Abstract
The lipid kinase Fab1 governs yeast vacuole homeostasis by generating PtdIns(3,5)P2 on the vacuolar membrane. Recruitment of effector proteins by the phospholipid ensures precise regulation of vacuole morphology and function. Cells lacking the effector Atg18p have enlarged vacuoles and high PtdIns(3,5)P2 levels. Although Atg18 colocalizes with Fab1p, it likely does not directly interact with Fab1p, as deletion of either kinase activator—VAC7 or VAC14—is epistatic to atg18Δ: atg18Δvac7Δ cells have no detectable PtdIns(3,5)P2. Moreover, a 2xAtg18 (tandem fusion) construct localizes to the vacuole membrane in the absence of PtdIns(3,5)P2, but requires Vac7p for recruitment. Like the endosomal PtdIns(3)P effector EEA1, Atg18 membrane binding may require a protein component. When the lipid requirement is bypassed by fusing Atg18 to ALP, a vacuolar transmembrane protein, vac14Δ vacuoles regain normal morphology. Rescue is independent of PtdIns(3,5)P2, as mutation of the phospholipid-binding site in Atg18 does not prevent vacuole fission and properly regulates Fab1p activity. Finally, the vacuole-specific type-V myosin adapter Vac17p interacts with Atg18p, perhaps mediating cytoskeletal attachment during retrograde transport. Atg18p is likely a PtdIns(3,5)P2“sensor,” acting as an effector to remodel membranes as well as regulating its synthesis via feedback that might involve Vac7p.
INTRODUCTION
Eukaryotic cells have evolved highly sophisticated macromolecular machinery to orchestrate the complex and dynamic process of membrane trafficking. These protein complexes vary widely in structure and function, but are all tightly coordinated in order to catalyze a sequence of specific events with the goal of transferring membrane cargo from one compartment to another. Despite the constant flux of membrane and proteins, organelles maintain distinct identities. To achieve the formation and maintenance of organelle size, shape, and function, trafficking complexes must be properly targeted and subsequently recycled (Munro, 2004; Behnia and Munro, 2005; Jordens et al., 2005). Lipids have been shown to play an increasingly important role in these processes, especially phosphoinositides (PtdInsPs).
PtdInsPs are phosphorylated derivatives of phosphatidylinositol, of which there are seven species that can be rapidly interconverted by phosphorylation or dephosphorylation reactions. The occurrence of a specific PtdInsP transiently recruits cognate effector proteins that bind the PtdInsP via short modular motifs such as the FYVE and PH domains (Lemmon, 2003; Balla, 2005; Behnia and Munro, 2005; Di Paolo and De Camilli, 2006; Takenawa and Itoh, 2006). Hence, PtdInsPs are particularly suited to serve as membrane surface tags. Importantly, PtdInsP-binding modules differ greatly in their affinity and specificity for the various PtdInsPs, which when coupled with protein partners, generates a specificity code for each organelle (Lawe et al., 2000; Balla, 2005).
PtdIns(3)P and PtdIns(3,5)P2, respectively, label early and late endosomal structures (Burd and Emr, 1998; Cooke et al., 1998; Gary et al., 1998; Gillooly et al., 2000; Jeffries et al., 2004; Kim et al., 2005). Although PtdIns(3)P predominantly controls protein sorting and membrane dynamics at the early endosome, PtdIns(3,5)P2 appears to govern events associated with later endosomal compartments such as the yeast vacuole (Schu et al., 1993; Cooke et al., 1998; Gary et al., 1998; Clague et al., 1999). Cells depleted for PtdIns(3,5)P2 exhibit numerous vacuolar defects including nonacidic vacuoles, impaired vacuole-to-endosome retrograde transport, and abnormal vacuolar inheritance, and most conspicuously, these cells possess a drastically enlarged, single-lobed vacuole (Yamamoto et al., 1995; Cooke et al., 1998; Gary et al., 1998; Jeffries et al., 2004). In contrast, increasing the levels of PtdIns(3,5)P2 during hypertonic shock or through genetic manipulation generates many small vacuole lobes (Dove et al., 1997; Gary et al., 2002). Together, this suggests that PtdIns(3,5)P2 principally modulates membrane traffic and dynamics of the vacuole.
PtdIns(3,5)P2 is generated from PtdIns(3)P phosphorylation by the yeast PtdIns(3)P 5-kinase Fab1p (PIKfyve in mammals; Cooke et al., 1998; Gary et al., 1998; Sbrissa et al., 1999). Synthesis of PtdIns(3,5)P2 is modulated by at least two vacuolar proteins, Vac7p and Vac14p. Vac7p is a transmembrane protein with no known homologues in higher eukaryotes, whereas Vac14p is likely an adaptor protein. PtdIns(3,5)P2 is reduced 10-fold or more in vac14Δ and vac7Δ and consequently, these cells exhibit a phenotype similar to fab1Δ cells (Bonangelino et al., 1997; Dove et al., 2002; Gary et al., 2002). Interestingly, Vac14p binds to Fig4p, a PtdIns(3,5)P2-specific 5′-phosphatase thought to antagonize Fab1p (Rudge et al., 2004). Indeed, a loss of function mutant of FIG4 was originally isolated as a suppressor of vac7Δ, partially rescuing the levels of PtdIns(3,5)P2 and vacuolar morphology (Gary et al., 2002). However, Fig4p appears to be necessary for maximal synthesis of PtdIns(3,5)P2 during hypertonic shock, pointing to a more complex role for Fig4 than to simply turnover PtdIns(3,5)P2 (Duex et al., 2006a).
Several putative protein effectors of PtdIns(3,5)P2 have been identified. The mammalian ESCRT-III component, mVps24 (Whitley et al., 2003), and the ENTH-containing proteins Ent3p/Ent5p were shown to bind PtdIns(3,5)P2 and are suggested effectors for PtdIns(3,5)P2-dependent sorting into MVBs (Friant et al., 2003; Eugster et al., 2004). In addition, Dove et al. (2004) identified SVP1 in a screen for cells with swollen vacuoles and demonstrated that Svp1p bound to PtdIns(3,5)P2 (Dove et al., 2004). Interestingly, Svp1p was shown to be the same as Atg18p (used herein), a previously identified component of the autophagic and the cytosol-to-vacuole transport (Cvt) pathways (Barth et al., 2001). It is noteworthy that PtdIns(3,5)P2 does not appear to play a direct role in autophagy or the Cvt pathways, suggesting that Atg18p has at least two distinct functions in the cell (Dove et al., 2004).
ATG18 encodes a multi-WD–containing protein that is postulated to fold into a β-barrel. It is related to the yeast Atg21p and Hsv2p and to the WIPI proteins in mammals (Jeffries et al., 2004). Atg18p does not possess a distinct PtdIns-binding module. Instead, an intact β-barrel comprising almost the entire length of Atg18p is required to associate with PtdIns(3,5)P2. However, the FRRG287 motif on the β-barrel is a strong candidate for the direct site of interaction with PtdIns(3,5)P2. Mutation of the double arginines disrupts binding to PtdIns(3,5)P2 and shifts Atg18p from the vacuole to the cytosol (Dove et al., 2004).
The precise role and molecular mechanism of Atg18p as a PtdIns(3,5)P2 effector remains uncertain. Dove et al. (2004) suggest that Atg18p is required for PtdIns(3,5)P2-dependent retrograde membrane traffic from the vacuole to the Golgi apparatus via the endosomes. This conclusion was deduced from the lack of the mature isoform of RS-ALP in the Golgi membrane fraction in atg18Δ cells (Bryant and Stevens, 1998; Dove et al., 2004). RS-ALP is an ALP isoform modified with a FXFXD motif that enables recycling from the vacuole, where it undergoes proteolytic maturation, to the Golgi (Bryant and Stevens, 1998; Dove et al., 2004). On the other hand, deletion of ATG18 causes an abnormal elevation in the levels of PtdIns(3,5)P2, which suggests that Atg18p is also a negative regulator of the Fab1 kinase pathway (Bryant and Stevens, 1998; Dove et al., 2004). Clearly, Atg18p is a key component of the PtdIns(3,5)P2 signaling network, being an apparent effector and modulator of this lipid.
