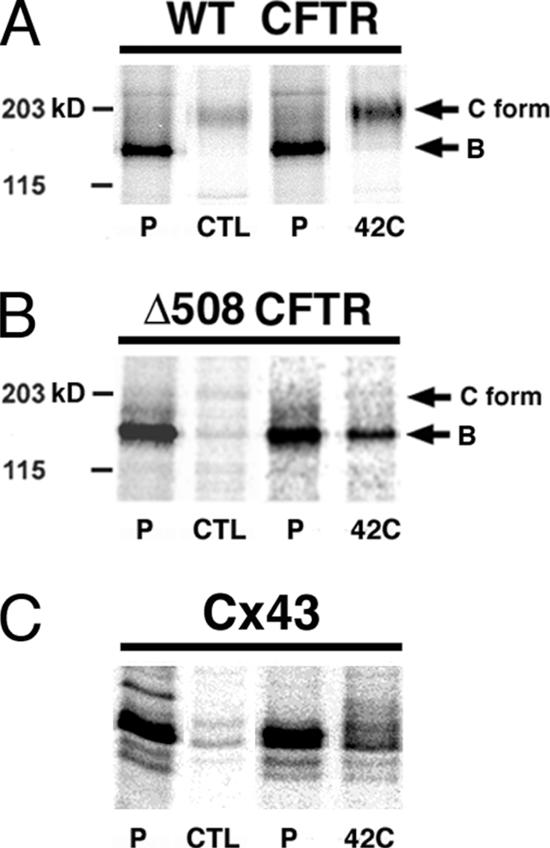
We extended our studies to a WT and ER-retained mutant form of another polytopic plasma membrane protein, the CFTR. In CHOs and most other cell types, ∼75% of WT CFTR is degraded by ERAD, as is nearly 99% of the Δ508 CFTR mutant (Younger et al., 2004). The immaturely glycosylated, ER-localized forms of both WT and mutant CFTR migrate at ∼140 kDa on SDS-PAGE and are referred to as the “B form.” Subjecting [35S]met-labeled cells to heat stress immediately after the pulse did not appreciably change the amount of WT or mutant [35S]met-CFTR recovered after a 6-h chase (not shown). Proteasome inhibitors are also ineffective in slowing CFTR turnover when present only during the chase period (Gelman et al., 2002), whereas significant stabilization of CFTR is obtained if cells are preincubated with the inhibitor. This behavior is in keeping with reports that CFTR is ubiquitinated and targeted for ERAD cotranslationally (Sato et al., 1998). We therefore incubated cells for 30 min at 42°C, followed by 4 h at 37°C, before subjecting them to pulse labeling and chase under unstressed conditions. Control experiments confirmed that this “preshock” stabilized endogenous Cx43 to the same extent as when hyperthermia was administered immediately after the pulse (VanSlyke and Musil, 2002; Figure 3C). This treatment also reduced the turnover of [35S]met-WT CFTR by 2.4-fold ± 0.22 (n = 4), which was recovered in the maturely glycosylated, plasma membrane Mr ∼170 kDa “C” form (Figure 3A). Δ508 CFTR was also spared from degradation by preshock when chased at either 37°C (not shown) or 27°C (by 3.24 ± 0.35-fold; n = 3; Figure 3B). The latter temperature has been reported to promote the proper folding of Δ508 CFTR, albeit in a process too slow and inefficient to result in detectable recovery of pulse-labeled [35S]met-Δ508CFTR in the C form (see Younger et al., 2004). Similar results were obtained if the experiment was repeated in the presence of actinomycin, ruling out a role for stress-induced heat shock proteins in CFTR stabilization (not shown). We conclude that H/O stress reduces the degradation of both WT and ER-retained mutant forms of Cx32 and CFTR, but that only WT proteins are competent to traverse the secretory pathway.
Figure 3.
H/O stress decreases the degradation of both WT and Δ508 CFTR, but only promotes the maturation of the former. CHO cells stably expressing either WT (A) or Δ508 (B and C) CFTR were either “preshocked” by a 30-min incubation at 42°C (42C), or mock-shocked at 37°C (CTL), and returned to 37°C for 4 h. The cells were then pulsed for 30 min with [35S]methionine and either lysed immediately (P) or chased for 6 h at 37°C (WT) or 27°C (Δ508) before immunoprecipitation of CFTR from the RIPA soluble cell lysate (A and B) or of endogenous Cx43 from the pooled RIPA soluble and insoluble fractions boiled in SDS (C). The contrast of the image of each pulse-chase pair was adjusted such that the intensity of all of the pulse (P) lanes was approximately equal.
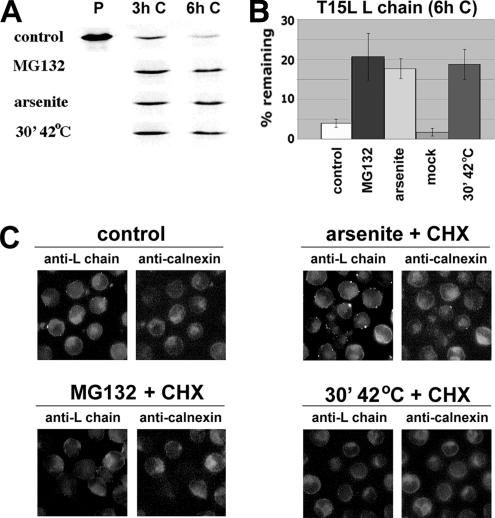
Secretory proteins lacking transmembrane domains are also substrates for ERAD. When stably expressed in SP2/0 myeloma cells, the T15L immunoglobulin light chain (L chain) cannot be transported to the Golgi due to the lack of its obligatory assembly partner and is instead rapidly degraded by the proteasome (O'Hare et al., 1999). Pulse-chase analysis of these transfectants demonstrated that H/O stress was as potent as a proteasome inhibitor in reducing [35S]met-T15L turnover (Figure 4, A and B). Immunoprecipitable [35S]met-L chain did not accumulate in the medium under any of the conditions tested, indicating it remained transport-incompetent (not shown). Although some forms of unassembled light chain have been reported to be able to accumulate in cytoplasmic aggresomes (Dul et al., 2001), anti-L chain immunocytochemistry revealed no evidence for their formation in either stressed or unstressed SP2/O cells. Instead, the staining pattern of T15L spared from degradation by cytosolic stress remained colocalized with calnexin in a distribution indistinguishable from that of the ER, and very different from that of the Golgi, as identified by O'Hare et al. (1999) (Figure 4C).
Figure 4.
H/O stress promotes the stability, but not the intracellular transport, of a soluble ERAD substrate. (A) SP2/0 myeloma cells stably expressing the unassembled, secretion-incompetent T15L immunoglobulin light chain were metabolically labeled for 20 min and lysed either immediately (P) or after being chased for the indicated period with no additions (control), MG132, sodium arsenite, or for 30 min at 42°C immediately after the pulse and returned to 37°C for the remainder of the chase period. The cells were treated with SDS, and immunoprecipitated with anti-L chain antibodies. (B) The amount of [35S]methionine-L chain recovered by immunoprecipitation from the cells after a 6-h chase under the indicated conditions was expressed as a percentage of [35S]methionine-L chain immunoprecipitable immediately after the pulse. For each experimental condition, n = 3 or 4. (C) The T15L-expressing cells were incubated for 4 h at 37°C with no additions (control) or for a total of 4 h in the presence of CHX + arsenite, CHX + MG132, or CHX for 30 min at 42°C followed by 3.5 h at 37°C. All cells were then fixed and immunostained with anti-L chain antibodies and antibodies specific for the ER marker calnexin.
Effect of H/O Stress on Degradation of Cytoplasmically Localized, non-ERAD Substrates of the Proteasome
The transcription factor MyoD is a naturally occurring resident of the cytosol and nucleus that is rapidly degraded by the UPS. As in myocytes and transfected COS cells (Thayer et al., 1989; Abu Hatoum et al., 1998; Breitschopf et al., 1998), the half-life of pulse-labeled [35S]met-MyoD in transiently transfected CHO cells was <1 h. The addition of MG132 to the chase medium greatly increased the recovery of [35S]met-MyoD after a 2–4-h chase, as did subjecting the cells to either oxidative or hyperthermic stress (Figure 5A).
