Abstract
Nitric oxide (NO) release from endothelial cells, via endothelial NO synthase (eNOS) activation, is central to the proangiogenic actions of vascular endothelial growth factor (VEGF). VEGF signaling to eNOS is principally mediated by an Akt-dependent phosphorylation of eNOS and by increased association of eNOS to the molecular chaperone, heat-shock protein 90 kDa (Hsp90). Herein, we report that VEGFR-2 activation induces tyrosine phosphorylation of VEGF receptor 2 (VEGFR-2)-associated Hsp90β. Tyrosine phosphorylation of Hsp90β in response to VEGF is dependent on internalization of the VEGFR-2 and on Src kinase activation. Furthermore, we demonstrate that c-Src directly phosphorylates Hsp90 on tyrosine 300 residue and that this event is essential for VEGF-stimulated eNOS association to Hsp90 and thus NO release from endothelial cells. Our work identifies Y300 phosphorylation of Hsp90 as a novel regulated posttranslational modification of the chaperone and demonstrates its importance in the proangiogenic actions of VEGF, namely by regulating NO release from endothelial cells.
INTRODUCTION
Vascular endothelial growth factor (VEGF) is a potent proangiogenic cytokine that activates three specific receptor tyrosine kinases (RTK): VEGF receptor (VEGFR)-1, -2, and -3. Gene knockout studies revealed that VEGF and VEGFRs are essential for the development of the vasculature in mouse embryos (Fong et al., 1995; Shalaby et al., 1995; Carmeliet et al., 1996; Dumont et al., 1998). In addition to its functional roles in vasculogenesis, VEGF plays key roles in adult physiological and pathological angiogenesis such as wound healing and tumor vascularization. VEGFR-2 mediates most of the functional and biochemical effects of VEGF in endothelial cells including proliferation, migration, survival, and nitric oxide (NO) release. These effects are produced through the activation of multiple signaling pathways, such as the phosphoinositide-3 kinase (PI-3 kinase)/Akt, p38 mitogen-activated protein kinase (MAPK), phospholipase C (PLC)-γ, and Erk/MAPK, after the activation of VEGFR-2 (Rousseau et al., 1997; Takahashi and Shibuya, 1997; Gerber et al., 1998; Takahashi et al., 2001). VEGF-mediated activation of the Ser/Thr kinase Akt leads to phosphorylation of serine 1179 on bovine endothelial NO synthase (eNOS; Ser1177 in the human form), which increases eNOS catalytic activity and results in release of NO from endothelial cells (Fulton et al., 1999; Dimmeler et al., 1999). In turn, NO released from endothelium contributes to the proangiogenic effects of VEGF. Indeed, VEGF-mediated vascular permeability, angiogenesis, and endothelial cell precursor mobilization are markedly impaired in eNOS-deficient mice (Fukumura et al., 2001; Aicher et al., 2003).
Heat-shock protein 90 kDa (Hsp90) is a multifunctional molecular chaperone, and its role in the prevention of protein aggregation and in the refolding of denatured proteins has been studied in much detail (Whitesell and Lindquist, 2005). Hsp90 is also a regulator of the activity of signaling molecules such as c-Src, Akt, and eNOS and contributes to their activation. Hsp90 is known to associate with eNOS and Akt after VEGF stimulation, which contributes to increase eNOS catalytic activity (Garcia-Cardena et al., 1998; Brouet et al., 2001a; Fontana et al., 2002). In addition to a direct allosteric effect of Hsp90 on eNOS, it is thought that binding of Hsp90 to Akt prevents the phosphatase 2A-mediated dephosphorylation of Thr308 of Akt, which maintains its positive functional effects on eNOS catalytic activity (Sato et al., 2000; Takahashi and Mendelsohn, 2003). Furthermore, Hsp90 has been proposed to facilitate the calcineurin-dependent dephosphorylation of Thr495 of eNOS, which contributes to enzyme activation (Kupatt et al., 2004). Hyperphosphorylation of serine and/or threonine residues on Hsp90 has been shown to negatively regulate Hsp90's role in the conformational maturation of oncogenic signaling proteins, including ErbB2, c-Src, Akt, and Raf-1 (Schulte et al., 1995; Mimnaugh et al., 1995; Xu et al., 2001; Zhao et al., 2001; Basso et al., 2002). However, details in the understanding of the role of these phosphorylation sites in Hsp90 function are still emerging (Ogiso et al., 2004). Furthermore, evidences for a role of regulated posttranslational modifications in intracellular signaling events are lacking.
Internalization of activated RTK from plasma membrane is a critical step of signal transduction either by terminating membrane signaling or by redirecting and sustaining signaling intracellularly. Many studies, including a recent report on VEGFR-2, suggest that receptor signaling is maintained in the endosomal compartment, which may serve as a mechanism for sustaining and amplifying membrane signaling (Urbe et al., 2000; Hayes et al., 2002; Lampugnani et al., 2006). In this study, we investigated the contribution of internalized VEGFR-2 on intracellular signaling pathways leading to NO release. We found that Hsp90β is a novel tyrosine-phosphorylated protein associated to VEGFR-2 and have obtained evidence indicating that the phosphorylation of Hsp90β bound to VEGFR-2 is mediated by c-Src, occurs on Y300, and is regulated by VEGFR-2 internalization. Furthermore, phosphorylation of Hsp90 on Y300 after VEGFR-2 activation regulates the association of Hsp90 with eNOS and thus NO release from endothelial cells in response to VEGF.
MATERIALS AND METHODS
Cell Culture
Bovine aortic endothelial cells (BAECs) and COS-7 cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT), 2.0 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Human umbilical vein endothelial cells (HUVECs; VEC Technologies, Rensselaer, NY) were plated on 0.1% gelatin-coated 60-mm dishes and cultured in M199 supplemented with 20% FBS (Invitrogen, Carlsbad, CA), 50 μg/ml endothelial cell growth supplement (Sigma, St. Louis, MO), 100 μg/ml heparin (Sigma), 2.0 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Recombinant human VEGF obtained from the BRB Preclinical Repository of the National Cancer Institute, Frederick Cancer Research and Development Center was used for cell stimulation throughout this study.
Adenoviruses and Cell Infections
Hemagglutinin (HA)-tagged K44A-dynamin adenoviruses were generously given by Dr. Jeffrey E. Pessin (SUNY Stony Brook). Expansion of the virus was done at the Animal Reproduction and Biotechnology Lab of Colorado State University. For cell infections, 80% confluent dishes of BAECs or HUVECs were used. Cells were infected, at a multiplicity of infection (MOI) of 10 for 8–16 h, with recombinant adenoviruses coding for β-galactosidase or HA-K44A-dynamin. The adenoviruses were removed, and cells were left to recover for 24–48 h in complete medium. These conditions resulted in uniform expression of the transgenes in close to 95% of the cells, as determined by β-galactosidase activity staining.
Receptor Internalization Studies by Fluorescence-activated Cell Sorting Analysis
HUVECs infected with either Ad-β-gal or Ad-K44A-dynamin were trypsinized and incubated with mouse anti-human VEGFR-2 antibody (Sigma) for 30 min on ice. Cells were then incubated with fluorescein isothiocyanate (FITC)-conjugated affinity-purified goat F(ab′)2 anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) on ice for 30 min analyzed using a FACScan (fluorescence-activated cell sorting) Cytometer as described in supplemental methods.