Here, we used several engineered isoforms of Atg18p that deliver it to the vacuole independently of PtdIns(3,5)P2 to explore the requirement for PtdIns(3,5)P2-binding in governing vacuolar morphology and lipid levels. We also found that Vac7 plays a role in membrane recruitment of Atg18. Finally, we identify Vac17 as a protein partner of Atg18p. Putative interaction with Vac7p may provide a model for Atg18p-dependent inhibition of the Fab1 kinase, whereas binding to Vac17p suggests a possible role of Atg18p in vacuole inheritance.
MATERIALS AND METHODS
Strains and Media
A list of all S. cerevisiae strains used in this study and their genotypes can be found in Table 1. Strains were grown in rich (YPD) or minimal (SD) media supplemented with the appropriate amino acids. Standard growth conditions and manipulations have been described previously by Rose et al. (1990).
Table 1.
S. cerevisiae strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| SEY6210 | Matα leu2-3, 112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | Robinson et al. (1988) |
| fab1Δ2 | SEY6210; fab1Δ::HIS3 | Gary et al. (1998) |
| JGY134 | SEY6210; vac7Δ::HIS3 | Gary et al. (2002) |
| JGY138 | SEY6210; fig4Δ::LEU2 | Gary et al. (2002) |
| JGY145 | SEY6210; vac14Δ::TRP1 | Gary et al. (2002) |
| SRY13 | SEY6210; atg18Δ::HIS3 | This study |
| SRY14 | SEY6210; Atg18-GFP:HIS3 | This study |
| SRY15 | SEY6210; Atg18-GFP:HIS3 fab1Δ::HIS3 | This study |
| JEY35 | SEY6210; Atg18-HA:TRP1 | This study |
| JEY38 | SEY6210; Atg18-GFP:HIS3 vac14Δ::TRP1 | This study |
| JEY40 | SEY6210; Atg18-GFP:HIS3 vac7Δ::HIS3 | This study |
| JEY41 | SEY6210; atg18Δ::HIS3 vac14Δ::TRP1 | This study |
| JEY48 | SEY6210; atg18Δ::HIS3 vac7Δ::HIS3 | This study |
| JEY53 | SEY6210; atg18Δ::HIS3 fig4Δ::LEU2 | This study |
| JEY66 | SEY6210; Atg18-FLAG:TRP1 | This study |
| JEY85 | SEY6210; Atg18-HA-mRFP:LEU2 Fab1-GFP:HIS3 | This study |
| JEY89 | SEY6210; Atg18-HA-mRFP:LEU2 Vac14-GFP:HIS3 | This study |
Genetic and DNA Manipulations
Restriction enzymes were purchased from New England Biolabs (Ipswich, MA), and PCR reactions were carried out with KOD polymerase (EMD Biosciences, San Diego, CA) or ExTaq (Takara Mirus Bio, Madison, WI) for cloning and diagnostic reactions, respectively. Standard molecular biology techniques as described by Maniatis et al. (1992) were used for all DNA manipulations. Yeast transformations were done by the method of Ito et al. (1983), and yeast genomic preparations were carried out as described by Hoffman and Winston (1987).
Deletions and chromosomal epitope-tagging were all done in the SEY6210 wild-type background (Table 1) using PCR-amplified genomic integrations as described by Longtine et al. (1998). All deletions and integrations were verified by PCR analysis, and expression (chromosomal and plasmid-based) of fusion proteins was confirmed by Western blot analysis. For all PCR-based cloning procedures, unique restriction enzyme sites were generated at the 5′ and 3′ ends of open reading frames (ORFs) by incorporating them into their respective primers.
Plasmids pJE181 (encoding GFP-Atg18) and pJE191 (GFP-Vac17) are based on the vector pBP73G, a kind gift from Dr. Willliam R. Parrish (UCSD, San Diego). Briefly, pBP73G was created by cloning the GPD1 promoter (beginning 500 base pairs upstream of start) between the SacI and XbaI sites of pRS416 (Sikorski and Hieter, 1989) by PCR amplification, followed by the subcloning of GFP between the XbaI and BamHI sites. pJE181 and pJE191 contain the full-length ATG18 and VAC17 ORFs amplified by PCR and ligated in-frame between the EcoRI/XhoI and BamHI/SalI site pairs, respectively. pJE182 was generated from pJE181 by mutating 285RR286 to 285GG286 in Atg18 using site-directed mutagenesis (Stratagene, La Jolla, CA). Plasmids pJE183 (encoding GFP-Atg18-ALP), pJE184 (GFP-Atg18-ALP with a 285GG286 mutation in Atg18), pJE185 (GFP-2xAtg18), and pJE186 (myc-2xAtg18) are all based on the vector pRB415A, a modified version of pBP74A (also a gift from Dr. William R. Parrish). Briefly, pBP74A was generated from pRS415 (Sikorski and Hieter, 1989) by cloning the ADH1 promoter (beginning 500 base pairs upstream of start) by PCR as a SacI-XbaI fragment, followed by ligation of GFP between the XbaI and BamHI sites. pRB415A modifies pBP74A by integrating the restriction sites BglII-AvrII-AatI-SpeI-NruI-NheI-BspEI-SacII between the HindIII and SalI sites of pBP74A. pJE183 contains full-length ATG18 and PHO8 ligated in-frame between the EcoRI/HindIII and AvrII/NruI site pairs, respectively. pJE184 was generated from pJE183 using site-directed mutagenesis as described above. pJE185 is identical to pJE183, except that a second copy of ATG18 was cloned in-frame between the XmaI and XhoI sites instead of PHO8 as above. pJE186 was generated form pJE185 by excision of GFP using XbaI and EcoRI, followed by ligation of a 12xmyc tag (amplified from pFA6a-13myc-TRP1; Longtine et al., 1998) into the gapped plasmid using the same sites.
Primer sequences for all of the above genetic manipulations are available upon request.
Fluorescence Microscopy
In vivo labeling of the vacuole limiting membrane with the lipophilic dye N-[3-triethylammoniumpropyl]-4-[p-diethylaminophenylhexa-trienyl] pyridinium dibromide (FM4-64; Molecular Probes, Eugene, OR) was done as outlined previously by Vida and Emr (1995). Vacuoles were also labeled with 100 nM 4-chloromethyl-7-aminocoumarin (CMAC) for 10 min and subsequently washed with fresh medium.
Fluorescence and differential interference contrast (DIC) images of cells labeled with FM4-64 and those expressing GFP fusion proteins were generated with a Delta Vision RT microscopy system (Applied Precision, Issaquah, WA). Specifically, data were acquired using an Olympus IX71 inverted microscope (Tokyo, Japan) equipped with fluorescein isothiocyanate (FITC) and rhodamine filters and coupled to a Photometrics CoolSNAP HQ camera (Tucson, AZ). Images were processed using Delta Vision deconvolution software (Applied Precision) and Adobe Photoshop 8.0 (Adobe Systems, San Jose, CA).
Quantification of Fluorescence Intensity and Coincidence
Fluorescence intensity of the FM4-64 (rhodamine) and green fluorescent protein (GFP; FITC) channels of unprocessed TIFF images was quantified by using ImageJ 1.36b (NIH, Bethesda, MD). Typically, plot profiles were acquired by drawing a five-pixel wide line from the cytosol to the vacuole lumen; lines started around the midpoint of the cytosol and were typically 20–40 pixels long. Line positioning across the vacuole was random, but puncta were avoided. Fluorescence intensity values were exported to Excel 2004 (Microsoft, Redmond, WA) where the background was subtracted. For graphical representation in Figures 1 and 5, intensity values were normalized against the highest intensity value to achieve a peak of 1. For the values depicted in Tables 2 and 3 intensity values were normalized against the value of the first cytosolic pixel. The vacuole membrane was defined as the pixel with the highest FM4-64 intensity, and the corresponding GFP intensity was used to measure the relative enrichment of the GFP-fusion protein between the vacuole membrane and the cytosol. The relative GFP fluorescence intensity of vacuole/cytosol for Atg18-GFP and GFP-2xAtg18 was statistically analyzed against that of soluble GFP using the one-tail, unpaired Student's t test. Note that the contrast and the brightness of the images shown in Figures 1 and 5 have been adjusted after acquiring line plot profiles.
Figure 1.