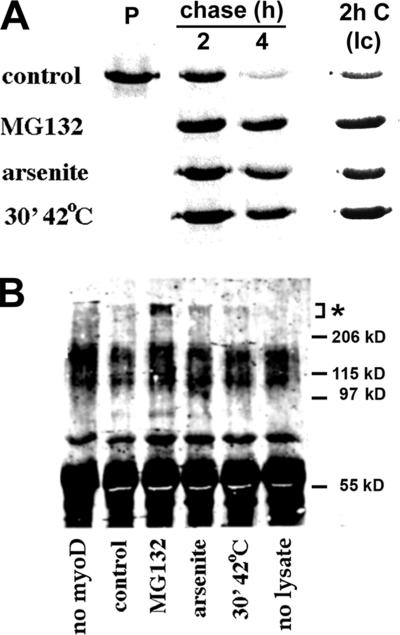
Figure 5.
Inhibition of degradation and polyubiquitination of the cytosolic/nuclear UPS substrate MyoD by H/O stress. (A) CHO cells transiently transfected with plasmids encoding MyoD were lysed and immunoprecipitated with anti-MyoD antibodies either immediately after a 20-min pulse with [35S]methionine (P) or after being chased for the indicated period with no additions (control), MG132, sodium arsenite, or for 30 min at 42°C immediately after the pulse and returned to 37°C for the remainder of the chase period. A lower contrast (lc) image of the 2-h chase data is included to indicate the extent to which MG132 and stress increase the recovery of MyoD at this time point; relative to the amount of [35S]methionine-MyoD recovered from control cells, the fold increase was: 2.09 ± 0.26 (MG132); 2.21 ± 0.62 (arsenite); and 3.65 ± 0.56 (hyperthermia). For each experimental condition, n = 3. (B) CHO cells transiently cotransfected with plasmids encoding MyoD and HA-tagged ubiquitin at a ratio of 1:5 were incubated for 4 h as in A before immunoprecipitation of MyoD. The samples were subjected to SDS-PAGE, transferred to PVDF membranes, and probed for polyubiquitinated MyoD (asterisk) with anti-HA antibodies. The no-MyoD sample was prepared from cells transfected with ubiquitin-HA only and treated as in lane 3; the no-lysate control shows the background signal from the immunoprecipitating antibody. Representative of three experiments. Anti-MyoD blots confirmed that the lack of polyubiquitinated MyoD from cells subjected to H/O stress was not due to decreased levels of MyoD protein.
The results obtained with MyoD demonstrated that stabilization by H/O stress is not confined to ER-localized proteins and raised the possibility that some process common to both membrane-associated and -unassociated UPS substrates is the target of stress. To examine the effects of H/O stress on polyubiquitination of MyoD, CHO cells cotransfected to express MyoD and HA epitope–tagged ubiquitin were subjected to either MG132 or H/O stress before immunoprecipitation of MyoD and detection of polyubiquitinated forms by Western blot. As expected, high-molecular-weight anti-HA immunoreactivity was readily observed in samples from cells incubated with MG132 for 4 h; MyoD plasmid-minus and no lysate negative controls demonstrated that the signal was specific (Figure 5B). In contrast, no increase in ubiquitinated MyoD was detected in immunoprecipitates from cells subjected to either oxidative or hyperthermic stress despite the fact that both treatments stabilized MyoD from degradation to the same extent as MG132.
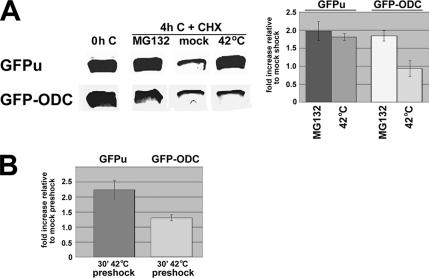
If polyubiquitination is the H/O stress–sensitive step in UPS-mediated degradation, then it would be expected that two closely related proteins would differ in their stress sensitivity if one required polyubiquitination to be degraded and the other did not. This was confirmed by comparing the effect of heat shock on two GFP-based constructs, GFPu and GFP-ODC (Figure 6). GFPu is enhanced GFP (EGFP) destabilized by the addition of the 16-amino acid CL1 degron to its carboxy terminus, which targets it for polyubiquitination and destruction by the 26S proteasome. It has been widely used as a unregulated reporter of the UPS (Bence et al., 2005). In contrast, degradation of mouse ODC by the 26S proteasome does not require its ubiquitination in either mammalian, yeast, or in vitro systems. Fusion of the COOH-terminal 37-amino acid degron of mouse ODC confers rapid, ubiquitin-independent turnover to EGFP, presumably because this domain allows the construct to bind to the 26S proteasome without an obligatory requirement for antizyme or any other targeting protein (Li et al., 1998; Hoyt et al., 2003). GFPu and GFP-ODC were transiently expressed in CHO cells, and their degradation was monitored by Western blotting after a 4–6-h incubation with CHX to block further protein synthesis (in our hands, immunoprecipitation by commercially available anti-GFP antibodies was inefficient). As expected, ∼70% GFPu and GFP-ODC was degraded over the chase period in a process partially inhibited by proteasome inhibitors such as MG132 (Figure 6A), but not by blockers of lysosomal degradation or autophagy (not shown). Turnover of GFPu was reduced by a 30-min, 42°C heat shock to an extent comparable to that obtained with MG132. In contrast, this treatment had no effect on the degradation of GFP-ODC constructs in 5/5 trials (Figure 6A). To rule out the possibility that this lack of effect was the result of more rapid targeting of ODC-GFP for degradation than inhibition of this process by hyperthermia, cells expressing either GFPu or GFP-ODC were mock (37°C)- or heat (42°C)-shocked for 30 min before being returned to 37°C for 3.5 h. Cells were then lysed either immediately or after a 4-h chase with CHX. As expected from results obtained with metabolically labeled ERAD substrates (Figure 3), “preshock” at 42°C slowed the degradation of GFPu to an extent comparable to, or slightly greater than, that observed under the standard assay conditions used in the experiment shown in Figure 6A. In contrast, the turnover of GFP-ODC in preshocked cells was very similar to that in mock preshocked cells (Figure 6B). Given that GFPu (H/O stress-sensitive) and GFP-ODC (stress-insensitive) are virtually identical except for the small destabilizing sequences appended to the carboxy terminus of GFP, it is highly unlikely that differences other than their dependence on ubiquitination are responsible for these results. This finding further rules out the possibility that H/O stress inhibits UPS-mediated degradation by interfering with the proteasome itself (by, for example, “choking” the proteasome with degradation-resistant, stress-induced unfolded proteins), because in that case the (proteasome-mediated) turnover of GFP-ODC and GFPu would be expected to be equally affected.
Figure 6.
Heat stress inhibits the degradation of EGFP bearing a ubiquitin-dependent degron, but not that of EGFP with a ubiquitin-independent degron. CHO cells were transiently transfected with plasmids encoding either GFPu or GFP-ODC. (A) Cells were lysed without any treatment (0h C), or incubated in the presence of cycloheximide for: 4 h at 37°C with MG132 (MG132), 30 min at 42°C followed by 3.5 h at 37°C (42°C), or 30 min at 37°C followed by 3.5 h at 37°C (mock). Total cell lysates were analyzed by Western blotting with anti-GFP antibodies. The amount of GFPu or GFP-ODC remaining after a 4-h chase in the presence of CHX under the indicated conditions was graphed as the fold increase relative to that obtained from CHX-treated mock-shocked cells. For each experimental condition, n = 3. (B) CHO cells transiently transfected with plasmids encoding either GFPu or GFP-ODC were “preshocked” by a 30-min incubation at 42°C or mock-shocked at 37°C and returned to 37°C for 3.5 h. The cells were then lysed without any treatment (0h C), or incubated for 4 h with CHX at 37°C and analyzed by Western blotting with anti-GFP antibodies. The amount of GFPu or GFP-ODC remaining after the CHX chase was calculated relative to the corresponding 0h C sample and was graphed relative to the value obtained from CHX-treated mock-shocked cells. For each experimental condition, n = 3.