Plasmids and Transfections
cDNA coding for bovine eNOS (in pcDNA3), human VEGFR-2 (in pRK7), β-galactosidase (in pcDNA3), and HA-Hsp90β (in pcDNA3) cDNAs were described previously (Fulton et al., 1999; Fontana et al., 2002). Akt and p110 expression plasmids were given by Dr. William C. Sessa (Yale University, New Haven, CT). HA-tagged wild-type (WT)-dynamin, K44A-dynamin, WT, and K298R-Src expression vectors were obtained from Dr. Stephane A. Laporte (McGill University, Montreal, QC, Canada). The Y300F and Y483F mutant myc-Hsp90β constructs were generated by site-directed mutagenesis (Stratagene, La Jolla, CA) using the following primers: Y300F, 5′-CACCCAAGAGGAGTTTGGAGAATTCTACAAGAGCC-3′; Y483F, 5′-CAGAAGTCCATCTTTTACATCACTGG-3′; and their respective reverse complement. Each construct was sequenced to confirm the presence of the mutation. VEGFR-2-GFP constructs was obtained by inserting in frame the VEGFR-2 cDNA in XhoI and SacII restriction sites of pEGFP-N3 vector. COS-7 and BAECs were transfected using Lipofectamine 2000 according to manufacturer's protocol (Invitrogen). Protein expression was confirmed (60 μg of total cell lysate) by SDS-PAGE and Western blot analysis 48 h after transfections.
NO Release
Cell medium was processed for the measurement of nitrite (NO2−), the stable breakdown product of NO in aqueous solution, by NO-specific chemiluminescence using a NO analyzer (Ionics Instruments, Boulder, CO; Sessa et al., 1995). Transfected COS-7 cells were processed for the measurement of nitrite, 48 h after transfection.
Antibodies, Immunoprecipitations, and Immunoblotting
The following primary antibodies were used: anti-VEGFR-2 mAb from Santa Cruz Biotechnology (Santa Cruz, CA); anti-eNOS, -Hsp90, -HA-Tag (pAbs) from BD Transduction Laboratories (Lexington, KY); anti-HA-Tag (mAb), -myc-Tag, -P-Tyr783-PLC-γ1, -PLC-γ1, -P-p44/42 MAPK (Thr202/Tyr204), -P-Akt (Ser473), -Akt and -P-Ser1179-eNOS from Cell Signaling (Beverly, MA); and anti-phosphotyrosine (4G10) from Upstate Biotechnology (Lake Placid, NY).
For immunoprecipitations, cells were solubilized with a lysis buffer containing 1% NP-40, 20 mM Tris-HCl, 100 mM NaCl, 2.5 mM EDTA, 20 mM sodium fluoride, 1 mM sodium pyrophosphate, 1 mM sodium orthovanadate, and protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Soluble proteins were incubated with the primary antibody (2 μg) at 4°C for 2 h. Protein A-Sepharose (Sigma; 50 μl of a 50% slurry) was then added and incubated for an additional hour. The immune complexes were precipitated by centrifugation, washed three times with lysis buffer, boiled in SDS sample buffer, separated by SDS-PAGE, transferred onto a nitrocellulose membrane (Hybond ECL, Amersham, Piscataway, NJ) and Western blotted. Antibody detection was performed by a chemiluminescence-based detection system (ECL, Amersham) or by an LI-COR Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE) using Alexa 680– and Alexa 800–labeled secondary antibodies (Invitrogen).
Immunofluorescence
BAECs were plated on gelatin-coated coverslips and were transfected as described above. The transiently transfected cells were serum-starved and after VEGF treatments, cells were fixed with 3.5% formaldehyde and permeabilized with 0.2% Triton X-100 in phosphate-buffered saline. VEGFR-2-green fluorescent protein (GFP) was detected using rabbit anti-GFP antibody (Cedarlane Laboratories, Hornby, ON, Canada). Myc and HA epitopes were detected using the mouse monoclonal antibodies mentioned above. The antigen–antibody complexes were detected using the corresponding Alexa 488– or 568–labeled anti-mouse or anti-rabbit secondary antibodies. The cells were examined using a LSM510 confocal microscope (Zeiss, Thornwood, NY).
In Vitro Kinase Assay
Purified human Hsp90 (1 μg; Stressgen Bioreagents, San Diego, CA) was incubated with 5.25 U of recombinant c-Src (Upstate Biotechnology) for 20 min at 30°C in buffer containing 20 mM HEPES, pH 7.4, 10 mM MgCl2, 10 mM MnCl2, 1 mM dithiothreitol, and 0.05 mM sodium orthovanadate with or without 100 μM ATP (Sigma). The kinase reaction was stopped by adding SDS sample buffer. Tyrosine phosphorylation was monitored by Western blotting using anti-pTyr antibody.
Protein Identification by LC/MS/MS
Immunoprecipitated proteins were separated on SDS-PAGE gel and revealed by silver staining. The band of interest was cut out from the gel and digested with trypsin (0.1 μg) for 45 min at 4°C. Peptides were extracted from the gel at room temperature, and the supernatants were transferred into a 96-well plate and then completely dried in a vacuum centrifuge. Before the analysis, peptides were dissolved under agitation for 15 min in 13 μl of trifluoroacetic acid 0.1%, then sonicated for 5 min, and centrifuged at 2000 rpm for 1 min. Analysis of the peptide mixture was done by liquid chromatography–mass spectrometry (LC/MS/MS) using a Q-Tof 2 Mass Spectrometer configured with an on-line CapLC system (Waters, Millipore, Milford, MA). Protein identification was obtained from the MS/MS spectra using Mascot analysis software (Matrix Science, Boston, MA).
Phosphopeptide Enrichment and Identification
After in vitro kinase assay (as described above), samples were subjected to SDS-PAGE, and then proteins were revealed by silver staining. Protein bands were cut out and trypsinized. Peptides were reconstituted with 50 μl of 1% n-octyl glucoside (NOG) buffer in Tris-buffered saline. PY99 agarose-conjugated antibody (Santa Cruz Biotechnology) was added and incubated for 2 h at 4°C in order to enrich in tyrosine-phosphorylated peptides. After centrifugation, the beads were washed once with NOG buffer and three times with water. Peptides were eluted from the beads with 50 μl of 1% formic acid/50% acetonitrile. Eluates were dried down and reconstituted for proteomic analysis using a LTQ Orbitrap equipped with a Surveyor LC system (Thermo Fischer Scientific, Waltham, MA).
RESULTS
Dynamin Mediates VEGF-induced Internalization of VEGFR-2
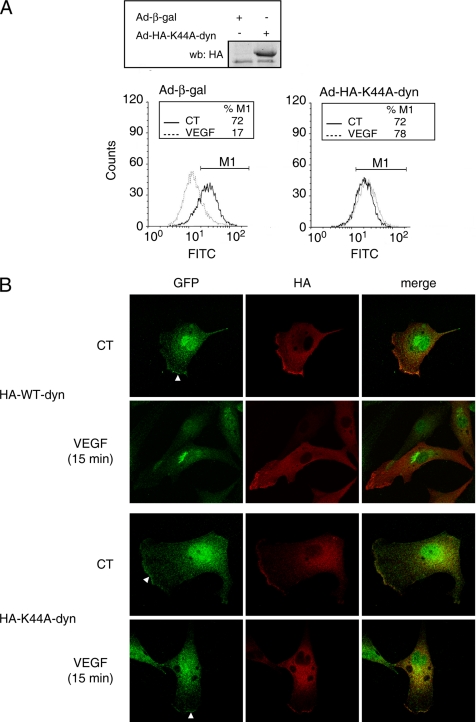
Internalization of activated receptor tyrosine kinases from the plasma membrane is a critical step of signal transduction. To investigate VEGFR-2 endocytosis, HUVECs were infected with adenoviruses coding for β-galactosidase (β-gal) as a control or for HA-tagged K44A-dynamin, a dominant-negative form of the GTPase to inhibit internalization. Immunodetection, using anti-HA antibody, was performed on lysates from HUVECs to confirm K44A-dynamin expression, and FACS analysis was used to detect the presence of VEGFR-2 at the cell surface of endothelial cells before and after VEGF stimulation (Figure 1A). In cells infected with Ad-β-gal, VEGF stimulation resulted in a loss of VEGFR-2 expression at the cell surface indicative of a VEGF-dependent internalization of VEGFR-2 (Figure 1A, left panel). Expression of K44A-dynamin completely inhibited VEGF-induced VEGFR-2 internalization (Figure 1A, right panel). Quantitatively, the percentage of mean surface fluorescence (FITC) was reduced by 40% (p < 0.05) after VEGF treatment of Ad-β-gal–infected cells and K44A-dynamin expression completely inhibited the VEGF-stimulated reduction of VEGFR-2 surface expression (Supplementary Figure 1). Fluorescence emitted from cells incubated with the secondary antibody alone was negligible (results not shown). Additionally, Ad-K44A-dynamin infection also inhibited the uptake of fluorescently labeled transferrin, a known dynamin-dependent process (results not shown). These findings indicate that VEGF induces a dynamin-dependent disappearance from endothelial cellular surface.