Atg18 is entirely cytosolic in fab1Δ, vac7Δ, and vac14Δ mutants. (A) Fluorescence microscopy was used to determine the localization of an Atg18-GFP fusion in relation to the vacuoles of wild-type and mutant cells, as labeled with FM4-64. Overlap with the vacuolar membrane was quantified with ImageJ software by plotting normalized fluorescence intensity along a path traversing the vacuole membrane (indicated by the white bars). For orientation purposes, the cytosol (left) is separated from the lumen of the vacuole (right) by a dotted line. (B) Atg18-RFP localizes to puncta on the vacuolar rim that partially overlap with Vac14-GFP and Fab1-GFP. RFP/GFP pairs were coexpressed and their localization was compared by fluorescence microscopy. Arrows in the merge panels indicate areas of extensive overlap. (C) Atg18-GFP puncta are highly mobile. Representative still images from a 64-s time-lapse movie (Supplementary Movie 1; figure 1C.mov) depict a patch of membrane enriched in Atg18-GFP (arrows) budding from the mother cell vacuole and traveling into the daughter cell. Simultaneously acquired FM4-64 fluorescence images highlight the vacuolar membranes. Bars, 4 μm.
Figure 5.
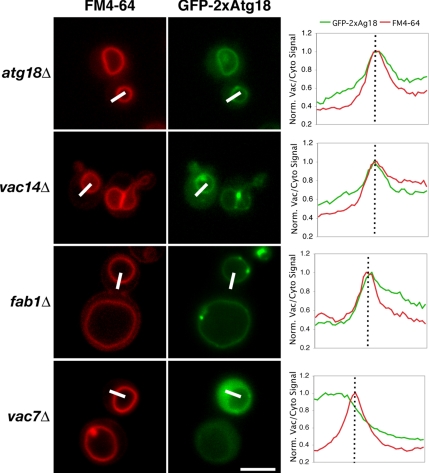
GFP-2xAtg18 can bind to the vacuole membrane in the absence of PtdIns(3,5)P2, but requires Vac7p for membrane localization. atg18Δ, vac14Δ, fab1Δ, and vac7Δ cells expressing GFP-2xAtg18 were labeled with the fluorescent dye FM4-64 to highlight vacuoles. Coincidence of the GFP signal with the vacuole membrane was quantified as described in the legend to Figure 1. Bars, 4 μm.
Table 2.
Ratio of the Atg18-GFP intensity on the vacuolar membrane vs. the cytosolic signal
| Strain | GFP vacuolar/cytosolic signala | t test, p valueb | Sample number (n) |
|---|---|---|---|
| Wt-GFP | 1.04 ± 0.05 | N/A | 13 |
| atg18Δ | 1.70 ± 0.27 | p < 0.05 | 13 |
| vac14Δ | 1.12 ± 0.1 | p < 0.05 | 17 |
| fab1Δ | 1.07 ± 0.08 | p > 0.05 | 11 |
| vac7Δ | 1.05 ± 0.03 | p > 0.05 | 16 |
a GFP signal intensity on the vacuolar membrane was defined by colocalization with the highest intensity of FM4–64 signal in line scan profiles.
b Student's t test, one tail was used to test the null hypothesis that the vacuole/cytosol ratio of Atg18-GFP was not significantly greater than the ratio obtained with soluble GFP expressed in wild-type cells. Values are normalized against first cytosolic pixel value.
Table 3.
Ratiometric measurement of GFP-2ξAtg18 signal present on the vacuolar membrane vs. that of the cytosol
| Strain | GFP vacuolar/cytosolic signala | t test, p valueb | Sample number (n) |
|---|---|---|---|
| Wt-GFP | 1.04 ± 0.05 | N/A | 13 |
| atg18Δ | 1.84 ± 0.22 | p < 0.05 | 10 |
| vac14Δ | 1.88 ± 0.30 | p < 0.05 | 13 |
| fab1Δ | 2.55 ± 0.54 | p < 0.05 | 11 |
| vac7Δ | 1.08 ± 0.13 | p >g 0.05 | 12 |
a GFP signal intensity on the vacuolar membrane was defined by colocalization with the highest intensity of FM4-64 signal in line scan profiles.
b Student's t test, one tail was employed to test the null hypothesis that the vacuole/cytosol ratio of GFP-2xAtg18 was not significantly greater than the ratio obtained with soluble GFP expressed in wild-type cells. Values are normalized against first cytosolic pixel value.
Immunoprecipitation and Western Blot Analysis
35S metabolic labeling and immunoprecipitations were performed as described previously (Gaynor et al., 1994). Briefly, midlog (OD600 ∼0.6) phase cultures were concentrated to 3 OD600 units/ml and labeled with 3 μl Tran-35S label per OD600 (Perkin Elmer Life and Analytical Sciences, Boston, MA) for 20 min in SD medium. Cells were chased with 20 mM methionine, 8 mM cysteine, and 0.8% yeast extract for 120 min. Proteins were precipitated in 10% trichloroacetic acid (TCA), and resulting pellets were washed twice with ice-cold acetone, dried, and processed for immunoprecipitation as described previously (Gaynor et al., 1994). Anti-APeI antibody was a kind gift from Dr. Daniel Klionsky (University of Michigan, Ann Arbor, MI). Immunoprecipitated proteins were resolved on SDS-PAGE gels and analyzed by autoradiography.
Cross-linking immunoprecipitations using 35S-labeled extracts were carried out essentially as described by Rieder and Emr (1997). Briefly, osmotic whole cell lysates were treated with the cross-linker DSP [dithiobis(succinimidyl-propionate); Pierce, Rockford, IL] and TCA-precipitated proteins were subjected to two successive overnight immunoprecipitations using anti-HA antibody (Roche Diagnostics, Indianapolis, IN). After cleavage of the cross-linker and resolution by SDS-PAGE, autoradiography was used to reveal any proteins cross-linked to Atg18-HA.
For coimmunoprecipitation of Atg18 and Vac17, cells (20 OD600 units) were grown at 26°C to midlogarithmic phase and spheroplasted (Darsow et al., 1997). Spheroplasts were resuspended in 1 ml ice-cold lysis buffer (200 mM sorbitol, 50 mM potassium acetate, 20 mM HEPES, pH 7.2, 2 mM EDTA) containing protease inhibitors, and lysed by douncing (10×). Tween-20 (Sigma, St. Louis, MO) was added to the 500 × g supernatants to a final concentration of 0.5%. After a 10-min incubation, detergent-insoluble material was pelleted with a 10-min. 13,000 × g spin. After removing and TCA-precipitating 5% of the supernatant to determine total protein content, anti-FLAG antibody (Sigma) and 20 μl Gammabind G-Sepharose beads (Amersham Biosciences, Piscataway, NJ) were added to the remainder and incubated at 4°C for 90 min. Protein complexes bound to the beads were recovered by washing twice with 1 ml ice-cold lysis buffer containing 0.5% Tween-20 and twice with detergent-free lysis buffer, followed by elution in boiling buffer (50 mM Tris, pH 6.8, 2% SDS, 5% β-mercaptoethanol, 10% glycerol, 0.005% bromophenol blue) at 100°C for 10 min. SDS-PAGE and Western blot analysis were used to detect Atg18-FLAG and GFP-Vac17.
In Vivo Analysis of Phosphoinositides
Phosphoinositide levels were analyzed as previously described by Rudge et al. (2004). Briefly, 5 OD600 units of cells (per strain) were labeled with 60 μCi of myo-[2-3H]inositol (Amersham Biosciences) in SD media lacking inositol for 1 h. After precipitation in 4.5% perchloric acid (final concentration) for 5 min, phospholipids were deacylated by incubation in methylamine reagent (10.7% methylamine, 45.7% methanol, 11.4% 1-butanol) for 50 min at 53°C. Excess methylamine was removed by drying in a vacuum chamber, followed by two washes (resuspension by sonication and subsequent drying) of the pellet in 300 μl sterile water. After a third resuspension in water, an equal volume of extraction reagent (1-butanol/ethyl-ether/formic acid ethyl ester at a ratio of 20:4:1) was added, and [3H]gylcero-phosphoinositides were extracted into the aqueous phase by vortexing for 5 min and centrifugation at 14,000 rpm for 2 min. The extraction was repeated twice more and the final aqueous phase was collected and dried as above.
For quantitative analyses, dried pellets were resuspended in sterile water and 1 × 107 cpm quantities of sample were separated on a Partisphere SAX column (Whatman, Florham Park, NJ) attached to a Shimadzu HPLC system (Shimadzu Manufacturing, Kyoto, Japan) and a 610TR on-line radiomatic detector (Perkin Elmer, Waltham, MA) using Ultima Flo scintillation fluid (Perkin Elmer). The HPLC and on-line detector were controlled with EZStart 7.2.1 and ProFSA 3.3 software, respectively, with final data analysis taking place in the latter.