Effect of H/O Stress and Proteasome Inhibitors on the Levels of Polyubiquitinated E208K Cx32
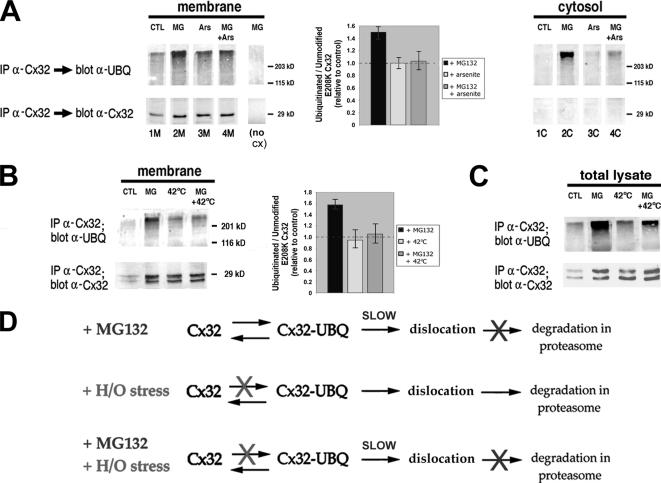
Using E208K Cx32 as a model protein, we next examined whether H/O stress inhibits ubiquitination of ERAD substrates. This mutant was chosen because it is confined to the ER and thus not subject to lysosomal degradation, contains the carboxyl terminal epitope (missing from 175fs and 142fs Cx32) recognized by the antibody most efficient for immunoprecipitation, does not appear to be cotranslationally ubiquitinated like WT or Δ508 CFTR, and (in contrast to T15L light chain or MyoD) can be reproducibly detected in a polyubiquitinated form under basal conditions in CHO cells. Cells transiently transfected with E208K Cx32 and HA-epitope tagged ubiquitin plasmids were subjected to various treatments before preparation of total membrane and cytosolic fractions under high salt conditions that leave only integrally associated connexin molecules in the membrane pellet (VanSlyke and Musil, 2002). The SDS-solubilized fractions were immunoprecipitated with rabbit anti-Cx32 antibodies and then analyzed by Western blotting for unmodified E208K Cx32 and for polyubiquitinated forms of the protein by probing with either a monoclonal anti-Cx32 antibody or anti-ubiquitin antibodies, respectively (blot-back with anti-ubiquitin instead of anti-Cx32 antibodies prevented any high-molecular-weight but nonubiquitinated Cx32 aggregates from being misidentified as Cx32-ubiquitin conjugates). Under basal conditions, both nonubiquitinated and polyubiquitinated E208K Cx32 species were recovered from the membrane fraction (Figure 7A, lane 1M, bottom and top, respectively). Plasmid-minus and no antibody controls verified that all signals were specific; anti-HA antibodies yielded results comparable to those obtained with the anti-ubiquitin antibody. Thus Cx32, like other membrane ERAD substrates, becomes ubiquitinated before its dislocation into the cytosol. Inhibitors of proteasome function such as MG132 block protein degradation, but not the activity of protein-ubiquitinating and -deubiquitinating enzymes. They also slow, but do not block, dislocation of connexins and many other proteins from the ER (Yu et al., 1997; Story et al., 1999; Mancini et al., 2000). As would be predicted from these properties, a 4-h exposure to MG132 increased the level of both ubiquitinated (by 1.96 ± 0.35-fold) and nonubiquitinated (by 1.3 ± 0.17-fold) E208K Cx32 in the membrane fraction relative to untreated controls (lane 2M). If cells were exposed to H/O stress (in Figure 7A, 80 μM sodium arsenite for 4 h) instead of MG132, the amount of unmodified E208K Cx32 in the membrane fraction was increased (1.5 ± 0.1-fold) to a level comparable to that obtained with the proteasome inhibitor, in keeping with our pulse-chase results (Figure 1). Remarkably, however, the level of ubiquitin-modified E208K Cx32 recovered in the membrane fraction after H/O stress was less than that observed in MG132-treated cells in 5 of 5 experiments (compare lane 3M to 2M). Given that proteasome inhibitors do not accelerate ubiquitination, the most likely explanation for these results is that H/O stress reduces polyubiquitination of connexins, acting at a step upstream of the site of action of MG132. If so, then it would be expected that treating cells with arsenite plus MG132 would reduce the amount of polyubiquitinated E208K Cx32 recovered in the membrane fraction relative to that obtained after exposure to MG132 alone. This is what was observed (lane 4M). Similar results were obtained when hyperthermia was used as the H/O stress (30 min at 42°C, followed by a 1.5-h chase in the presence of CHX to prevent artifacts due to differences in protein synthesis between the heat-shocked and mock-shocked cells; Figure 7B). An H/O stress-induced reduction in the intensity of the anti-ubiquitin signal of immunoprecipitated E208K Cx32 on Western blots could be the result of a decrease in either the number of E208K Cx32 molecules bearing polyubiquitination chains or in the number and/or length of polyubiquitination chains per molecule. Because ubiquitination is a readily reversible modification and is required for the association of UPS substrates with the 26S proteasome, either type of alteration would be expected to reduce the probability that a substrate becomes productively engaged with the 26S proteasome (Elsasser and Finley, 2005), thereby slowing its rate of degradation. The graphs in Figure 7, A and B, summarize the effects of arsenite, heat shock, and/or proteasome inhibitors on recovery of polyubiquitinated E208K Cx32 in membrane fractions; all data were normalized to unmodified E208K Cx32 recovered from the same sample. It is appreciated that recognition of polyubiquitinated proteins by Western blot may be inefficient. This caveat would apply to samples prepared from cells exposed to either MG132 or stress and would, if anything, cause us to underestimate the extent to which E208K Cx32 is polyubiquitinated in the presence of MG132 alone.
Figure 7.
Membrane-associated and total E208K Cx32 spared from ERAD by H/O stress is less highly ubiquitinated than E208K Cx32 saved by proteasome inhibitors. CHO cells were transiently cotransfected with plasmids encoding E208K Cx32 and HA-tagged ubiquitin at a ratio of 1:5. (A) The cells were incubated for 4 h without additions (control; CTL), or with MG132 (MG), arsenite (Ars), or both. All cells were then lysed without detergent and fractionated into cytosolic (C) and membrane (M) fractions before solubilization in SDS and immunoprecipitation of Cx32. Immunoprecipitates were subjected to SDS-PAGE on 12% gels and transferred to PVDF membranes. The blots were cut in half, and the lower portion was probed for unmodified E208K Cx32 with anti-Cx32 antibodies and the upper portion probed for polyubiquitinated E208K Cx32 with anti-ubiquitin antibodies. A “no cx” sample was prepared from cells transfected with ubiquitin-HA only and treated as in lane 2M. For each membrane sample from cells treated with MG132 and/or arsenite, the signal obtained for polyubiquitinated E208K Cx32 was calculated as a percentage of that obtained for polyubiquitinated E208K Cx32 from control cells in the same experiment. A similar calculation was performed for unmodified E208K Cx32 in the membrane. The ratio of the percentage obtained for polyubiquitinated E208K Cx32 to the percentage obtained for unmodified E208K Cx32 was calculated, and graphed relative to the ratio obtained from controls (defined as 100%/100%; 1). Data from three independent experiments. (B) Cotransfected cells were incubated in the presence of CHX for 2 h at 37°C with no other additions (control), 2 h at 37°C with MG132, 30 min at 42°C and returned to 37°C for 1.5 h, or 30 min at 42°C and returned to 37°C for 1.5 h in the continuous presence of MG132. Cells were lysed, and membrane fractions were analyzed as in A. Data from three independent experiments. (C) Cotransfected cells were incubated as in B, except that the CHX treatment was extended from 2 to 4 h. Cells were lysed in the presence of SDS, and whole cell lysates were analyzed for both polyubiquitinated and unmodified E208K Cx32. (D) Schematic of the effect of MG132 and/or H/O stress on ERAD of E208K Cx32. Top, proteasome inhibitors slow, but do not completely abolish, dislocation, and have a stronger inhibitory effect on proteolysis within the 20S proteasome core. Under these conditions, E208K Cx32 accumulates in the membrane in both ubiquitinated and nonubiquitinated forms and is also slowly dislocated into the cytosol after ubiquitination but is not degraded. Middle, cytosolic stress reduces ubiquitination of E208K Cx32, thereby inhibiting its dislocation and causing E208K Cx32 to accumulate in the membrane in a less ubiquitinated form than in the presence of proteasome inhibitors. Any E208K Cx32 that does becomes ubiquitinated and dislocated is degraded by the proteasome. Bottom, ubiquitination of membrane-associated E208K Cx32 is also reduced when cells are subjected to H/O stress in the presence of MG132. The latter blocks degradation of the small amount of E208K Cx32 that becomes dislocated, allowing it to be recovered from the medium.