Figure 1.
Dynamin-dependent internalization of VEGFR-2. (A) K44A-dynamin inhibits VEGFR-2 surface expression. HUVECs infected with adenoviruses coding for β-galactosidase (Ad-β-gal) or HA-K44A-dynamin (Ad-HA-K44A-dyn) were serum-starved and stimulated with VEGF (40 ng/ml, 30 min). The cell surface expression of VEGFR-2 was analyzed by FACS. Inset, cells were lysed, and HA-K44A-dyn expression was monitored by Western blot (wb) against HA. (B) Inhibition of VEGF-stimulated subcellular relocalization of VEGFR-2 by K44A-dynamin. BAECs were transfected with VEGFR-2-GFP and HA-tagged WT- or K44A-dynamin. Transfected BAECs were stimulated or not with VEGF (40 ng/ml; 30 min). Cell were fixed permeabilized, and immunofluorescence was performed using anti-GFP (green) and anti-HA (red) antibodies. Transfected BAECs, expressing both proteins (merged image), were visualized using a confocal microscope. White arrows point to membrane localized VEGFR-2-GFP. Note that the nuclear anti-GFP staining is nonspecific (not shown).
To document the effect of dynamin inhibition on VEGFR-2 cellular localization, we performed confocal microscopy experiments on BAECs transiently transfected with VEGFR-2-GFP and HA-tagged WT- or K44A-dynamin. In BAECs expressing WT-dynamin, VEGF stimulation induced a relocalization of VEGFR-2-GFP from the cell membrane, easily detectable in membrane ruffles, to a concentrated perinuclear punctate distribution indicative of an internalization of the receptor after stimulation (Figure 1B, top panels). VEGF treatment of BAECs expressing K44A-dynamin did not induce this relocalization of VEGFR-2-GFP. Indeed, VEGFR-2-GFP remained mostly at the plasma membrane, again visible in membrane ruffles, after VEGF stimulation, suggesting that dynamin inhibition prevents internalization of the VEGFR-2 after activation.
K44A-Dynamin Expression Inhibits VEGFR-2 Signaling to Akt and NO Release
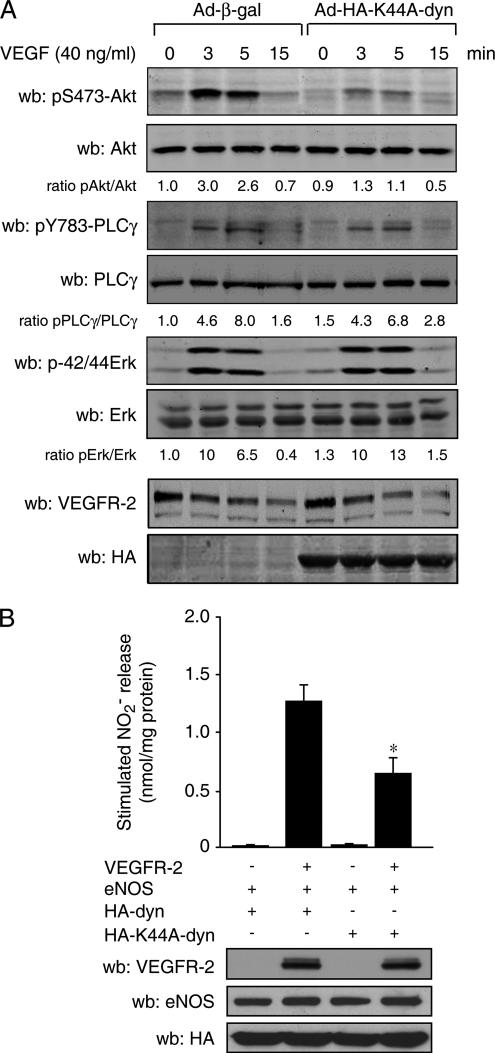
To evaluate the contribution of VEGFR-2 internalization on VEGF-induced activation of intracellular signaling pathways in endothelial cells, we infected BAECs with Ad-K44A-dynamin or Ad-β-gal adenoviruses and monitored the activation of well-established VEGF-sensitive signaling pathways. Ad-K44A-dynamin expression, as monitored by Western blotting against HA (Figure 2A, bottom), resulted in a marked decrease of VEGF-induced activation of Akt, as monitored by Ser 473 phosphorylation levels (Figure 2A, top). Interestingly, activation of PLC-γ and MAPK pathways as well as the expression level of VEGFR-2 were unaltered by this level of expression of K44A-dynamin in BAECs. These data suggest that VEGF-mediated Akt activation depends, at least in part, on internalization of VEGFR-2.
Figure 2.
Dynamin-dependent internalization of VEGFR-2 is required for Akt activation and NO release. (A) K44A-dynamin expression in endothelial cells inhibits Akt activation. Adenovirus-infected BAECs were stimulated with VEGF (40 ng/ml) for the indicated time. Cell lysates were separated by SDS-PAGE and immunoblotted using the indicated antibodies. (B) K44A-dynamin inhibits VEGFR-2–stimulated NO release. COS-7 cells were transfected with VEGFR-2, eNOS, HA-dynamin (HA-dyn), and HA-K44A-dynamin (HA-K44A-dyn) as indicated. Forty-eight hours after transfection, cell culture medium was processed for the measurement of NO. *p < 0.05 versus cells expressing HA-dyn, ANOVA followed by Bonferroni.
It is well established that VEGF-stimulated NO release from endothelial cells is downstream from the PI-3 kinase/Akt signaling pathway (Dimmeler et al., 1999; Fulton et al., 1999). We thus investigated the contribution of internalized VEGFR-2 on stimulated NO release. COS-7 cells were transfected with VEGFR-2 and eNOS, and the effect of HA-tagged WT- and K44A-dynamin expression on NO release was monitored. Coexpression of VEGFR-2 with eNOS and WT-dynamin significantly increased NO production when compared with cells transfected with β-gal, eNOS, and WT-dynamin. This confirms that VEGFR-2 stimulates eNOS-dependent NO release in transfected COS-7 cells. Interestingly, coexpression of K44A-dynamin with VEGFR-2 and eNOS inhibited by more than 50% VEGFR-2–stimulated NO release from COS-7 cells (Figure 2B). This effect of the mutant dynamin was specific for VEGFR-2–mediated effects because stimulation of NO release by the calcium ionophore ionomycin or, via the direct activation of eNOS by PI-3 kinase or Akt (Supplementary Figure 2), was not affected by K44A-dynamin expression.