RESULTS
Atg18p Colocalizes with the PtdIns(3,5)P2 Core Synthesis Machinery on Vacuolar Foci
Using a fab1Δ strain, Dove et al. (2004) have previously shown that the localization of Atg18-GFP to the vacuole limiting membrane depends on the presence of the Fab1 kinase. However, this result does not establish whether the observed failure to localize is solely due to a lack of PtdIns(3,5)P2. The Fab1 protein could be playing at least as important a role in recruiting Atg18p to the vacuole membrane as its phospholipid product, especially because these proteins interact in two-hybrid experiments (Georgakopoulos et al., 2001). To differentiate between the two possibilities, we examined Atg18-GFP localization in strains deleted for VAC7 or VAC14, which encode the two upstream activators of the Fab1 kinase. In both vac7Δ and vac14Δ strains, Fab1p properly localizes to the vacuole membrane (Bonangelino et al., 2002; Dove et al., 2002). We found that Atg18-GFP is entirely cytosolic in both cases, indicating that PtdIns(3,5)P2 is necessary for recruitment even in the presence of a fully functional (but inactive) Fab1 kinase on the vacuole membrane (Figure 1A and Table 2).
In wild-type cells, Atg18-GFP does not appear uniformly distributed along the vacuolar membrane; prominent punctate structures are frequently seen as well (Guan et al., 2001). It has been speculated that these puncta represent autophagic membrane compartments and an association with PtdIns3P may be necessary for recruitment to them (Guan et al., 2001; Stromhaug et al., 2004); however, it is important to note that punctate enrichment on the vacuole membrane is also characteristic of most components of the PtdIns(3,5)P2 synthesis machinery (i.e., Fab1p, Vac14p, and Fig4p). Importantly, there is a significant degree of overlap between them (our unpublished observations), and we have found that Atg18-mRFP puncta frequently coincide with foci of Fab1-GFP or Vac14-GFP (Figure 1B).
Although Atg18-GFP puncta are moderately motile, their movement is almost always confined to the vacuole membrane. In the rare instances that we do observe cytosolic foci of Atg18, it is in the context of what appears to be retrograde vesicular membrane transport from the vacuole. Time-lapse microscopy clearly shows an FM4-64–positive vesicle budding from a site of Atg18-GFP enrichment, followed by movement of this vesicle into the daughter cell and subsequent fusion with endocytic and/or vacuolar compartment(s) (Figure 1C and Supplementary Movie 1 [figure 1C.mov]).
Hyperactivation of the Fab1 Kinase in atg18Δ Requires Both Activators of the Kinase as Well as the Fig 4 Phosphatase
Figure 4.
GFP-Atg18-ALP can alleviate atg18Δ phenotypes even if the putative PtdIns(3,5)P2 binding site is mutated. (A) GFP-Atg18 and GFP-Atg18-ALP fusions harboring either wild-type or an 285RR286-to-285GG286 point mutant of Atg18 were transformed into atg18Δ cells and visualized by fluorescence microscopy. Bars, 4 μm. (B) PtdIns(3,5)P2 levels in the same set of transformants were measured as described in the legend to Figure 2.
Unlike the deletion of genes encoding the core machinery for PtdIns(3,5)P2 synthesis and turnover, the atg18Δ strain has dramatically elevated levels of this phosphoinositide (Dove et al., 2004). This observation, coupled with the finding that Atg18p is recruited to its site of function by PtdIns(3,5)P2, suggests that this protein is part of a negative feedback pathway regulating synthesis of this phospholipid. More specifically, we hypothesized that Atg18p most likely inhibits Fab1 kinase function rather than upregulating Fig4 phosphatase activity, because phosphoinositide levels and vacuole morphology are unaltered in a fig4Δ strain (Gary et al., 2002; Efe et al., 2005). Although a two-hybrid interaction between FAB1 and ATG18 suggests Atg18p might directly inhibit Fab1 kinase function (Georgakopoulos et al., 2001), indirect regulation via one or both of the kinase's upstream regulators, Vac7p and Vac14p, is also possible. To address this issue, we examined PtdIns(3,5)P2 levels in atg18Δvac7Δ and atg18Δvac14Δ strains. In both cases, deletion of the upstream activator was epistatic to atg18Δ (Figure 2A). The precipitous drop in PtdIns(3,5)P2 levels associated with the individual deletion of VAC7 or VAC14 is not even partially suppressed by additionally deleting ATG18 (which, on its own, elevates lipid levels about eightfold) (Dove et al., 2004). The atg18Δvac14Δ strain also displays a very severe synthetic phenotype: compared with either single deletion, the double mutant grows approximately three times more slowly and is temperature-sensitive (practically no growth is observed at 30°C; data not shown). Based on these results, Atg18p most likely inhibits Fab1p function indirectly via one or both of its upstream regulators. However, a direct interaction with Fab1p cannot be ruled out at this time.
Figure 2.
Deletion of FIG 4, VAC7, or VAC14 is epistatic to that of ATG18. PtdIns(3)P and PtdIns(3,5)P2 levels in (A) vac7Δ/atg18Δ and vac14Δ/atg18Δ or (B) atg18Δ/fig4Δ strains were analyzed and compared with those of the single mutant parents and a wild-type strain. 3H-labeled phosphoinositides were isolated and measured by HPLC as described in Materials and Methods.
Paradoxically, atg18Δ cells also lacking the Fig4 phosphatase exhibit a very similar phenotype: in an atg18Δfig4Δ strain PtdIns(3,5)P2 levels drop to 10% of wild type, or just over 1% of atg18Δ (Figure 2B). However, this result is in agreement with a recent study showing that activation of the Fab1 kinase by Vac14p is largely dependent on the presence of Fig4p, either as a coactivator of the kinase itself or a scaffold for a putative kinase complex (Duex et al., 2006a).
Tethering Atg18p to the Vacuole Membrane Restores Normal Vacuole Morphology in the vac14Δ Mutant
Although wild-type S. cerevisiae vacuoles are small and multilobed, the vacuoles of cells lacking Atg18p are significantly enlarged and almost exclusively single-lobed (Dove et al., 2004). Thus, in addition to the Fab1 kinase hyperactivation previously described, atg18Δ cells show a defect in vacuole size control similar to that of a fab1Δ strain. Numerous studies have established a firm and clear link between high levels of PtdIns(3,5)P2 and excessive vacuole fission, especially in the context of hyperosmotic shock (Gary et al., 1998, 2002; Bonangelino et al., 2002). To date, atg18Δ is the only mutant strain in which very high levels of PI(3,5)P2 and enlarged vacuoles can coexist. In light of this observation, we have proposed that Atg18p might be the PtdIns(3,5)P2 effector involved in vacuole fission (Efe et al., 2005). To test this hypothesis, we asked whether tethering Atg18p to the vacuole limiting membrane is sufficient to confer normal morphology to vacuoles lacking PtdIns(3,5)P2 on their surface. Membrane anchoring was achieved by fusing GFP-Atg18p C-terminally to alkaline phosphatase (ALP), a transmembrane protein of the vacuole (Klionsky and Emr, 1989). The requirement for the phospholipid in the proper localization of Atg18p is thus bypassed, and the stable vacuolar association of Atg18 is sufficient to shrink and fission not only atg18Δ vacuoles, but also the grossly enlarged vacuoles of a vac14Δ strain. Conversely, overexpression of GFP-Atg18 without a membrane anchor fails to restore wild-type vacuole morphology in the same strain (Figure 3A). Interestingly, the Atg18-ALP fusion does not rescue the vacuoles of vac7Δ or fab1Δ cells (data not shown). It is important to note that, unlike these strains, vac14Δ cells do synthesize a small amount of PtdIns(3,5)P2—∼10% of wild-type, and this residual phospholipid is most likely what allows for effective function of Atg18p on the membrane, either via allosteric regulation or recruitment of an additional PtdIns(3,5)P2 effector required for vacuole size regulation.
Figure 3.
A GFP-Atg18-ALP fusion restores wild-type vacuole morphology in a vac14Δ strain. (A) Fluorescence microscopy comparison of GFP-Atg18 and GFP-Atg18-ALP fusion protein localization and the resulting vacuole morphology in a vac14Δ/atg18Δ strain. Bars, 4 μm. (B) GFP-Atg18-ALP is defective in the cytoplasm-to-vacuole and macroautophagy pathways. APe1 was immunoprecipitated from whole cell lysates of cells metabolically labeled with [35S]methionine (chase time is 2 h for all samples) in the presence or absence of rapamycin. Proteolytic maturation of APe1 was analyzed by SDS-PAGE.