Analysis of the cytosolic fractions from these experiments supports the contention that H/O stress and proteasome inhibitors have distinct effects on ERAD (Figure 7A). Under control (no stress or MG132) conditions, neither polyubiquitinated nor unmodified forms of E208K Cx32 were detectable in the cytosol, as expected given the rapid rate at which most dislocated proteins are degraded (lane 1C). In MG132-treated cells, E208K Cx32 was recovered in the cytosol, in keeping with our previous data demonstrating gradual accumulation of dislocated connexin in the cytosol in the presence of proteasome inhibitors (VanSlyke and Musil, 2002; lane 2C). Unlike E208K Cx32 in membranes (lane 2M), this material is visible by Western blot only as a polyubiquitinated species, although both modified and unmodified forms are detected by the more sensitive radiolabeling/immunoprecipitation technique (see Figure 8). In cells subjected to arsenite plus MG132, ∼57 ± 5.2% (n = 4) less (polyubiquitinated) connexin was recovered in the cytosol than from cells treated with MG132 alone (compare lane 4C to 2C). The finding that virtually no connexin was detectable in the cytosol of cells treated with arsenite alone (lane 3C) is consistent with the concept that H/O stress, unlike proteasome inhibitors, does not block degradation within the proteasome core. Comparable results were obtained when hyperthermia was used as the H/O stressor (not shown).
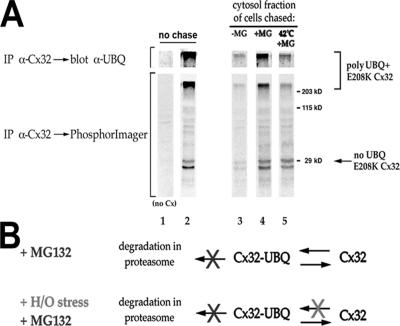
Figure 8.
H/O stress promotes the recovery of dislocated, MG132-stabilized E208K Cx32 in a nonubiquitinated form. (A) CHO cells transiently cotransfected with plasmids encoding E208K Cx32 and HA-tagged ubiquitin were metabolically labeled for 4 h in the presence of MG132. The cells were lysed without detergent, and a cytosolic fraction was prepared, either immediately after labeling (lane 2) or after chasing in the presence of CHX as follows: for 4 h with no other additions (lane 3), for 4 h with MG132 (lane 4), or for 30 min at 42°C followed by 3.5 h at 37°C in the continuous presence of MG132 (lane 5). Cx32 was immunoprecipitated from the cytosolic fraction, subjected to SDS-PAGE on 12% gels, and transferred to PVDF membranes. The membranes were analyzed for radiolabeled E208K Cx32 by phosphorImaging and were probed with anti-ubiquitin antibodies. Only the top portion of the anti-ubiquitin blot (Mr >200 kDa) showed specific anti-ubiquitin immunoreactivity and is included. Lane 1, sample prepared from cells transfected with ubiquitin-HA only and treated as in lane 2. (B) Schematic of the effect of H/O stress on post-dislocation pools of E208K Cx32. When degradation of dislocated, ubiquitinated E208K Cx32 (Cx32-UBQ) is blocked by MG132, E208K Cx32 accumulates in the cytosol in a less highly ubiquitinated form if the cells are also subjected to H/O stress due to inhibition of connexin reubiquitination by stress.
The ratio of ubiquitinated to unmodified E208K Cx32 in the membrane fraction of cells exposed to MG132 for 2–4 h is never greater than 1.7-fold that from untreated control cells (Figure 7, A and B), presumably because molecules that become more highly ubiquitinated are preferentially dislocated into the cytosolic fraction. If the analysis is repeated using total (unfractionated) lysates, the ratio of ubiquitinated to nonubiquitinated E208K Cx32 recovered from MG132-treated cells increases to an average of 4.1 ± 1.4-fold over control (Figure 7C). In contrast, this ratio is not appreciably increased if cells are exposed to cytosolic stress alone (either 30 min at 42°C followed by 3.5 h at 37°C, or 80 μM arsenite for 4 h; 1.08 ± 0.15-fold over control; all n = 6). Thus when both membrane and cytosolic pools of E208K Cx32 are taken into account, the difference between H/O stress and MG132 in the recovery of E208K Cx32 in a polyubiquitinated form is significantly increased. These findings further support our contention that H/O stress reduces substrate ubiquitination and rule out the possibility that the conclusions drawn from Figure 7, A and B, are affected by the removal of the postnuclear pellet fraction before analysis.
Note that the ratio of ubiquitinated to unmodified E208K Cx32 in either the membrane fraction (Figure 7, A and B) or total cell lysates (Figure 7C) of cells exposed to H/O stress is similar to that obtained from untreated control cells. This would be expected if E208K Cx32 molecules that attain a higher level of ubiquitination are degraded and lost to the analysis. Because more connexin molecules reach this threshold level of ubiquitination in control than in stressed cells, the ratio of ubiquitinated to unmodified E208K Cx32 calculated from control cells is an underestimate relative to the value from stressed cells. The fraction of E208K Cx32 that is recovered in a polyubiquitinated form from stressed cells can only be meaningfully compared with that obtained from cells subjected to conditions in which the turnover rate of E208K Cx32 is comparable, namely in the presence of a proteasome inhibitor. For E208K Cx32 (Figures 7 and 8) as well as MyoD (Figure 5B), the amount polyubiquitinated conjugates recovered from stressed cells is consistently less than that from MG132-treated cells.