VEGFR-2 Internalization Promotes Tyrosine Phosphorylation of Hsp90β
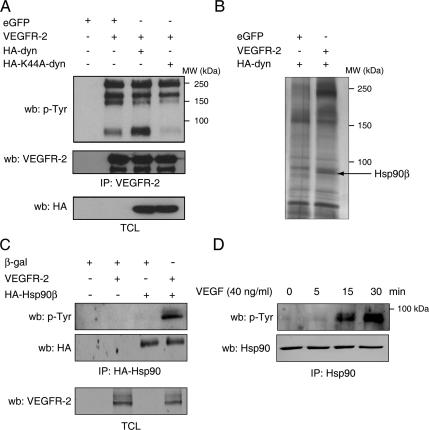
Because K44A-dynamin expression specifically affects VEGFR-2 signaling, we evaluated if the expression of K44A-dynamin affects the autophosphorylation on tyrosine residues of VEGFR-2. COS-7 cells were cotransfected with VEGFR-2 and WT- or K44A-dynamin. Cell lysates were immunoprecipitated with antibodies against VEGFR-2, and tyrosine phosphorylation levels of VEGFR-2 were monitored. Transient expression of VEGFR-2 in COS-7 cells results in constitutive autophosphorylation of the receptor; Western blot analysis showed that the levels of tyrosine phosphorylation of VEGFR-2 were not affected by the coexpression of K44A-dynamin, indicating that inhibition of VEGFR-2 internalization does not affect autophosphorylation and activation per se of the receptor (Figure 3A, top, 200- and 250-kDa bands). Interestingly, we also observed the presence of a highly tyrosine phosphorylated protein of ∼85 kDa associated to the VEGFR-2. This phospho-protein band was absent when VEGFR-2 internalization was inhibited by K44A-dynamin and was enhanced in presence of WT-dynamin (Figure 3A). We used LC-MS/MS analysis to identify this protein. COS-7 cells were transfected with plasmids coding for VEGFR-2 or enhanced GFP (eGFP), as a control, and HA-tagged WT-dynamin. Cell lysates were immunoprecipitated for VEGFR-2; proteins were resolved by SDS-PAGE and detected by silver staining (Figure 3B). The ≈85-kDa protein, present only in the immunoprecipitate from cells expressing VEGFR-2, was analyzed by tandem MS. Protein database search using Mascot analysis (Matrix Science) revealed the presence of seven peptides with a total Mascot score of 241, corresponding to human Hsp90β (Supplementary Table S1).
Figure 3.
VEGF induces tyrosine phosphorylation of Hsp90β. (A) COS-7 cells were cotransfected with VEGFR-2, eGFP, as a control, and HA-dynamin (HA-dyn) and HA-K44A-dynamin (HA-K44A-dyn) expression vectors as indicated. Cell lysates were immunoprecipitated (IP) with an anti-VEGFR-2 antibody and Western blotted (wb) using anti-phosphotyrosine antibodies (p-Tyr). Equal protein expression was determined by Western blotting the total cell lysates (TCL). (B) VEGFR-2 internalization is necessary for Hsp90 phosphorylation. VEGFR-2 immunoprecipitate from COS-7 cells, cotransfected as in A, were separated by SDS-PAGE, and proteins were detected by silver staining. The unique ≈85-kDa protein present in the VEGFR-2–transfected cells (arrow) was cut, subjected to LC/MS/MS analysis, and identified as Hsp90β. (C) VEGFR-2 stimulates tyrosine phosphorylation of Hsp90β. HA-Hsp90β immunoprecipitation was done in COS-7 cell lysates using and levels of tyrosine phosphorylation detected by anti-p-Tyr Western blotting. (D) VEGF stimulation of endothelial cells induces tyrosine phosphorylation of Hsp90. BAECs were stimulated with VEGF (40 ng/ml) for the indicated time and Hsp90 was immunoprecipitated from the cell extracts and Western blotted using anti-p-Tyr.
We have previously reported a regulated association of Hsp90 with VEGFR-2 (Le Boeuf et al., 2004). However, this is the first report of VEGFR-2–mediated tyrosine phosphorylation of the chaperone. To further confirm that Hsp90 is tyrosine phosphorylated upon VEGFR-2 activation, HA-tagged Hsp90β was transfected in presence or in absence of the VEGFR-2, and anti-phosphotyrosine Western blotting of the HA-tag immunoprecipitate revealed that Hsp90β is phosphorylated on tyrosine residues when coexpressed with VEGFR-2 (Figure 3C). To investigate if tyrosine phosphorylation of Hsp90 occurs in endothelial cells in response to VEGF stimulation, Hsp90 was immunoprecipitated from VEGF-stimulated BAECs. Phosphotyrosine immunoblotting done on Hsp90 immunoprecipitates showed a marked increase in the levels of phosphorylation of Hsp90 on tyrosine residues at 15 min of VEGF stimulation and that the maximum was reached at 30 min of stimulation (Figure 3D). These results confirm that VEGFR-2 activation results in increased tyrosine phosphorylation of Hsp90β.
Association of Hsp90 to VEGFR-2 Is Required for Tyrosine Phosphorylation
We then studied the relationship between Hsp90 phosphorylation and VEGFR-2 internalization; we first looked at endogenous Hsp90 association to VEGFR-2 in COS-7 cells. Cells were cotransfected with VEGFR-2 and WT dynamin or K44A-dynamin. VEGFR-2 immunoprecipitation showed that endogenous Hsp90 is associated to VEGFR-2 irrespectively of the presence of WT- or K44A-dynamin. In contrast, immunoprecipitation of Hsp90 from these same lysates revealed that tyrosine phosphorylation of Hsp90 is increased only in cells expressing both VEGFR-2 and HA-dynamin. Conversely, K44A-dynamin expression completely abolished the VEGFR-2–stimulated tyrosine phosphorylation of Hsp90 (Figure 4A). To demonstrate in endothelial cells that internalization of VEGFR-2 is necessary for VEGF-dependent tyrosine phosphorylation of Hsp90, we infected BAECs with β-gal or Ad-HA-K44A-dynamin and monitored the increase in Hsp90 phosphorylation levels in response to VEGF stimulation (Figure 4B). VEGF stimulation induced an increase in the tyrosine phosphorylation levels of Hsp90 in cells infected with Ad-β-gal. In contrast, K44A-dynamin expression in BAECs completely inhibited the VEGF-stimulated increase in Hsp90 tyrosine phosphorylation levels (Figure 4B). These findings support internalization of the VEGFR-2 as being a prerequisite for the phosphorylation of Hsp90 in endothelial cells.
Figure 4.
VEGFR-2 internalization is required for tyrosine phosphorylation of Hsp90. (A) Lysates from transfected COS-7 cells were immunoprecipitated (IP) using anti-VEGFR-2 or anti-Hsp90 antibodies. Tyrosine phosphorylation levels of Hsp90 were monitored by anti-p-Tyr immunodetection and Hsp90 association to the VEGFR-2 was revealed by anti-Hsp90 Western blotting (wb) on the VEGFR-2 immunoprecipitate. Anti-HA antibodies were used to control for dynamin expression in total cell lysates (TCL). (B) BAECs were infected with adenoviruses coding for HA-K44A-dynamin (HA-K44A-dyn) or β-gal. Serum-starved cells were stimulated with VEGF (40 ng/ml, 15 min). Cell lysates were immunoprecipitated for Hsp90, and the tyrosine phosphorylation level of Hsp90 was detected with anti-phosphotyrosine antibody (p-Tyr). (C) Geldanamycin (GA) inhibits VEGF-stimulated tyrosine phosphorylation of Hsp90. HUVECs were pretreated with GA and stimulated with VEGF. Tyrosine phosphorylation levels of Hsp90 immunoprecipitates were monitored by Western blot using anti-p-Tyr antibodies.