The Atg18-ALP fusion construct also allows one to assess whether restricting Atg18p activity to the vacuole limiting membrane has any adverse effects on autophagy. To gauge the efficiency of both the Cvt and macroautophagy pathways, we examined the proteolytic maturation of aminopeptidase I (APeI) that takes place upon its delivery to the vacuole in Cvt vesicles or autophagosomes (Nair and Klionsky, 2005). In the atg18Δ strain, metabolic labeling followed by a 120-min chase shows no maturation of APeI, both under vegetative (Cvt) and starvation (macroautophagy) conditions, where the latter is induced by treatment with rapamycin (Figure 3B). Interestingly, although adding back GFP-Atg18 rescues both defects, the GFP-Atg18-ALP fusion is incapable of Cvt transport: 50% of APe1 remains in the unprocessed form following a 2-h chase. Thus, Atg18p must be able to detach from the vacuole membrane in order to effectively mediate its autophagic function(s), a requirement that we do not observe in the context of PtdIns(3,5)P2 homeostasis and vacuole size regulation.
The Putative Phosphoinositide-binding Site in Atg18p Is Not Critical for Its Regulation of the Fab1 Kinase and Vacuole Morphology
Because PtdIns(3,5)P2 might be required for Atg18 activity at the vacuole membrane, we sought to determine if the putative phosphoinositide-binding region identified by Dove et al. (2004) as critical for recruitment of Atg18 also plays a role the activation of Atg18p. We first made a 284FRRG287-to-284FGGG287 mutation in GFP-Atg18 and showed that this construct, even when overexpressed, is not recruited to the vacuole membrane in an atg18Δ strain and cannot properly regulate vacuole size (Figure 4A). However, when we made the same mutation in the context of our GFP-Atg18-ALP chimera—thereby bypassing the recruitment step and highlighting any adverse effect(s) on activity—we observed a complete rescue of vacuole morphology. Moreover, the ability to attenuate Fab1 kinase activity also remained intact in the chimeric point mutant (Figure 4B). These results indicate that the 284FRRG287 basic patch of Atg18, although absolutely necessary for proper localization, is not required for its vacuole size regulation function or for suppression of Fab1 kinase activity.
Membrane Recruitment of a GFP-2xAtg18 Fusion Requires Vac7p
As alluded to above, it is unclear whether Atg18p directly interacts with the Fab1 kinase or its upstream regulators; a strict requirement for PtdIns(3,5)P2 in proper localization of Atg18p makes it impossible to determine the role of protein–protein interactions, if any, in the process. To test for the potential contribution of protein–protein interactions, we expressed a chimera consisting of GFP fused N-terminally to two tandem copies of Atg18 (GFP-2xAtg18) in the fab1Δ, vac7Δ, and vac14Δ strains. We hypothesized that the increased avidity an Atg18 dimer would have for putative protein interactor(s) might allow recruitment to the vacuole-limiting membrane even in the absence of PtdIns(3,5)P2. Indeed, we observed that GFP-2xAtg18 localizes predominantly to the vacuole membrane in the fab1Δ and vac14Δ strains, indicating that 1) PtdIns(3,5)P2 is not required for localization of the chimera and 2) any potential interaction with Fab1p or Vac14p is also not required for recruitment (Figure 5A and Table 3). In agreement with our previous results indicating a requirement for PtdIns(3,5)P2 in activation of Atg18p, GFP-2xAtg18 was only able to rescue the vacuole size defect in vac14Δ cells. Strikingly, the chimera was entirely cytosolic in the vac7Δ strain, indicating that Vac7p likely constitutes a protein component of Atg18p's interaction with the vacuole membrane.
Atg18p may negatively regulate Fab1p activity by sequestering or masking Vac7p. We could not directly test if Atg18 interacts with Vac7 biochemically because Vac7p proved unstable under the conditions we used. Nevertheless, consistent with this hypothesis, cells that overexpressed Vac7 exhibited an approximately threefold increase in PtdIns(3,5)P2 lipid levels. Notably, this exacerbation in PtdIns(3,5)P2 lipid levels was suppressed by co-overexpression of the tandem 2xAtg18 protein (data not shown).
Atg18p Interacts with Vac17p
To visualize various proteins interacting with Atg18, we metabolically labeled cells with 35S and carried out cross-linking experiments. We found only one protein clearly coimmunoprecipitating with Atg18-HA under these conditions, and it migrated to ∼50 kDa on a PAGE gel (Figure 6A). Of the previously identified interactors of Atg18p (putative or biochemically demonstrated), three proteins fit this electrophoretic profile: Bio3p, Rtg3p, and Vac17p. In a two-hybrid screen, Georgakopoulos et al. (2001) found that VAC17 was one of the most frequently recovered genes and the highest-ranking gene encoding a protein localizing to the vacuole membrane (Ishikawa et al., 2003).
Figure 6.
Atg18p interacts with Vac17p in vivo. (A) Osmotic lysates from cells metabolically labeled with [35S]methionine for 1 h were cross-linked with DSP for 30 min and Atg18-HA was immunoprecipitated as described in Materials and Methods. After cleavage of the cross-linker, associated proteins were visualized by SDS-PAGE and subsequent autoradiography. (B) Twenty OD600 units of the indicated strains were spheroplasted and osmotically lysed. Atg18-FLAG was immunoprecipitated from detergent-treated (0.2% Tween-20) whole cell lysates, and association with GFP-Vac17 was determined by SDS-PAGE.
We were able to replicate this result using a synthetic two-hybrid library engineered by PCR amplification of all Saccharomyces cerevisiae ORFs. More than 100,000 colonies were screened using high stringency, ultimately resulting in the isolation of 10 clones, all of which turned out to harbor VAC17 plasmids (data not shown). More importantly, we also found that Vac17p and Atg18p interact in vivo by coimmunoprecipitation analysis (Figure 6B).
Vac17p is the vacuole-specific myosin V adapter in yeast that regulates the process of vacuole inheritance, during which membrane from the mother cell vacuole is transported into the nascent bud (Weisman, 2003). To probe potential role(s) of the novel Atg18p-Vac17p complex, we examined vacuole morphology and GFP-Atg18 recruitment in the vac17Δ strain. No defects were observed in either case (data not shown). Moreover, at the population level, the atg18Δ strain showed no gross vacuole inheritance defects (Table 4). Interestingly, we did not observe any Atg18-enriched vesicles budding from vacuole membranes in the vac17Δ strain (data not shown), suggesting a possible role in vesicular traffic from the vacuole.
Table 4.
Quantitative analysis of vacuole inheritance in vac17Δ and atg18Δ mutants
| Strain | Buds with vacuoles | Buds with no vacuoles | Unbroken vacuoles across mother-bud | Total n |
|---|---|---|---|---|
| 6210 | 33 | 4 | 8 | 45 |
| vac17Δ | 6 | 39 | 0 | 45 |
| atg18Δ | 34 | 1 | 13 | 48 |
| vac17Δ atg18Δ | 4 | 41 | 0 | 45 |
Vacuoles were labeled with CMAC as described in Materials and Methods. Budding cells were then scored for the presence of CMAC-positive vacuoles. We differentiated between vacuoles in the buds that appeared unconnected to the vacuole in the mother cell (first data column) and vacuoles that seemed to stretch across the mother and bud cells (unbroken, third data column).
DISCUSSION
Atg18p was first identified as an essential component of the autophagy, Cvt, and pexophagy pathways in S. cerevisiae (Guan et al., 2001). Its vacuolar rim localization is unique among proteins in these pathways, and it was subsequently found to bind PtdIns3P and PtdIns(3,5)P2, which plays a key role in its membrane association (Dove et al., 2004; Stromhaug et al., 2004). Moreover, deletion of ATG18 results in a ninefold increase in PtdIns(3,5)P2 levels. Here we show that Atg18p colocalizes with components of the PtdIns(3,5)P2 synthesis machinery on the vacuole and that vacuole recruitment of a tandem 2xAtg18 fusion requires Vac7p, suggesting a possible mechanism of Fab1 kinase inhibition. Further, Atg18p is necessary and sufficient for proper vacuole morphology in wild-type and vac14Δ strains. By tethering wild-type Atg18 or a mutant defective in PtdIns(3,5)P2 binding to the vacuole membrane, we show that PtdIns(3,5)P2 binding is not required for vacuole fission or restoration of wild-type levels of this phosphoinositide in these strains. Finally, we show that Atg18p coimmunoprecipitates with the vacuole inheritance protein Vac17p and might be involved in a vesicular transport pathway originating at the vacuole membrane.