We next examined the effect of H/O stress on E208K Cx32 no longer associated with membranes (Figure 8). CHO cells doubly transfected with E208K Cx32 and HA-ubiquitin plasmids were metabolically labeled for 4 h in the presence of MG132, conditions under which ER-dislocated Cx32 accumulates in the cytosol (VanSlyke and Musil, 2002). A cytosolic fraction was prepared and analyzed by anti-Cx32 immunoprecipitation followed by SDS-PAGE and phosphorImaging. Both high-molecular-weight [35S]met-E208K Cx32 (verified as being polyubiquitinated by anti-ubiquitin or anti-HA Western blot) and unmodified E208K Cx32 were recovered from the cytosol (Figure 8A, lane 2). Because polyubiquitination is an obligatory prerequisite for dislocation, the presence of both unmodified and modified E208K Cx32 is likely a consequence of repeated cycles of de- and reubiquitination under conditions (i.e., plus MG132) in which the protein cannot be degraded (Shamu et al., 1999; Flierman et al., 2003). Cells pulsed in the presence of MG132 were then chased for 4 h in medium containing CHX under various conditions before analysis of E208K Cx32 in the cytosolic fraction. If the chase was conducted in the absence of MG132, both ubiquitinated and nonubiquitinated forms of E208K Cx32 were rapidly lost from the cytosol, as would be predicted from the reversible nature of the inhibitor and because the dislocated connexin is likely to be severely misfolded and therefore a high-affinity target for reubiquitination and degradation (lane 3). In cells chased in the continuous presence of MG132 (lane 4), the ratio of ubiquitinated to unmodified [35S]met-E208K Cx32 recovered in the cytosol was similar to that immunoprecipitated from cells not subjected to a chase (lane 2), suggesting that the activity of ubiquitinating and deubiquitinating enzymes had reached a steady state during the first 4 h. If cells were chased with MG132 and also subjected to H/O stress (30 min at 42°C), 56 ± 5.3% (n = 3) less polyubiquitinated [35S]met-E208K Cx32 was recovered than from cells chased with MG132 but no stress (compare lane 5 to lane 4). Quantitation of the corresponding anti-ubiquitin Western blots revealed a 65 ± 19% (n = 3) reduction in the amount of polyubiquitinated connexin from heat-shocked cells; in 2 of 2 experiments, an even greater reduction was obtained when arsenite was used as the H/O stress (76 and 81%; not shown). Because the amount of unmodified, ∼30 kDa [35S]met-E208K Cx32 immunoprecipitated from the cytosol of cells subjected to H/O stress plus MG132 is approximately the same as that from cells treated with MG132 alone (compare lanes 4 and 5), this indicates that H/O stress reduced the fraction of cytosolic connexin in a polyubiquitinated form. Polyubiquitinated [35S]met-E208K Cx32 is metabolically labeled on its ubiquitin as well as its connexin moieties. Deubiquitination of E208K Cx32 results in the loss of the ubiquitin-derived [35S]methionine signal, likely explaining why the amount of radioactivity in the total anti-Cx32 immunoprecipitant in lane 5 is less than that in lane 4. The simplest explanation is that H/O stress inhibits the reubiquitination of dislocated, MG132-stabilized E208K Cx32, analogous to how treating cells with H/O stress plus MG132 reduces the accumulation of ubiquitinated E208K Cx32 in the membrane fraction relative to cell treated with MG132 alone (Figure 7).
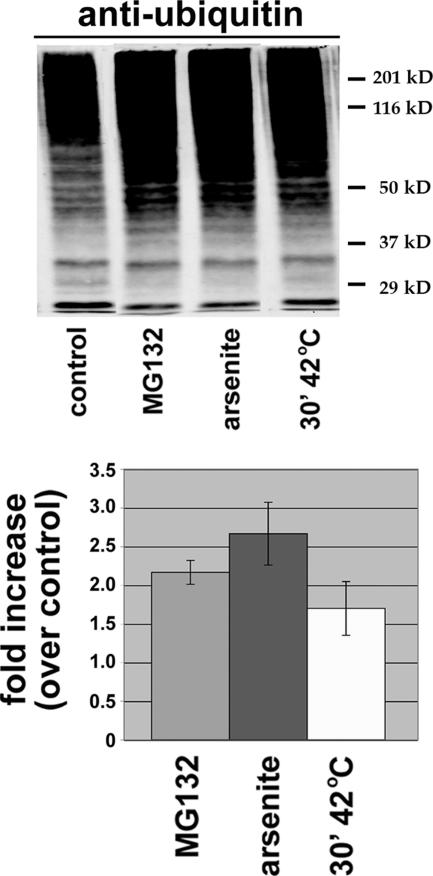
Effect of H/O Stress on the Level of Total Cellular Ubiquitinated Proteins
Finally, we addressed whether the reduction in recovery of E208K Cx32 in a polyubiquitinated form in H/O stressed cells compared with proteasome inhibitor-treated cells reflected a general shut-down of the cellular ubiquitination machinery under stress conditions. Anti-ubiquitin Western blots of total cellular protein lysates revealed a approximately twofold increase in polyubiquitinated conjugates in cells incubated for 4 h with MG132, as expected (Figure 9). An even greater, or slightly smaller, stimulation in total anti-ubiquitin immunoreactivity was observed in cells exposed to either oxidative or hyperthermic stress, respectively. Increased anti-ubiquitin immunoreactivity in lysates from heat-shocked or arsenite-treated cells relative to unstressed cells has also been observed by others (e.g., Bond et al., 1988) and is likely due to unfolding or misfolding of a subset of especially thermally or oxidatively sensitive cytosolic and nuclear proteins (Lepock et al., 1993; Senisterra et al., 1997). The increased levels of such high-affinity substrates for polyubiquitination machinery could explain, at least in part, why H/O stress inhibits the turnover of E208K Cx32 and of the other stress-sensitive proteins examined (see Discussion).
Figure 9.
Total cellular ubiquitin conjugates are increased by H/O stress. CHO cells transiently cotransfected with plasmids encoding E208K Cx32 and HA-tagged ubiquitin were incubated for 4 h with no additions (control), MG132, sodium arsenite, or for 30 min at 42°C followed by 3.5 h at 37°C. Equal amounts of total, SDS-solubilized cell lysates were analyzed by Western blotting using anti-ubiquitin antibodies, and the results were quantitated as the fold increase in anti-ubiquitin immunoreactivity under the indicated conditions relative to untreated controls. For each experimental condition, n = 3.
DISCUSSION
We have discovered a previously unrecognized aspect of the heat shock response, namely a reduction in the rate at which multiple proteins are degraded by the UPS. The stresses imposed did not cause cell death or compromise ER quality control, indicating they did not induce general cell dysfunction. They also do not appear to stabilize mutant or unassembled proteins by promoting their folding into a mature, less proteolytically labile state, because in that case T15L light chain and the Cx32 and CFTR mutants would be expected to become competent to traverse the secretory pathway. This was not observed.
Because UPS substrates located in two topologically separate compartments (the ER lumen and cytosol) are both stabilized by H/O stress, H/O stress cannot inhibit degradation by affecting the binding of a common recognition protein. We can also exclude proteolysis in the 20S core as the critical target for H/O stress given that exposure of cells to the levels of H/O stress used in our studies does not appreciably reduce the hydrolysis of proteasome substrate peptides (Pratt et al., 1989; Stanhill et al., 2006). For almost all substrates, the step in ERAD after recognition and before complete dislocation is polyubiquitination. We found that although degradation of membrane-associated E208K Cx32 was inhibited by exposure of cells to either H/O stress or MG132, the amount of polyubiquitinated E208K Cx32 recovered in both the membrane or cytosolic fractions was markedly greater after treatment with MG132 alone than after H/O stress either by itself or in combination with MG132. Given that there is no evidence that proteasome inhibitors such as MG132 increase the activity of the ubiquitination machinery, the simplest interpretation of our results with E208K Cx32 and myoD is that H/O stress perturbs the function of one or more components of this system. Among all of the proteasome substrates examined, the only one that was not stabilized by hyperthermia was the sole protein whose degradation is independent of ubiquitination (GFP-ODC). This finding further supports our contention that H/O stress affects polyubiquitination and not post-ubiquitination processes.
H/O stress rapidly raises the amount of thermally or oxidatively denatured proteins in the cytosol. Such misfolded proteins are highly susceptible to ubiquitination and are responsible for the increased levels of total polyubiquitinated proteins detected under these conditions (Figure 9; Parag et al., 1987; Bond et al., 1988). Stress-denatured proteins could titer a limiting component of the ubiquitination machinery away from pre-existing UPS substrates to an extent that depends on the substrate's abundance, how prone it is to deubiquitination, and (especially) its inherent affinity for the ubiquitination machinery. The ability of H/O stress to reduce connexin ERAD is transient, most likely reflecting the time required to return the level of unfolded protein in the cytosol to basal levels (VanSlyke and Musil, 2002). The turnover of UPS substrates with half-lives greater than ∼8 h would therefore not be expected to be markedly affected by H/O stress, which could explain why such conditions do not substantially reduce the rate of degradation of total cellular proteins (Westwood and Steinhardt, 1989; Bond et al., 1988; Luo et al., 2000).