We previously reported that inhibition of Hsp90 with geldanamycin (GA) impairs the recruitment of Hsp90 to VEGFR-2 (Le Boeuf et al., 2004). Interestingly, we now show that treatment of HUVECs with GA also results in the inhibition of the VEGF-stimulated tyrosine phosphorylation of Hsp90 (Figure 4C). However, internalization of VEGFR-2, measured by FACS analysis, was not impaired by GA pretreatment of endothelial cells (data not shown). Thus, receptor internalization is independent of Hsp90 association and phosphorylation because GA abrogated recruitment of Hsp90 to the VEGFR-2 but did not affect the VEGF-dependent internalization process. These results further suggest that tyrosine phosphorylation of Hsp90 occurs after internalization of the receptor.
c-Src Mediates in VEGF-stimulated Tyrosine Phosphorylation of Hsp90
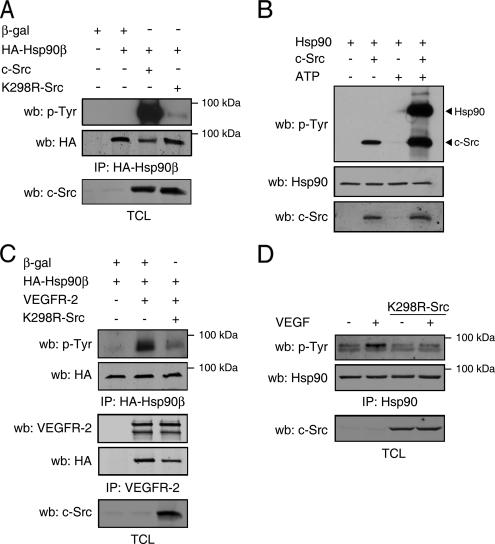
The tyrosine kinase c-Src is involved in various aspects of VEGF signaling. We thus explored the possibility of a contribution of Src kinase activity in VEGF-mediated tyrosine phosphorylation of Hsp90. In this context, COS-7 cells were cotransfected with plasmids coding for HA-tagged Hsp90β, β-gal, c-Src, or the dominant negative form K298R-Src (Figure 5A). In c-Src–overexpressing COS-7 cells, an important increase in tyrosine phosphorylation levels of Hsp90β was observed. Conversely, tyrosine phosphorylation of Hsp90β was barely detectable in cells expressing K298R-Src (Figure 5A). An in vitro kinase assay was also performed in order to confirm the ability of Src to directly phosphorylate Hsp90. Purified Hsp90 and recombinant c-Src were incubated in kinase buffer in the presence or absence of ATP, and tyrosine phosphorylation was detected by immunoblotting with anti-phosphotyrosine antibody. We show that tyrosine phosphorylation levels of Hsp90 are increased only when c-Src and ATP are both present in the kinase reaction (Figure 5B). We then wondered if K298R-Src might inhibit VEGFR-2–mediated tyrosine phosphorylation of Hsp90 in transfected COS-7 cells and in BAECs. In transfected COS-7 cells, phosphotyrosine immunodetection of the HA-Hsp90β immunoprecipitate showed that concomitant expression of K298R-Src with VEGFR-2 significantly inhibited the VEGFR-2–stimulated tyrosine phosphorylation of Hsp90β (Figure 5C, top). Simultaneously, VEGFR-2 was immunoprecipitated from these cell extracts and a slight reduction (≈30% reduction, n = 3) in the association of HA-Hsp90β to VEGFR-2 was observed in cells expressing the dominant negative form of c-Src (Figure 5C, middle).
Figure 5.
c-Src mediates the VEGF-stimulated tyrosine phosphorylation of Hsp90. (A) c-Src expression induces tyrosine phosphorylation of Hsp90β. COS-7 cells were transfected as indicated and cell extracts were immunoprecipitated (IP) using anti-HA. HA-Hsp90β tyrosine phosphorylation was determined by Western blotting using anti-p-Tyr. (B) c-Src phosphorylates Hsp90 in vitro. Purified Hsp90 was incubated with recombinant c-Src in the presence or absence of ATP. The kinase reaction was then immunoblotted with anti-p-Tyr, anti-Hsp90, or anti-c-Src antibodies. (C) Src activity is necessary for VEGFR-2–stimulated phosphorylation of Hsp90β. COS-7 cells were transfected with HA-Hsp90β, VEGFR-2, and K298R-Src. Lysates were immunoprecipitated with anti-HA or anti-VEGFR-2 antibodies and immunoblotted with anti-p-Tyr or anti-HA. (D) c-Src activity is necessary for VEGF-stimulated phosphorylation of Hsp90 in endothelial cells. BAECs were transiently transfected with K298R-Src–expressing vector. Serum-starved cells were stimulated with VEGF (40 ng/ml, 15 min,) and cell lysates were immunoprecipitated using anti-Hsp90 antibody and immunoblotted using anti-p-Tyr.
We also monitored, in endothelial cells, the effect of K298R-Src expression on VEGF-stimulated increase in tyrosine phosphorylation levels of Hsp90. K298R-Src was thus transfected in BAECs, and Hsp90 immunoprecipitation from VEGF-stimulated BAECs revealed that overexpression of the dominant negative Src inhibited the VEGF-stimulated increase tyrosine phosphorylation of Hsp90 (Figure 5D). Overall, these results indicate that Src activity is essential for the tyrosine phosphorylation of Hsp90.
Hsp90β Is Phosphorylated on Tyrosine 300 by c-Src
To identify the target tyrosyl residue of Src phosphorylation on Hsp90, we performed a Src kinase assay as above, separated the phosphorylated Hsp90 by SDS-PAGE, and submitted it to a tryptic digest. The tyrosine phosphorylated tryptic peptides were enriched by PY-99 immunoprecipitation, and peptide identification was done by MS/MS analysis. We identified a phosphorylated peptide, NPDDITQEEpYGEFYK, which corresponds to amino acids 291-305 of Hsp90β that was phosphorylated on tyrosine 300 of the Hsp90β sequence (Supplementary Figure 3). This phosphorylated peptide was only detected in enriched peptide samples originating from in vitro Src kinase assays performed in presence of ATP. To confirm that this residue could be a target of Src phosphorylation in cells, we generated a myc-tagged mutant of Hsp90β, where the tyrosine 300 residue was substituted for phenylalanine (Y300F). Recently, analysis of the whole phosphoproteome of the anaplastic large cell lymphoma line SU-DHL1 revealed the presence of a peptide that suggested tyrosine 483 of Hsp90 as basally phosphorylated in these cells (Rush et al., 2005). Even though we did not detect the presence of this phosphorylated residue in our assays, we generated a mutated version (Y483F). To confirm that Y300 is a phosphorylation site for c-Src, WT-, Y300F-, or Y483F-Hsp90β were transfected in presence of c-Src in COS-7 cells (Figure 6A). Anti-myc immunoprecipitation revealed that c-Src expression increased the levels of tyrosine phosphorylation of WT-Hsp90β, but the mutant Y300F was markedly less phosphorylated compared with WT-Hsp90β. Mutation of the Y483 residue did not alter the increase in tyrosine phosphorylation induced by c-Src. These results strongly suggest that tyrosine 300 of Hsp90β is a major phosphorylation site for c-Src.
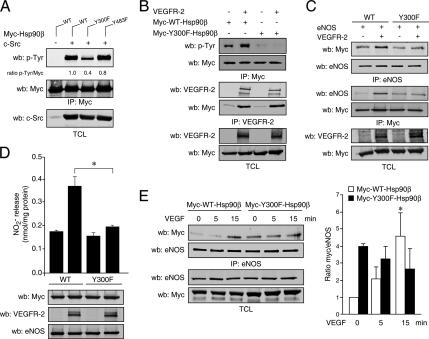
Figure 6.