Regulation of PtdIns(3,5)P2 Levels by Atg18p
Atg18-mRFP partially colocalizes with Fab1-GFP and Vac14-GFP on areas of punctate enrichment along the vacuole membrane. As has been previously suggested (Rudge et al., 2004), these puncta may represent a large number of protein complexes capable of rapidly phosphorylating PtdIns3P to PtdIns(3,5)P2. The cell could efficiently regulate this process by recruiting Atg18p to a subset of these sites as a direct or indirect inhibitor of the Fab1 kinase.
Although it has been reported that Atg18p and Fab1p interact by yeast two-hybrid analysis (Georgakopoulos et al., 2001), it is noteworthy that this interaction was weak compared with others listed. Instead, our data suggest that Atg18p regulates Fab1 activity indirectly. Epistasis tests show that the dramatic rise in PtdIns(3,5)P2 levels observed upon deletion of ATG18 requires VAC7, VAC14, and Fig4. The latter result confirms a previously reported role for the Fig4 phosphatase in Fab1 kinase activation (Duex et al., 2006a), especially under circumstances that require dramatic and/or sustained increases in phosphoinositide levels. Furthermore, the very low level of PtdIns(3,5)P2 in the atg18Δvac14Δ strain is especially striking, as vac14Δ cells have detectable but low (10% of wild-type) steady-state PtdIns(3,5)P2 and can still partially respond to hyperosmotic shock by elevating levels of this phosphoinositide (Bonangelino et al., 2002; Duex et al., 2006b).
Importantly, Vac7p is the only known protein in the PtdIns(3,5)P2 synthesis pathway that is required for recruitment of GFP-2xAtg18 to the vacuole membrane. GFP-2xAtg18 is associated with the vacuole even in the complete absence of PtdIns(3,5)P2 (i.e., in a fab1Δ strain), and we can rule out recruitment by PtdIns3P alone because the vac7Δ strain actually produces slightly higher than normal levels of this phosphoinositide. Therefore, the data support a model in which PtdIns(3,5)P2 and the transmembrane protein Vac7 function together on the vacuole to recruit Atg18 specifically to this organelle. A similar synergistic requirement is observed for the recruitment of EEA1 to endosomes; proper localization requires both PI(3)P and the small GTPase Rab5 (Simonsen et al., 1998). Conceivably, Atg18p might inhibit PtdIns(3,5)P2 synthesis by sequestering Vac7p away from Fab1p or by recruiting an unknown inhibitor of Vac7p.
Although we have found that overexpression of Vac7p does not lead to loss of Atg18p function in the Cvt pathway, it led to a threefold increase in PtdIns(3,5)P2 lipid levels. Notably, co-overexpression of the tandem 2xAtg18 and Vac7p returned lipid levels to near wild-type levels (our unpublished results). These results indicate that Atg18 negatively regulates Fab1p activity by sequestering or masking Vac7p from Fab1p. Nevertheless, in vivo coimmunoprecipitation experiments (both native and cross-linking) have thus far only shown a very weak interaction between a myc-2xAtg18 construct and HA-tagged Vac7 (data not shown). However, Vac7 is highly unstable under these conditions, and it is quite possible that rapid degradation is masking a stronger physical association between the two proteins.
Mechanistically, a plausible hypothesis is that the putative phosphoinositide binding pocket of Atg18p, 284FRRG287, is involved in calibrating lipid levels by an allosteric mechanism. However, making the 284FGGG287 mutation in the Atg18-ALP fusion construct did not ablate its ability to suppress hyperactivation of the Fab1 kinase in the atg18Δ background. Our data support a model in which the binding pocket 284FRRG287 is implicated in Atg18p-recruitment to the vacuole. However, this does not rule out allosteric regulation by PtdIns(3,5)P2 at the vacuole membrane; there might be still other site(s) of phospholipid interaction, albeit these sites are not sufficient for vacuolar localization in a 284FGGG287 Atg18 mutant.
Atg18p Control of Vacuolar Morphology
We and others have consistently observed an inverse relationship between vacuole size and steady-state PtdIns(3,5)P2 levels in yeast: low or nonexistent PtdIns(3,5)P2 gives rise to enlarged and single-lobed vacuoles, whereas excessive production of this phospholipid results in many small vacuoles. The atg18Δ strain is the only exception to this rule, and we have found that vacuoles in this strain remain enlarged even when expressing fab1-5, a hyperactive allele of the Fab1 kinase (lipid levels are at least 20 times higher than wild-type in this case; our unpublished results). This finding corroborates the report by Dove et al. (2004) that hypertonic shock of atg18Δ cells does not alter vacuole morphology either, even though PtdIns(3,5)P2 levels rise to an unprecendented 60 times that of wild-type. These results indicate that atg18Δ cells are not able to properly respond to the synthesis of the phospholipid and implicate Atg18p as a scission-specific PtdIns(3,5)P2 effector—in addition to its role in a negative feedback loop constraining Fab1 kinase activity.
In agreement with the above model, we found that Atg18p is not only required for the process of vacuole fission, but is also sufficient: a membrane-anchored Atg18-ALP fusion bypasses the requirement for PtdIns(3,5)P2 in recruitment to the vacuole and ameliorates the large vacuole phenotype of atg18Δ and vac14Δ strains. Interestingly, this construct fails to rescue the large vacuole defect in a vac7Δ or fab1Δ strain. Possible explanations for this observation include the following: 1) Atg18p-mediated fission might require a small amount of PtdIns(3,5)P2 for allosteric activation and the residual amount in vac14Δ is sufficient [vac7Δ and fab1Δ have no detectable PtdIns(3,5)P2]; 2) another protein functioning at the same level or downstream of Atg18p is not properly localized in vac7Δ and fab1Δ strains; or 3) deleting VAC7 or FAB1 has a more pleiotropic effect on vacuole homeostasis, rendering fission physiologically impossible. Indeed, Vac7p and Fab1p have very poorly acidified and larger vacuoles that appear more rigid. These proteins may eventually be found to have roles above and beyond PtdIns(3,5)P2 synthesis and regulation.
The ability to localize Atg18p to the vacuole membrane in the absence of PtdIns(3,5)P2 by fusing it to ALP also enabled us to ascertain that the putative phosphoinositide binding pocket 284FRRG287 is not relevant for Atg18-mediated regulation of vacuole morphology. Making the 284FGGG287 mutation in the Atg18-ALP fusion construct did not ablate its ability to restore wild-type morphology to atg18Δ and vac14Δ vacuoles. These results clearly show that the putative phosphoinositide-binding pocket is not required for lipid or vacuole morphology regulation by Atg18p after its recruitment to the membrane.
Autophagy and Vacuole Size Regulation Functions of Atg18p Are Distinct
Atg18 has been shown to bind both PtdIns3P and PtdIns(3,5)P2 (Dove et al., 2004; Stromhaug et al., 2004). However, because only one phosphoinositide binding site has been found in Atg18 to date—and mutation of this site ablates binding to both lipids, it was unclear whether the ability to bind different phospholipids is simply promiscuity resulting from structural similarity or whether it actually signifies a dual role on two different membrane compartments. Our results confirm the latter hypothesis, insofar as Atg18p is fully competent for vacuole size control when tethered to the vacuole membrane, but either partially (macroautophagy) or completely (Cvt) loses its autophagic functionality under these circumstances. An 284FTTG287 point mutation that renders Atg18p completely cytosolic results in essentially the same deficiencies in these pathways (Krick et al., 2006). Thus, it would appear that Atg18p must be able to cycle on and off one or more target membrane compartment(s) to be fully functional, at least in the context of macroautophagy. We have shown that no such requirement exists for vacuole size control and PtdIns(3,5)P2 by Atg18p, providing definitive evidence for two distinct areas of function.