Ubiquitination of cytosolic as well as ERAD substrates of the UPS requires the activity of E1 (ubiquitin-activating), E2 (ubiquitin-conjugating; Ubc), and, for most proteins, E3 (ubiquitin ligase) enzymes (Glickman and Ciechanover, 2002). Exogenous overexpression of ubiquitin does not appreciably affect stabilization by H/O stress, indicating that H/O stress does not act by reducing the level of ubiquitin available for polyubiquitination (not shown). Although oxidative stress causes dysfunction of the WT E3 ligase Parkin, mild (<46°C) hyperthermia does not (Winklhofer et al., 2003). Moreover, given the high level of substrate specificity of most of the >500 known E3 enzymes, it is very unlikely that degradation of all of the proteins we have found to be stress-sensitive involves the same E3 ligase. Selective inhibition of the ubiquitination and/or turnover of some, but not the majority, of cellular UPS substrates has previously been shown to result from partial inhibition of E1 or E2 activity (Gonen et al., 1999; Salvat et al., 2000). For the latter, this is likely due to the ability of multiple, but not all, E2s to modify a particular UPS substrate; only if the down-regulated E2 cannot be fully compensated for by another E2 is the substrate's ubiquitination (and thus its turnover) reduced (Saville et al., 2004; Younger et al., 2004). Similar to what we have observed after H/O stress (Figure 3), inhibiting the activity of the E2 UbcH5a decreases the degradation of both pulse-labeled Δ508 and WT forms of CFTR, but detectably increased the maturation of only the latter during a short (<8 h) chase (Younger et al., 2004). Salvat et al. (2000) have reported that levels of E1 that are ∼10–15% of WT support the degradation of p53 and the ubiquitination of most other proteins, but not that of another well-characterized UPS substrate, c-jun. Evidence that E1 activity may not be in excess under basal conditions (Pickart, 2004) and becomes limiting for a subset of ubiquitination substrates after only 10 min at 42°C (Kulka et al., 1988) has been discussed. Moreover, certain E2s, and to a lesser extent E1, are transcriptionally up-regulated in yeast by both heat shock and oxidative stressors, suggesting that they become limiting under these conditions (Gasch et al., 2000). Further experiments will be required to determine whether E1 and/or E2s become saturated and/or partially inactivated during H/O stress and whether the function of deubiquitinating enzymes is altered. Kopito and coworkers have established that expression of aggresome-forming proteins inhibits the degradation of multiple ER-associated and cytosolic UPS substrates. Under these conditions, however, substrates accumulate in a polyubiquitinated form, in some cases (e.g., CFTR) after their dislocation (Johnston et al., 1998). The effect of H/O stress is also distinct from that of inhibition of p97, which reduces UPS-mediated degradation at a postubiquitination step (Kobayashi et al., 2002; Dalal et al., 2004; Wojcik et al., 2006), and from that of ataxin-3 overexpression, which affects the degradation of ERAD, but not fast-turnover cytosolic, UPS substrates (Zhong and Pittman, 2006). We can also exclude saturation or inactivation of HSP70 as the likely mechanism by which H/O stress exerts the effects observed given that overexpression of HSP70 does not block the ability of stress to stabilize mutant Cx32 (not shown), and because turnover of ER-lumenal soluble ERAD substrates appears to be independent of HSP70 (Taxis et al., 2003; Huyer et al., 2004). Although binding of HSP90 protects certain proteins from ubiquitination under basal conditions (e.g., Kim et al., 2006), to our knowledge inhibition of ubiquitination of multiple proteins in response to mild, nonlethal hyperthermic and oxidative stress has not previously been reported.
Physiologically, the heat shock response is induced by a variety of conditions including hypoxia/reoxygenation, high fever, circulatory and hemorrhagic shock, energy depletion, acidosis, hypothermia, and viral infection. Moreover, hyperthermia and inorganic arsenite compounds (e.g., arsenic trioxide) at levels comparable to those used in this study have been administered in the treatment of several types of cancer (Douer and Tallman, 2005; Dewhirst et al., 2006). A well-known aspect of the heat shock response is a temporary inhibition of general protein synthesis (Brostrom and Brostrom, 1998) Stress stabilization might serve as a means to help preserve the function of fast-turnover UPS substrates under conditions in which they cannot be replaced. H/O stress also increases the level of ER-retained, misfolded proteins, which might act as dominant-negative inhibitors of WT proteins by binding to them or by otherwise interfering with their function. Either scenario would be a novel means by which genetic and environmental factors interact to influence disease progression.
ACKNOWLEDGMENTS
This work was supported by Grant R01 NS40740-01 to L.S.M. and the National Eye Institute Immunology/Molecular Biology Training Grant in Ophthalmology (T32-EY07123-16) to S.M.K.
Abbreviations used:
- BFA
brefeldin A
- CHX
cycloheximide
- Cx32
connexin32
- Cx43
connexin43
- DTT
dithiothreitol
- ERAD
ER-associated degradation
- H/O stress
hyperthermic/oxidative stress
- UPS
ubiquitin-proteasome system
- WT
wild-type.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-05-0487) on August 15, 2007.
REFERENCES
- Abel A., Bone L. J., Messing A., Scherer S. S., Fischbeck K. H. Studies in transgenic mice indicate a loss of connexin32 function in X-linked Charcot-Marie-Tooth disease. J. Neuropathol. Exp. Neurol. 1999;58:702–710. doi: 10.1097/00005072-199907000-00004. [DOI] [PubMed] [Google Scholar]
- Abu Hatoum O., Gross-Mesilaty S., Breitschopf K., Hoffman A., Gonen H., Ciechanover A., Bengal E. Degradation of myogenic transcription factor MyoD by the ubiquitin pathway in vivo and in vitro: regulation by specific DNA binding. Mol. Cell. Biol. 1998;18:5670–5677. doi: 10.1128/mcb.18.10.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bence N. F., Bennett E. J., Kopito R. R. Application and analysis of the GFPu family of ubiquitin-proteasome system reporters. Methods Enzymol. 2005;399:481–490. doi: 10.1016/S0076-6879(05)99033-2. [DOI] [PubMed] [Google Scholar]
- Bond U., Agell N., Haas A. L., Redman K., Schlesinger M. J. Ubiquitin in stressed chicken embryo fibroblasts. J. Biol. Chem. 1988;263:2384–2388. [PubMed] [Google Scholar]
- Breitschopf K., Bengal E., Ziv T., Admon A., Ciechanover A. A novel site for ubiquitination: the N-terminal residue, and not internal lysines of MyoD, is essential for conjugation and degradation of the protein. EMBO J. 1998;17:5964–5973. doi: 10.1093/emboj/17.20.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brostrom C. O., Brostrom M. A. Regulation of translational initiation during cellular responses to stress. Prog. Nucleic Acid Res. Mol. Biol. 1998;58:79–125. doi: 10.1016/s0079-6603(08)60034-3. [DOI] [PubMed] [Google Scholar]