Phosphorylation of Y300 of Hsp90β by c-Src mediates VEGFR-2–stimulated NO release. (A) COS-7 cells were transfected with c-Src and myc-WT-, Y300F-, or Y483F-Hsp90β, as indicated. Anti-myc immunoprecipitates were Western blotted (wb) with anti-p-Tyr and anti-Myc antibodies. (B) VEGFR-2–stimulated phosphorylation of Y300 is not required for Hsp90 association to the receptor. COS-7 cells were transfected with VEGFR-2, myc-WT-Hsp90β, and myc-Y300F-Hsp90β as indicated. Cell extracts were immunoprecipitated using anti-myc or anti-VEGFR-2 antibodies. Tyrosine phosphorylation of the anti-myc immunoprecipitate was revealed by anti-p-Tyr– and VEGFR-2–associated Hsp90 was monitored by anti-myc Western blotting. TCL, total cell lysates. (C) Expression of Y300F inhibits the association of Hsp90 and eNOS. VEGFR-2, eNOS, myc-WT-Hsp90β, and myc-Y300F-Hsp90β were expressed in COS-7 cells as indicated. Cell lysates were immunoprecipitated using anti-myc or anti-eNOS antibodies. Antibodies against myc or eNOS were use to detect protein association. (D) Inhibition of VEGFR-2–stimulated NO release by Y300F-Hsp90. COS-7 cells were transfected with VEGFR-2, eNOS, myc-WT-Hsp90β, or myc-Y300F-Hsp90β. Cell culture medium was processed for NO measurement. *p< 0.05, ANOVA followed by Bonferroni. (E) Reduced VEGF-stimulated association of Y300F-Hsp90 and eNOS in endothelial cells. BAECs were transfected with myc-WT-Hsp90β or myc-Y300F-Hsp90β. Serum-starved cells were stimulated with VEGF (40 ng/ml), and anti-eNOS immunoprecipitates were blotted with anti-myc antibodies. Bar graph, quantification of the immunoprecipitation presented as densitometric ratio of anti-myc and eNOS Western blots; values are mean ± SEM, n = 5. *p< 0.05 versus time 0 min.
We next sought to determine the phosphorylation of the Y300 residue when stimulated by VEGFR-2. As previously, tyrosine phosphorylation of myc-WT-Hsp90 is markedly increased when cells are cotransfected with VEGFR-2. In contrast, tyrosine phosphorylation of Y300F-Hsp90 was undetectable even in presence of VEGFR-2, indicating that the Y300 residue is a major tyrosine phosphorylation site on Hsp90 in response to VEGFR-2 stimulation (Figure 6B, top panels). In similar experiments, we studied if Y300 phosphorylation influences the association of Hsp90β to the VEGFR-2. Immunoprecipitation of VEGFR-2 revealed that WT-Hsp90β is associated with the receptor and that mutation of Y300 residue did not affect the extent of association of Hsp90 to VEGFR-2 (Figure 6B, bottom panels). In addition, immunofluorescence studies revealed that the cellular localization of both myc-WT-Hsp90 and myc-Y300F-Hsp90 is mostly cytoplasmic and that VEGF stimulation did not alter the cellular distribution of both WT- and Y300F-Hsp90 (Supplementary Figure 4). Overall, these experiments indicate that tyrosine phosphorylation is not necessary for Hsp90 association to the receptor and for proper cellular localization but suggests that it may be important for downstream signaling from VEGFR-2.
Y300 Phosphorylation of Hsp90 Is Necessary for VEGF Signaling to eNOS
To investigate the involvement of Y300 phosphorylation of Hsp90β in VEGFR-2 signaling to eNOS, we studied the capacity of VEGFR-2 to induce Hsp90 association to eNOS and stimulate NO release from cells. Immunoprecipitation of myc-WT-Hsp90β or myc-Y300F-Hsp90β in COS-7 cells cotransfected with eNOS in absence or in the presence of VEGFR-2 showed that the association of eNOS to Y300F-Hsp90β is not increased in the presence of VEGFR-2, in contrast to the association of eNOS to WT-Hsp90β, which is increased in the presence of VEGFR-2 (Figure 6C). The converse eNOS immunoprecipitation also revealed that Y300F-Hsp90β does not associate to eNOS in response to VEGFR-2 activation (Figure 6C).
To study the consequence of this incapacity of Y300F to associate to eNOS in a VEGFR-2–dependent manner, we performed NO release experiments in presence of the phosphorylation-defective Hsp90. VEGFR-2–stimulated NO release in eNOS-transfected COS-7 cells is completely inhibited when cotransfected with Y300F-Hsp90 in contrast to WT-Hsp90β–transfected cells (Figure 6D). We also monitored the effect of myc-Y300F-Hsp90β expression on the VEGF-stimulated association of Hsp90 to eNOS in endothelial cells. BAECs were transfected with myc-tagged WT- or Y300F-Hsp90 and stimulated with VEGF. Anti-eNOS immunoprecipitation revealed that VEGF failed to increase the association of eNOS to myc-Y300F-Hsp90β in contrast to cells expressing the WT version of Hsp90β (Figure 6E). Interestingly, the basal association of Y300F-Hsp90 to eNOS, in unstimulated conditions, is higher than the basal association of WT-Hsp90 with eNOS. However, the association of Y300F-Hsp90 to eNOS was not significantly increase upon VEGF stimulation, in contrast to WT-Hsp90–transfected cells (Figure 6E, bar graph). This may suggest a differential implication of Y300 phosphorylation in the chaperoning functions of Hsp90 (basal association) versus its role in VEGF signal transduction to eNOS. Nonetheless, these results underscore the importance Y300 phosphorylation of Hsp90 in the regulation of VEGFR-2–stimulated signaling to eNOS.
DISCUSSION
Herein, we delineate an intracellular VEGF signaling axis that begins with the internalization of the VEGFR-2 and culminates with the activation of eNOS and NO release from endothelial cells and in which the phosphorylation of Y300 of the chaperone Hsp90 by c-Src acts as an important regulator. Our data demonstrate that VEGFR-2–bound Hsp90β is phosphorylated in response to VEGF stimulation in a c-Src–dependent manner on Y300 and that this event is necessary for VEGF-induced eNOS association to Hsp90 resulting in stimulated NO production.
We have previously shown that Hsp90 interacts with the VEGFR-2 and this VEGF-promoted effect contributes to signaling to FAK and actin remodeling (Rousseau et al., 2000; Masson-Gadais et al., 2003). Using MS/MS analysis, we have now identified Hsp90β as a tyrosine-phosphorylated protein associated to the VEGFR-2. We thus provide novel data suggesting that tyrosine phosphorylation of Hsp90β is an early event in signal transduction leading to VEGF-mediated effects in endothelial cells. The phosphorylation of the chaperone is increased after VEGF stimulation of endothelial cells and is present only when the internalization of the VEGFR-2 is allowed. We have shown that Hsp90 is recruited to the last 130 AA of the VEGFR-2 in response to VEGF stimulation of endothelial cells (Le Boeuf et al., 2004). On the basis of our current understanding, we can speculate that chronologically, after VEGFR-2 activation, a pool of nonphosphorylated Hsp90 quickly associates with the last 130 AA of the receptor in response to VEGF and that, after internalization, this pool of Hsp90 becomes tyrosine-phosphorylated by c-Src. We also identified phosphorylation of Y300 as a regulated posttranslational modification that alters the capacity of Hsp90 to interact with eNOS and to promote its activation by VEGF in endothelial cells. Interestingly, all the components of this pathway have been previously shown to interact directly; it is thus logical that they all depend on the internalization process for efficient signaling.