However, there is also a significant parallel between the otherwise distinct roles of Atg18p. Our model suggests Atg18p may mediate vesicular budding and transport from the vacuole membrane, and previous studies have found that Atg18p has a similar role in autophagy: it is required for the recycling of the transmembrane protein Atg9p from the pre-autophagosomal structure (PAS; Reggiori et al., 2004, 2005). It is tempting to postulate that Atg18p, once recruited to the PAS and the vacuole by PtdIns3P and PtdIns(3,5)P2, respectively, is essentially carrying out the same function on different membranes: recycling of protein(s) and membrane.
Atg18p May Mediate Retrograde and/or Vacuole Inheritance–related Vesicular Traffic
Atg18p-containing foci are not entirely confined to the vacuole membrane. Very rarely, a membrane patch enriched in Atg18-GFP can be observed budding from the vacuole membrane and traveling into a nascent bud. The relatively slow speed of these vesicles that allows them to be clearly visualized, as well as the non-Brownian nature of the movement suggest they might be moving along cytoskeletal track(s). Our results showing that Atg18p associates with Vac17p in vivo provide a mechanistic model for this type of vesicular membrane traffic. Vac17p is the vacuole inheritance-specific adapter for the type-V myosin Myo2p, which enables the transport of vacuoles into buds on actin cables in a cell cycle–coordinated manner (Weisman, 2003). Although we have observed a lack of this type of membrane traffic in a vac17Δ strain, this result is inconclusive at best because it is too rare to reliably quantify. Thus, it remains at present unclear whether Vac17p is required for this process.
We have found that Vac17p plays no role in PtdIns(3,5)P2 synthesis or regulation (our unpublished results). However, it has been long known that proper vacuole inheritance requires PtdIns(3,5)P2, and others have postulated that Atg18p might be required for membrane fission during the formation and/or termination of the segregation structure (Weisman, 2003). Yet, we failed to observe a defect in vacuolar inheritance in atg18Δ cells. Alternatively, Vac17p may, as a separate but related function, facilitate the budding and transport of vesicles enriched in Atg18p from the vacuole membrane for recycling purposes; further testing of this hypothesis awaits the discovery of a reliable endogenous cargo protein as a marker to probe the integrity of a vacuolar retrograde transport pathway.
We propose (Figure 7) that that Atg18p regulates vacuole morphology by modulating the activity of the Fab1 lipid kinase, possibly via sequestration of Vac7p to alter PtdIns(3,5)P2 levels on the vacuole membrane, and by acting as an effector of the phospholipid to induce vacuole fission by an as of yet uncharacterized mechanism. Finally, Atg18p is involved in a form of vesicular transport originating at the vacuole membrane, and this function most likely requires cytoskeletal associations mediated by Vac17p and Myo2p (Figure 7). In coordinately performing these various functions, Atg18 is essentially acting as a “sensor” of PtdIns(3,5)P2, remodeling membranes and concurrently regulating phosphoinositide synthesis as it dynamically cycles on and off the vacuole membrane.
Figure 7.
(A) PtdIns(3)P and PtdIns(3,5)P2 most likely recruit Atg18 to two distinct membrane compartments, where it is required for autophagy (PAS) and regulation of organelle morphology (vacuole), respectively. On the vacuole, we envision Atg18 acting as a “sensor” of PtdIns(3,5)P2, continually cycling between the cytosol (Atg18CYTO) and the limiting membrane (Atg18VAC) in response to changes in phosphoinositide levels. One function of Atg18 on the membrane is to inhibit the Fab1 kinase, thus establishing a negative feedback loop. This circuit forms the integral part of an elegant system that allows dynamic control of vacuole morphology. (B) Model for Atg18 function as it cycles on and off the vacuole membrane. During and after recruitment by the phospholipid PtdIns(3,5)P2, Atg18 also associates with Vac7 and Vac17. Atg18 may indirectly regulate activity of the Fab1 kinase by sequestering Vac7. Vac17 most likely functions as a myosin-specific adapter for Atg18, thus enabling retrograde membrane transport from the vacuole along actin tracks. Membrane deformation could be directly or indirectly mediated by Atg18, e.g., via recruitment of an as-of-yet unidentified fission factor.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Simon Rudge for his contributions in the early stages of the project as well as helpful discussions throughout. This work was supported in part by the Jean-François St.-Denis Fellowship from the Canadian Institutes of Health Research to R.J.B. S.D.E is an investigator of the Howard Hughes Medical Institute.
Abbreviations used:
- PtdIns
phosphatidylinositol
- ALP
alkaline phosphatase
- Cvt
cytoplasm-to-vacuole transport.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-04-0301) on August 15, 2007.

 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Balla T. Inositol-lipid binding motifs: signal integrators through protein-lipid and protein-protein interactions. J. Cell Sci. 2005;118:2093–2104. doi: 10.1242/jcs.02387. [DOI] [PubMed] [Google Scholar]
- Barth H., Meiling-Wesse K., Epple U. D., Thumm M. Autophagy and the cytoplasm to vacuole targeting pathway both require Aut10p. FEBS Lett. 2001;508:23–28. doi: 10.1016/s0014-5793(01)03016-2. [DOI] [PubMed] [Google Scholar]
- Behnia R., Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. doi: 10.1038/nature04397. [DOI] [PubMed] [Google Scholar]
- Bonangelino C. J., Catlett N. L., Weisman L. S. Vac7p, a novel vacuolar protein, is required for normal vacuole inheritance and morphology. Mol. Cell. Biol. 1997;17:6847–6858. doi: 10.1128/mcb.17.12.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonangelino C. J., Nau J. J., Duex J. E., Brinkman M., Wurmser A. E., Gary J. D., Emr S. D., Weisman L. S. Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. J. Cell Biol. 2002;156:1015–1028. doi: 10.1083/jcb.200201002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant N. J., Stevens T. H. Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole. Microbiol. Mol. Biol. Rev. 1998;62:230–247. doi: 10.1128/mmbr.62.1.230-247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C. G., Emr S. D. Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol. Cell. 1998;2:157–162. doi: 10.1016/s1097-2765(00)80125-2. [DOI] [PubMed] [Google Scholar]
- Clague M. J., Jones A. T., Mills I. G., Walker D. M., Urbe S. Regulation of early-endosome dynamics by phosphatidylinositol 3-phosphate binding proteins. Biochem. Soc. Trans. 1999;27:662–666. doi: 10.1042/bst0270662. [DOI] [PubMed] [Google Scholar]
- Cooke F. T., Dove S. K., McEwen R. K., Painter G., Holmes A. B., Hall M. N., Michell R. H., Parker P. J. The stress-activated phosphatidylinositol 3-phosphate 5-kinase Fab1p is essential for vacuole function in S. cerevisiae. Curr. Biol. 1998;8:1219–1222. doi: 10.1016/s0960-9822(07)00513-1. [DOI] [PubMed] [Google Scholar]
- Darsow T., Rieder S. E., Emr S. D. A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J. Cell Biol. 1997;138:517–529. doi: 10.1083/jcb.138.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G., De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Dove S. K., Cooke F. T., Douglas M. R., Sayers L. G., Parker P. J., Michell R. H. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature. 1997;390:187–192. doi: 10.1038/36613. [DOI] [PubMed] [Google Scholar]
- Dove S. K., McEwen R. K., Mayes A., Hughes D. C., Beggs J. D., Michell R. H. Vac14 controls PtdIns(3,5)P(2) synthesis and Fab1-dependent protein trafficking to the multivesicular body. Curr. Biol. 2002;12:885–893. doi: 10.1016/s0960-9822(02)00891-6. [DOI] [PubMed] [Google Scholar]