- Castro C., Gomez-Hernandez J. M., Silander K., Barrio L. C. Altered formation of hemichannels and gap junction channels caused by C- terminal connexin-32 mutations. J. Neurosci. 1999;19:3752–3760. doi: 10.1523/JNEUROSCI.19-10-03752.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Martin T. M., Stevens S., Rittenberg M. B. Defective secretion of an immunoglobulin caused by mutations in the heavy chain complementarity determining region 2. J. Exp. Med. 1994;180:577–586. doi: 10.1084/jem.180.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal S., Rosser M., Cyr M. D., Hanson P. Distinct roles for the AAA ATPases NSF and p97 in the secretory pathway. Mol. Biol. Cell. 2004;15:637–648. doi: 10.1091/mbc.E03-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes S. M., Walcott J. L., Wexler T. L., Scherer S. S., Fischbeck K. H. Altered trafficking of mutant connexin32. J. Neurosci. 1997;17:9077–9084. doi: 10.1523/JNEUROSCI.17-23-09077.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst M. W., Jones E., Samulski T., Vujaskovic Z., Li C., Prosnitz L. Hyperthermia. In: Kufe D. W., Bast R. C., Hait W. W., Hong W. K., Pollack R. E., Weichselbaum R. R., Holland J. F., Frei E., editors. Holland-Frei Cancer Medicine. Vol. 7. Hamilton, BC, Canada: Decker; 2006. pp. 549–561. [Google Scholar]
- Douer D., Tallman M. S. Arsenic trioxide: new clinical experience with an old medication in hematologic malignancies. J. Clin. Oncol. 2005;23:2396–2410. doi: 10.1200/JCO.2005.10.217. [DOI] [PubMed] [Google Scholar]
- Dul J. L., Davis D. P., Williamson E. K., Stevens F. J., Argon Y. Hsp70 and antifibrillogenic peptides promote degradation and inhibit intracellular aggregation of amyloidogenic light chains. J. Cell Biol. 2001;152:705–716. doi: 10.1083/jcb.152.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsasser S., Finley D. Delivery of ubiquitinated substrates to protein-unfolding machines. Nat. Cell Biol. 2005;7:742–749. doi: 10.1038/ncb0805-742. [DOI] [PubMed] [Google Scholar]
- Fairweather N., Bell C., Cochrane S., Chelly J., Wang S., Mostacciuolo M. L., Monaco A. P., Haites N. E. Mutations in the connexin 32 gene in X-linked dominant Charcot-Marie-Tooth disease (CMTX1) Hum. Mol. Genet. 1994;3:29–34. doi: 10.1093/hmg/3.1.29. [DOI] [PubMed] [Google Scholar]
- Flierman D., Ye Y., Dai M., Chau V., Rapoport T. A. Polyubiquitin serves as a recognition signal, rather than a ratcheting molecule, during retrotranslocation of proteins across the endoplasmic reticulum membrane. J. Biol. Chem. 2003;278:34774–34782. doi: 10.1074/jbc.M303360200. [DOI] [PubMed] [Google Scholar]
- Gasch A. P., Spellman P. T., Kao C. M., Carmel-Harel O., Eisen M. B., Storz G., Botstein D., Brown P. O. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman M. S., Kannegaard E. S., Kopito R. R. A principal role for the proteasome in endoplasmic reticulum-associated degradation of misfolded intracellular cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 2002;277:11709–11714. doi: 10.1074/jbc.M111958200. [DOI] [PubMed] [Google Scholar]
- Glickman M. H., Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- Gonen H., Bercovich B., Orian A., Carrano A., Takizawa C., Yamanaka K., Pagano M., Iwai K., Ciechanover A. Identification of the ubiquitin carrier proteins, E2s, involved in signal-induced conjugation and subsequent degradation of IkappaBalpha. J. Biol. Chem. 1999;274:14823–14830. doi: 10.1074/jbc.274.21.14823. [DOI] [PubMed] [Google Scholar]
- Goodenough D. A., Goliger J. A., Paul D. L. Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- Groll M., Huber R. Inhibitors of the eukaryotic 20S proteasome core particle: a structural approach. Biochim. Biophys. Acta. 2004;1695:33–44. doi: 10.1016/j.bbamcr.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Hoyt M. A., Zhang M., Coffino P. Ubiquitin-independent mechanisms of mouse ornithine decarboxylase degradation are conserved between mammalian and fungal cells. J. Biol. Chem. 2003;278:12135–12143. doi: 10.1074/jbc.M211802200. [DOI] [PubMed] [Google Scholar]
- Huyer G., Piluek W., Fansler Z., Kreft S., Hochstrasser M., Brodsky J., Michaelis S. Distinct machinery is required in Saccharomyces cerevisiae for the endoplasmic reticulum-associated degradation of a multispanning membrane protein and a soluble lumenal protein. J. Biol. Chem. 2004;279:38369–38378. doi: 10.1074/jbc.M402468200. [DOI] [PubMed] [Google Scholar]
- Jarosch E., Lenk U., Sommer T. Endoplasmic reticulum-associated protein degradation. Int. Rev. Cytol. 2003;223:39–81. doi: 10.1016/s0074-7696(05)23002-4. [DOI] [PubMed] [Google Scholar]
- Johnston J. A., Ward C. L., Kopito R. R. Aggresomes: a cellular response to misfolded proteins. J. Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T. S, Jang C. Y., Kim H. D., Lee J. Y., Ahn B. Y., Kim J. Interaction of Hsp90 with ribosomal proteins protects from ubiquitination and proteasome-dependent degradation. Mol. Biol. Cell. 2006;17:824–833. doi: 10.1091/mbc.E05-08-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Tanaka K., Inoue K., Kakizuka A. Functional ATPase activity of p97/valosin-containing protein (VCP) is required for the quality control of endoplasmic reticulum in neuronally differentiated mammalian PC12 cells. J. Biol. Chem. 2002;277:47358–47365. doi: 10.1074/jbc.M207783200. [DOI] [PubMed] [Google Scholar]
- Kulka R. G., Raboy B., Schuster R., Parag H. A., Diamond G., Ciechanover A., Marcus M. A Chinese hamster cell cycle mutant arrested at G2 phase has a temperature-sensitive ubiquitin-activating enzyme, E1. J. Biol. Chem. 1988;263:15726–15731. [PubMed] [Google Scholar]
- Laird D. W. Life cycle of connexins in health and disease. Biochem. J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepock J. R., Frey H. E., Ritchie K. P. Protein denaturation in intact hepatocytes and isolated cellular organelles during heat shock. J. Cell Biol. 1993;122:1267–1276. doi: 10.1083/jcb.122.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhao X., Fang Y., Jiang X., Duong T., Fan C., Huang C. C., Kain S. R. Generation of destabilized green fluorescent protein as a transcription reporter. J. Biol. Chem. 1998;273:34970–34975. doi: 10.1074/jbc.273.52.34970. [DOI] [PubMed] [Google Scholar]
- Lukacs G. L., Mohamed A., Kartner N., Chang X. B., Riordan J. R., Grinstein S. Conformational maturation of CFTR but not its mutant counterpart (delta F508) occurs in the endoplasmic reticulum and requires ATP. EMBO J. 1994;13:6076–6086. doi: 10.1002/j.1460-2075.1994.tb06954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G. J., Sun X., Hasselgren P. O. Hyperthermia stimulates energy-proteasome-dependent protein degradation in cultured myotubes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;278:R749–R756. doi: 10.1152/ajpregu.2000.278.3.R749. [DOI] [PubMed] [Google Scholar]
- Mancini R., Fagioli C., Fra A. M., Maggioni C., Sitia R. Degradation of unassembled soluble Ig subunits by cytosolic proteasomes: evidence that retrotranslocation and degradation are coupled events. FASEB J. 2000;14:769–778. doi: 10.1096/fasebj.14.5.769. [DOI] [PubMed] [Google Scholar]
- Meusser B., Hirsch C., Jarosch E., Sommer T. ERAD: the long road to destruction. Nat. Cell Biol. 2005;7:766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- Musil L. S., Cunningham B. A., Edelman G. M., Goodenough D. A. Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines. J. Cell Biol. 1990;111:2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil L. S., Goodenough D. A. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993;74:1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- Musil L. S., Le A. C., VanSlyke J. K., Roberts L. M. Regulation of connexin degradation as a mechanism to increase gap junction assembly and function. J. Biol. Chem. 2000;275:25207–25215. doi: 10.1074/jbc.275.33.25207. [DOI] [PubMed] [Google Scholar]