Phosphorylation of Hsp90 on Ser and/or Thr residues has clearly been shown to be involved in its function. Hyperphosphorylation at these sites has been shown to result in the inhibition of the chaperoning function of Hsp90 (Mimnaugh et al., 1995; Zhao et al., 2001; Ogiso et al., 2004). Similarly, our results suggest that, under basal conditions, Y300 phosphorylation could reduce the chaperoning role of Hsp90 with client proteins (Figure 6E). However, VEGF-induced phosphorylation of the Y300 residue is clearly involved in the stimulated activation of eNOS via Hsp90. Interestingly, the Y300 residue of Hsp90β is located at the beginning of the middle domain of Hsp90, and this region has previously been shown to mediate the interaction of Hsp90 with eNOS and Akt (Sato et al., 2000; Fontana et al., 2002). In addition, the Y300 residue of Hsp90β corresponds to Y308 of Hsp90α; in fact, this tyrosine residue is extremely conserved from the yeast to the human form. This argues for the possibility that phosphorylation at this site may govern a wide variety of functions in multiple organisms. Also, the region encompassed by Y308α or Y300β has been demonstrated to be immunogenic, suggesting that it is exposed to the outer surface of the molecule thus accessible to kinases (Nemoto et al., 1997). Recently, the proximally located K294 of Hsp90α (K286β) has been proposed to be acetylated and that acetylation at this site decreases its affinity for most client proteins and certain cochaperones (Scroggins et al., 2007). This further suggests that posttranslational modifications in this region of the molecule contribute to the regulation of Hsp90 functions as a chaperone and as a signaling intermediate by modulating, in this case, the activity of eNOS. The direct and regulated binding of Hsp90 to eNOS as been shown to increase eNOS activity in endothelial cells (Garcia-Cardena et al., 1998). It is noteworthy that before the identification of Hsp90 as a modulator of eNOS activity, an unknown tyrosine-phosphorylated protein was identified in complex with eNOS (Venema et al., 1996). Further studies then suggested that this initially identified phosphorylated protein might in fact be Hsp90 and that tyrosine phosphorylation of Hsp90 correlates with statin-stimulated formation of the Akt/eNOS/Hsp90 complex (Harris et al., 2000; Brouet et al., 2001b). We now clearly demonstrate that tyrosine phosphorylation of Hsp90 occurs on Y300 via c-Src and that it is a regulated process. The use of the nonphosphorylatable Y300F-Hsp90β mutant also reveals that this is a crucial event for the allosteric modulation of eNOS in endothelial cells after VEGFR-2 activation. It is well known that pharmacological inhibition of Hsp90 by GA, attenuates Akt and eNOS activity, leading to inhibition of angiogenesis (Brouet et al., 2001b; Sun and Liao, 2004). On the basis of our data, we now suggest that Y300 phosphorylation of Hsp90, after receptor internalization, is a necessary step leading to eNOS association and activation and NO release from endothelial cells.
Our study demonstrates that internalization of the VEGFR-2 is dependent on the GTPase activity of dynamin because the overexpression of the K44A mutant of dynamin inhibits VEGF-stimulated VEGFR-2 internalization from the endothelial cell surface, tyrosine phosphorylation of Hsp90, and eNOS activation. It is known that the VEGFR-2 internalizes after activation and that full kinase activity of the receptor is necessary for internalization (Dougher and Terman, 1999). Interestingly, the VEGFR-2 has previously been shown to colocalize with dynamin−2, and expression of dominant negative dynamin regulates VEGFR-2 protein expression and VEGF-mediated survival of endothelial cells. (Bhattacharya et al., 2005). In this study, we have used levels of K44A-dynamin expression that inhibit the internalization of VEGFR-2 without altering its levels. It is possible that prolonged and robust inhibition of VEGFR-2 internalization at high K44A-dynamin expression levels prevents membrane turnover of the receptor and eventually targets it to the degradation machinery (Duval et al., 2003). However, in line with our present study, a recent report suggests that the VEGFR-2 internalizes in a clathrin and VE-cadherin–dependent manner and that the internalized receptors contribute to VEGF signaling from the endosomal compartment (Lampugnani et al., 2006). We now show that internalization of VEGFR-2, via a dynamin-dependent process, regulates intracellular signaling to Hsp90, Akt, and eNOS, which has never been demonstrated. Although we do not provide here direct evidence for endosomal signaling from internalized VEGFR-2, we demonstrate, however, that VEGFR-2 internalization is required for VEGF signaling to eNOS. In corollary, the fact that the Hsp90 inhibitor GA inhibits the recruitment of Hsp90 to VEGFR-2, and consequently its phosphorylation, but does not affect the VEGF-dependent internalization of the receptor indicates that association and phosphorylation of Hsp90 are not required for the internalization of VEGFR-2. In fact, this suggests that both processes possibly occur during internalization. In addition, our results are consistent with our previous observations that GA does not inhibit Src activation in response to VEGF, which further suggests that the phosphorylation of Hsp90 occurs after internalization (Le Boeuf et al., 2004).
Finally, c-Src has been implicated in various signaling pathways downstream of the VEGFR-2, such as NO and prostacyclin release (He et al., 1999). In addition, Src kinase activity is necessary for VEGF-mediated vascular permeability involved in angiogenesis, tumor cell extravasation, and metastasis (Eliceiri et al., 1999; Weis et al., 2004). Recently, eNOS as been shown to be tyrosine-phosphorylated by c-Src in response to increases in intracellular calcium concentration (Fulton et al., 2005). This phosphorylation of eNOS on Y83 increases the activity of the enzyme. However, it seems specific for calcium mobilizing agonists because VEGF stimulation did not increase the tyrosine phosphorylation levels of eNOS (Fulton et al., 2005). We now provide evidence that c-Src catalyzes the tyrosine phosphorylation of Hsp90 on Y300 in response to VEGF stimulation. c-Src is a well known Hsp90 client protein, and Hsp90 activity is essential for proper maturation of the tyrosine kinase (Xu et al., 1999). Hsp90 is thought to direct the folding of Src kinases into the inactive conformation, which prevents their degradation (Xu and Lindquist, 1993; Xu et al., 1999). Interestingly, inhibition of Hsp90 with GA rapidly disrupts Src association with Hsp90, leading to transient activation of c-Src (Koga et al., 2006). We now demonstrated that Hsp90 is also a bona fide Src substrate in response to VEGFR-2 activation, which results in activation of Hsp90, suggesting a reciprocal influence between the two proteins that regulates both their activity in endothelial cells. Moreover, the possibility that the phosphorylation of Hsp90 by c-Src influences the tyrosine phosphorylation of eNOS by c-Src stimulated by agonists other than VEGF remains to be explored.
In summary, our data demonstrate that Hsp90 is phosphorylated on Y300 by c-Src in response to VEGFR-2 activation, and this leads to increase Hsp90 and eNOS association, and thus NO release from endothelial cells. These results hint toward a better understanding of the intracellular events leading to the proangiogenic signals activated by VEGF and delineate a role for a regulated posttranslational modification of Hsp90. In this context, one may envision that the inhibition of Hsp90 phosphorylation by GA derivatives may contribute to their antiangiogenic and anticancer effects.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Denis Faubert from the Institut de recherches cliniques de Montréal proteomic core facility for protein identifications by MS and Daniela Baggio for secretarial assistance. This work was supported by grants from the Canadian Institutes of Health Research (MOP-15402 to J.H. and MOP-53295 to J.P.G.). M.D. is a recipient of a studentship from the Heart and Stroke Foundation of Canada. F.L.B. was in receipt of a studentship from Le Fonds de la Recherche en Santé du Québec. J.P.G. holds a Canada Research Chair.