- Dove S. K., et al. Svp1p defines a family of phosphatidylinositol 3,5-bisphosphate effectors. EMBO J. 2004;23:1922–1933. doi: 10.1038/sj.emboj.7600203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duex J. E., Nau J. J., Kauffman E. J., Weisman L. S. Phosphoinositide 5-phosphatase Fig 4p is required for both acute rise and subsequent fall in stress-induced phosphatidylinositol 3,5-bisphosphate levels. Eukaryot. Cell. 2006a;5:723–731. doi: 10.1128/EC.5.4.723-731.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duex J. E., Tang F., Weisman L. S. The Vac14p-Fig4p complex acts independently of Vac7p and couples PI3,5P2 synthesis and turnover. J. Cell Biol. 2006b;172:693–704. doi: 10.1083/jcb.200512105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efe J. A., Botelho R. J., Emr S. D. The Fab1 phosphatidylinositol kinase pathway in the regulation of vacuole morphology. Curr. Opin. Cell Biol. 2005;17:402–408. doi: 10.1016/j.ceb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Eugster A., Pecheur E. I., Michel F., Winsor B., Letourneur F., Friant S. Ent5p is required with Ent3p and Vps27p for ubiquitin-dependent protein sorting into the multivesicular body. Mol. Biol. Cell. 2004;15:3031–3041. doi: 10.1091/mbc.E03-11-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friant S., Pecheur E. I., Eugster A., Michel F., Lefkir Y., Nourrisson D., Letourneur F. Ent3p Is a PtdIns(3,5)P2 effector required for protein sorting to the multivesicular body. Dev. Cell. 2003;5:499–511. doi: 10.1016/s1534-5807(03)00238-7. [DOI] [PubMed] [Google Scholar]
- Gary J. D., Sato T. K., Stefan C. J., Bonangelino C. J., Weisman L. S., Emr S. D. Regulation of Fab1 phosphatidylinositol 3-phosphate 5-kinase pathway by Vac7 protein and Fig4, a polyphosphoinositide phosphatase family member. Mol. Biol. Cell. 2002;13:1238–1251. doi: 10.1091/mbc.01-10-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary J. D., Wurmser A. E., Bonangelino C. J., Weisman L. S., Emr S. D. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J. Cell Biol. 1998;143:65–79. doi: 10.1083/jcb.143.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor E. C., te Heesen S., Graham T. R., Aebi M., Emr S. D. Signal-mediated retrieval of a membrane protein from the Golgi to the ER in yeast. J. Cell Biol. 1994;127:653–665. doi: 10.1083/jcb.127.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakopoulos T., Koutroubas G., Vakonakis I., Tzermia M., Prokova V., Voutsina A., Alexandraki D. Functional analysis of the Saccharomyces cerevisiae YFR021w/YGR223c/YPL100w ORF family suggests relations to mitochondrial/peroxisomal functions and amino acid signalling pathways. Yeast. 2001;18:1155–1171. doi: 10.1002/yea.764. [DOI] [PubMed] [Google Scholar]
- Gillooly D. J., Morrow I. C., Lindsay M., Gould R., Bryant N. J., Gaullier J. M., Parton R. G., Stenmark H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000;19:4577–4588. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J., Stromhaug P. E., George M. D., Habibzadegah-Tari P., Bevan A., Dunn W. A., Jr, Klionsky D. J. Cvt18/Gsa12 is required for cytoplasm-to-vacuole transport, pexophagy, and autophagy in Saccharomyces cerevisiae and Pichia pastoris. Mol. Biol. Cell. 2001;12:3821–3838. doi: 10.1091/mbc.12.12.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman C. S., Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Ishikawa K., Catlett N. L., Novak J. L., Tang F., Nau J. J., Weisman L. S. Identification of an organelle-specific myosin V receptor. J. Cell Biol. 2003;160:887–897. doi: 10.1083/jcb.200210139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries T. R., Dove S. K., Michell R. H., Parker P. J. PtdIns-specific MPR pathway association of a novel WD40 repeat protein, WIPI49. Mol. Biol. Cell. 2004;15:2652–2663. doi: 10.1091/mbc.E03-10-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordens I., Marsman M., Kuijl C., Neefjes J. Rab proteins, connecting transport and vesicle fusion. Traffic. 2005;6:1070–1077. doi: 10.1111/j.1600-0854.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- Kim Y., Chattopadhyay S., Locke S., Pearce D. A. Interaction among Btn1p, Btn2p, and Ist2p reveals potential interplay among the vacuole, amino acid levels, and ion homeostasis in the yeast Saccharomyces cerevisiae. Eukaryot. Cell. 2005;4:281–288. doi: 10.1128/EC.4.2.281-288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Emr S. D. Membrane protein sorting: biosynthesis, transport and processing of yeast vacuolar alkaline phosphatase. EMBO J. 1989;8:2241–2250. doi: 10.1002/j.1460-2075.1989.tb08348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krick R., Tolstrup J., Appelles A., Henke S., Thumm M. The relevance of the phosphatidylinositolphosphat-binding motif FRRGT of Atg18 and Atg21 for the Cvt pathway and autophagy. FEBS Lett. 2006;580:4632–4638. doi: 10.1016/j.febslet.2006.07.041. [DOI] [PubMed] [Google Scholar]
- Lawe D. C., Patki V., Heller-Harrison R., Lambright D., Corvera S. The FYVE domain of early endosome antigen 1 is required for both phosphatidylinositol 3-phosphate and Rab5 binding. Critical role of this dual interaction for endosomal localization. J. Biol. Chem. 2000;275:3699–3705. doi: 10.1074/jbc.275.5.3699. [DOI] [PubMed] [Google Scholar]
- Lemmon M. A. Phosphoinositide recognition domains. Traffic. 2003;4:201–213. doi: 10.1034/j.1600-0854.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Fritsch E. F., Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- Munro S. Organelle identity and the organization of membrane traffic. Nat. Cell Biol. 2004;6:469–472. doi: 10.1038/ncb0604-469. [DOI] [PubMed] [Google Scholar]
- Nair U., Klionsky D. J. Molecular mechanisms and regulation of specific and nonspecific autophagy pathways in yeast. J. Biol. Chem. 2005;280:41785–41788. doi: 10.1074/jbc.R500016200. [DOI] [PubMed] [Google Scholar]
- Reggiori F., Shintani T., Nair U., Klionsky D. J. Atg9 cycles between mitochondria and the pre-autophagosomal structure in yeasts. Autophagy. 2005;1:101–109. doi: 10.4161/auto.1.2.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F., Tucker K. A., Stromhaug P. E., Klionsky D. J. The Atg1-Atg13 complex regulates Atg9 and Atg23 retrieval transport from the pre-autophagosomal structure. Dev. Cell. 2004;6:79–90. doi: 10.1016/s1534-5807(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Rieder S. E., Emr S. D. A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole. Mol. Biol. Cell. 1997;8:2307–2327. doi: 10.1091/mbc.8.11.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Winston F., Hieter P. A Laboratory Course Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1990. Methods in Yeast Genetics. [Google Scholar]
- Rudge S. A., Anderson D. M., Emr S. D. Vacuole size control: regulation of PtdIns(3,5)P2 levels by the vacuole-associated Vac14-Fig 4 complex, a PtdIns(3,5)P2-specific phosphatase. Mol. Biol. Cell. 2004;15:24–36. doi: 10.1091/mbc.E03-05-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbrissa D., Ikonomov O. C., Shisheva A. PIKfyve, a mammalian ortholog of yeast Fab1p lipid kinase, synthesizes 5-phosphoinositides. Effect of insulin. J. Biol. Chem. 1999;274:21589–21597. doi: 10.1074/jbc.274.31.21589. [DOI] [PubMed] [Google Scholar]
- Schu P. V., Takegawa K., Fry M. J., Stack J. H., Waterfield M. D., Emr S. D. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A., Lippe R., Christoforidis S., Gaullier J. M., Brech A., Callaghan J., Toh B. H., Murphy C., Zerial M., Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Stromhaug P. E., Reggiori F., Guan J., Wang C. W., Klionsky D. J. Atg21 is a phosphoinositide binding protein required for efficient lipidation and localization of Atg8 during uptake of aminopeptidase I by selective autophagy. Mol. Biol. Cell. 2004;15:3553–3566. doi: 10.1091/mbc.E04-02-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenawa T., Itoh T. Membrane targeting and remodeling through phosphoinositide-binding domains. IUBMB Life. 2006;58:296–303. doi: 10.1080/15216540600732039. [DOI] [PubMed] [Google Scholar]
- Vida T. A., Emr S. D. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman L. S. Yeast vacuole inheritance and dynamics. Annu. Rev. Genet. 2003;37:435–460. doi: 10.1146/annurev.genet.37.050203.103207. [DOI] [PubMed] [Google Scholar]
- Whitley P., Reaves B. J., Hashimoto M., Riley A. M., Potter B. V., Holman G. D. Identification of mammalian Vps24p as an effector of phophatidylinositol-3,5-bisphosphate-dependent endosome compartmentalization. J. Biol. Chem. 2003;278:38786–38795. doi: 10.1074/jbc.M306864200. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., DeWald D. B., Boronenkov I. V., Anderson R. A., Emr S. D., Koshland D. Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol. Biol. Cell. 1995;6:525–539. doi: 10.1091/mbc.6.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.