- Nijman S. M., Luna-Vargas M. P., Velds A., Brummelkamp T. R., Dirac A. M., Sixma T. K., Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- O'Hare T., Wiens G. D., Whitcomb E. A., Enns C. A., Rittenberg M. B. Cutting edge: proteasome involvement in the degradation of unassembled Ig light chains. J. Immunol. 1999;163:11–14. [PubMed] [Google Scholar]
- Parag H. A., Raboy B., Kulka R. G. Effect of heat shock on protein degradation in mammalian cells: involvement of the ubiquitin system. EMBO J. 1987;6:55–61. doi: 10.1002/j.1460-2075.1987.tb04718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart C. M. Back to the future with ubiquitin. Cell. 2004;116:181–190. doi: 10.1016/s0092-8674(03)01074-2. [DOI] [PubMed] [Google Scholar]
- Pratt G., Hough R., Rechsteiner M. Proteolysis in heat-stressed HeLa cells. Stabilization of ubiquitin correlates with the loss of proline endopeptidase. J. Biol. Chem. 1989;264:12526–12532. [PubMed] [Google Scholar]
- Salvat C., Acquaviva C., Scheffner M., Robbins I., Piechaczyk M., Jariel-Encontre I. Molecular characterization of the thermosensitive E1 ubiquitin-activating enzyme cell mutant A31N-ts20. Requirements upon different levels of E1 for the ubiquitination/degradation of the various protein substrates in vivo. Eur. J. Biochem. 2000;267:3712–3722. doi: 10.1046/j.1432-1327.2000.01404.x. [DOI] [PubMed] [Google Scholar]
- Sato S., Ward C. L., Kopito R. R. Cotranslational ubiquitination of cystic fibrosis transmembrane conductance regulator in vitro. J. Biol. Chem. 1998;273:7189–7192. doi: 10.1074/jbc.273.13.7189. [DOI] [PubMed] [Google Scholar]
- Saville M. K., Sparks A., Xirodimas D. P., Wardrop J., Stevenson L. F., Bourdon J. C., Woods Y. L., Lane D. P. Regulation of p53 by the ubiquitin-conjugating enzymes UbcH5B/C in vivo. J. Biol. Chem. 2004;279:42169–42181. doi: 10.1074/jbc.M403362200. [DOI] [PubMed] [Google Scholar]
- Senisterra G. A., Huntley S. A., Escaravage M., Sekhar K. R., Freeman M. L., Borrelli M., Lepock J. R. Destabilization of the Ca2+-ATPase of sarcoplasmic reticulum by thiol-specific, heat shock inducers results in thermal denaturation at 37°C. Biochemistry. 1997;36:11002–11011. doi: 10.1021/bi9711590. [DOI] [PubMed] [Google Scholar]
- Shamu C. E., Story C. M., Rapoport T. A., Ploegh H. L. The pathway of US11-dependent degradation of MHC class I heavy chains involves a ubiquitin-conjugated intermediate. J. Cell Biol. 1999;147:45–58. doi: 10.1083/jcb.147.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhill A., Haynes C. M., Zhang Y., Min G., Steele M. C., Kalinina J., Martinez E., Pickart C. M., Kong X. P., Ron D. An arsenite-inducible 19S regulatory particle-associated protein adapts proteasomes to proteotoxicity. Mol. Cell. 2006;23:875–885. doi: 10.1016/j.molcel.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Story C. M., Furman M. H., Ploegh H. L. The cytosolic tail of class I MHC heavy chain is required for its dislocation by the human cytomegalovirus US2 and US11 gene products. Proc. Natl. Acad. Sci. USA. 1999;96:8516–8521. doi: 10.1073/pnas.96.15.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxis C, Hitt R., Park S. H., Deak P. M., Kostova Z., Wolf D. H. Use of modular substrates demonstrates mechanistic diversity and reveals differences in chaperone requirement of ERAD. J. Biol. Chem. 2003;278:35903–35913. doi: 10.1074/jbc.M301080200. [DOI] [PubMed] [Google Scholar]
- Thayer M. J., Tapscott S. J., Davis R. L., Wright W. E., Lassar A. B., Weintraub H. Positive autoregulation of the myogenic determination gene MyoD1. Cell. 1989;58:241–248. doi: 10.1016/0092-8674(89)90838-6. [DOI] [PubMed] [Google Scholar]
- Treier M., Staszewski L. M., Bohmann D. Ubiquitin-dependent c-Jun degradation in vivo is mediated by the delta domain. Cell. 1994;78:787–798. doi: 10.1016/s0092-8674(94)90502-9. [DOI] [PubMed] [Google Scholar]
- Valiunas V., Bukauskas F. F., Weingart R. Conductances and selective permeability of connexin43 gap junction channels examined in neonatal rat heart cells. Circ. Res. 1997;80:708–719. doi: 10.1161/01.res.80.5.708. [DOI] [PubMed] [Google Scholar]
- VanSlyke J. K., Deschenes S. M., Musil L. S. Intracellular transport, assembly, and degradation of wild-type and disease-linked mutant gap junction proteins. Mol. Biol. Cell. 2000;11:1933–1946. doi: 10.1091/mbc.11.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanSlyke J. K., Musil L. S. Analysis of connexin intracellular transport and assembly. Methods. 2000;20:156–164. doi: 10.1006/meth.1999.0933. [DOI] [PubMed] [Google Scholar]
- VanSlyke J. K., Musil L. S. Dislocation and degradation from the ER are regulated by cytosolic stress. J. Cell Biol. 2002;157:381–394. doi: 10.1083/jcb.200111045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E. D., Brodsky J. L., McCracken A. A. Proteasome-dependent endoplasmic reticulum-associated protein degradation: an unconventional route to a familiar fate. Proc. Natl. Acad. Sci. USA. 1996;93:13797–13801. doi: 10.1073/pnas.93.24.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood J. T., Steinhardt R. A. Effects of heat and other inducers of the stress response on protein degradation in Chinese hamster and Drosophila cells. J. Cell. Physiol. 1989;139:196–209. doi: 10.1002/jcp.1041390127. [DOI] [PubMed] [Google Scholar]
- Wojcik C., Rowicka M., Kudlicki A., Nowis D., McConnell E., Kujawa M., DeMartino G. N. Valosin-containing protein (p97) is a regulator of endoplasmic reticulum stress and of the degradation of N-end rule and ubiquitin-fusion degradation pathway substrates in mammalian cells. Mol. Biol. Cell. 2006;17:4606–4618. doi: 10.1091/mbc.E06-05-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Chong E., Skach W. R. Evidence that endoplasmic reticulum (ER)-associated degradation of cystic fibrosis transmembrane conductance regulator is linked to retrograde translocation from the ER membrane. J. Biol. Chem. 1999;274:2616–2624. doi: 10.1074/jbc.274.5.2616. [DOI] [PubMed] [Google Scholar]
- Ye Y., Meyer H., Rapoport T. The AAA-ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature. 2001;414:652–656. doi: 10.1038/414652a. [DOI] [PubMed] [Google Scholar]
- Younger J. M., Ren H. Y., Chen L., Fan C. Y., Fields A., Patterson C., Cyr D. M. A foldable CFTR{Delta}F508 biogenic intermediate accumulates upon inhibition of the Hsc70-CHIP E3 ubiquitin ligase. J. Cell Biol. 2004;167:1075–1085. doi: 10.1083/jcb.200410065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Kaung G., Kobayashi S., Kopito R. R. Cytosolic degradation of T-cell receptor alpha chains by the proteasome. J. Biol. Chem. 1997;272:20800–20804. doi: 10.1074/jbc.272.33.20800. [DOI] [PubMed] [Google Scholar]
- Winklhofer K. F., Henn I. H., Kay-Jackson P. C., Heller U., Tatzelt J. Inactivation of parkin by oxidative stress and C-terminal truncations: a protective role of molecular chaperones. J. Biol. Chem. 2003;278:47199–47208. doi: 10.1074/jbc.M306769200. [DOI] [PubMed] [Google Scholar]
- Zhong X., Pittman R. N. Ataxin-3 binds VCP/p97 and regulates retrotranslocation of ERAD substrates. Hum. Mol. Genet. 2006;15:2409–2420. doi: 10.1093/hmg/ddl164. [DOI] [PubMed] [Google Scholar]