Abbreviations used:
- BAECs
bovine aortic endothelial cells
- eNOS
endothelial nitric oxide synthase
- GA
geldanamycin
- HA
hemagglutinin
- Hsp90
heat-shock protein 90 kDa
- HUVECs
human umbilical vein endothelial cells
- NO
nitric oxide
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-05-0467) on September 12, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Aicher A., Heeschen C., Mildner-Rihm C., Urbich C., Ihling C., Technau-Ihling K., Zeiher A. M., Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat. Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- Basso A. D., Solit D. B., Munster P. N., Rosen N. Ansamycin antibiotics inhibit Akt activation and cyclin D expression in breast cancer cells that overexpress HER2. Oncogene. 2002;21:1159–1166. doi: 10.1038/sj.onc.1205184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya R., Kang-Decker N., Hughes D. A., Mukherjee P., Shah V., McNiven M. A., Mukhopadhyay D. Regulatory role of dynamin-2 in VEGFR-2/KDR-mediated endothelial signaling. FASEB J. 2005;19:1692–1694. doi: 10.1096/fj.05-3889fje. [DOI] [PubMed] [Google Scholar]
- Brouet A., Sonveaux P., Dessy C., Balligand J. L., Feron O. Hsp90 ensures the transition from the early Ca2+-dependent to the late phosphorylation-dependent activation of the endothelial nitric-oxide synthase in vascular endothelial growth factor-exposed endothelial cells. J. Biol. Chem. 2001a;276:32663–32669. doi: 10.1074/jbc.M101371200. [DOI] [PubMed] [Google Scholar]
- Brouet A., Sonveaux P., Dessy C., Moniotte S., Balligand J. L., Feron O. Hsp90 and caveolin are key targets for the proangiogenic nitric oxide-mediated effects of statins. Circ. Res. 2001b;89:866–873. doi: 10.1161/hh2201.100319. [DOI] [PubMed] [Google Scholar]
- Carmeliet P., et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A. M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Dougher M., Terman B. I. Autophosphorylation of KDR in the kinase domain is required for maximal VEGF-stimulated kinase activity and receptor internalization. Oncogene. 1999;18:1619–1627. doi: 10.1038/sj.onc.1202478. [DOI] [PubMed] [Google Scholar]
- Dumont D. J., Jussila L., Taipale J., Lymboussaki A., Mustonen T., Pajusola K., Breitman M., Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- Duval M., Bedard-Goulet S., Delisle C., Gratton J. P. Vascular endothelial growth factor-dependent down-regulation of Flk-1/KDR involves Cbl-mediated ubiquitination. Consequences on nitric oxide production from endothelial cells. J. Biol. Chem. 2003;278:20091–20097. doi: 10.1074/jbc.M301410200. [DOI] [PubMed] [Google Scholar]
- Eliceiri B. P., Paul R., Schwartzberg P. L., Hood J. D., Leng J., Cheresh D. A. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol. Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- Fong G. H., Rossant J., Gertsenstein M., Breitman M. L. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Fontana J., Fulton D., Chen Y., Fairchild T. A., McCabe T. J., Fujita N., Tsuruo T., Sessa W. C. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ. Res. 2002;90:866–873. doi: 10.1161/01.res.0000016837.26733.be. [DOI] [PubMed] [Google Scholar]
- Fukumura D., Gohongi T., Kadambi A., Izumi Y., Ang J., Yun C. O., Buerk D. G., Huang P. L., Jain R. K. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. USA. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D., Church J. E., Ruan L., Li C., Sood S. G., Kemp B. E., Jennings I. G., Venema R. C. Src kinase activates endothelial nitric-oxide synthase by phosphorylating Tyr-83. J. Biol. Chem. 2005;280:35943–35952. doi: 10.1074/jbc.M504606200. [DOI] [PubMed] [Google Scholar]
- Fulton D., Gratton J. P., McCabe T. J., Fontana J., Fujio Y., Walsh K., Franke T. F., Papapetropoulos A., Sessa W. C. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cardena G., Fan R., Shah V., Sorrentino R., Cirino G., Papapetropoulos A., Sessa W. C. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- Gerber H. P., McMurtrey A., Kowalski J., Yan M., Keyt B. A., Dixit V., Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- Harris M. B., Ju H., Venema V. J., Blackstone M., Venema R. C. Role of heat shock protein 90 in bradykinin-stimulated endothelial nitric oxide release. Gen. Pharmacol. 2000;35:165–170. doi: 10.1016/s0306-3623(01)00104-5. [DOI] [PubMed] [Google Scholar]
- Hayes S., Chawla A., Corvera S. TGF beta receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J. Cell Biol. 2002;158:1239–1249. doi: 10.1083/jcb.200204088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Venema V. J., Gu X., Venema R. C., Marrero M. B., Caldwell R. B. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J. Biol. Chem. 1999;274:25130–25135. doi: 10.1074/jbc.274.35.25130. [DOI] [PubMed] [Google Scholar]
- Koga F., Xu W., Karpova T. S., McNally J. G., Baron R., Neckers L. Hsp90 inhibition transiently activates Src kinase and promotes Src-dependent Akt and Erk activation. Proc. Natl. Acad. Sci. USA. 2006;103:11318–11322. doi: 10.1073/pnas.0604705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupatt C., Dessy C., Hinkel R., Raake P., Daneau G., Bouzin C., Boekstegers P., Feron O. Heat shock protein 90 transfection reduces ischemia-reperfusion-induced myocardial dysfunction via reciprocal endothelial NO synthase serine 1177 phosphorylation and threonine 495 dephosphorylation. Arterioscler. Thromb. Vasc. Biol. 2004;24:1435–1441. doi: 10.1161/01.ATV.0000134300.87476.d1. [DOI] [PubMed] [Google Scholar]
- Lampugnani M. G., Orsenigo F., Gagliani M. C., Tacchetti C., Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J. Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Boeuf F., Houle F., Huot J. Regulation of vascular endothelial growth factor receptor 2-mediated phosphorylation of focal adhesion kinase by heat shock protein 90 and Src kinase activities. J. Biol. Chem. 2004;279:39175–39185. doi: 10.1074/jbc.M405493200. [DOI] [PubMed] [Google Scholar]
- Masson-Gadais B., Houle F., Laferriere J., Huot J. Integrin alphavbeta3, requirement for VEGFR2-mediated activation of SAPK2/p38 and for Hsp90-dependent phosphorylation of focal adhesion kinase in endothelial cells activated by VEGF. Cell Stress. Chaperones. 2003;8:37–52. doi: 10.1379/1466-1268(2003)8<37:ivrfva>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimnaugh E. G., Worland P. J., Whitesell L., Neckers L. M. Possible role for serine/threonine phosphorylation in the regulation of the heteroprotein complex between the hsp90 stress protein and the pp60v-src tyrosine kinase. J. Biol. Chem. 1995;270:28654–28659. doi: 10.1074/jbc.270.48.28654. [DOI] [PubMed] [Google Scholar]
- Nemoto T., Sato N., Iwanari H., Yamashita H., Takagi T. Domain structures and immunogenic regions of the 90-kDa heat-shock protein (HSP90). Probing with a library of anti-HSP90 monoclonal antibodies and limited proteolysis. J. Biol. Chem. 1997;272:26179–26187. doi: 10.1074/jbc.272.42.26179. [DOI] [PubMed] [Google Scholar]
- Ogiso H., Kagi N., Matsumoto E., Nishimoto M., Arai R., Shirouzu M., Mimura J., Fujii-Kuriyama Y., Yokoyama S. Phosphorylation analysis of 90 kDa heat shock protein within the cytosolic arylhydrocarbon receptor complex. Biochemistry. 2004;43:15510–15519. doi: 10.1021/bi048736m. [DOI] [PubMed] [Google Scholar]
- Rousseau S., Houle F., Kotanides H., Witte L., Waltenberger J., Landry J., Huot J. Vascular endothelial growth factor (VEGF)-driven actin-based motility is mediated by VEGFR2 and requires concerted activation of stress-activated protein kinase 2 (SAPK2/p38) and geldanamycin-sensitive phosphorylation of focal adhesion kinase. J. Biol. Chem. 2000;275:10661–10672. doi: 10.1074/jbc.275.14.10661. [DOI] [PubMed] [Google Scholar]
- Rousseau S., Houle F., Landry J., Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- Rush J., Moritz A., Lee K. A., Guo A., Goss V. L., Spek E. J., Zhang H., Zha X. M., Polakiewicz R. D., Comb M. J. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat. Biotechnol. 2005;23:94–101. doi: 10.1038/nbt1046. [DOI] [PubMed] [Google Scholar]
- Sato S., Fujita N., Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. USA. 2000;97:10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T. W., Blagosklonny M. V., Ingui C., Neckers L. Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J. Biol. Chem. 1995;270:24585–24588. doi: 10.1074/jbc.270.41.24585. [DOI] [PubMed] [Google Scholar]
- Scroggins B. T., et al. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol. Cell. 2007;25:151–159. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa W. C., Garcia-Cardena G., Liu J., Keh A., Pollock J. S., Bradley J., Thiru S., Braverman I. M., Desai K. M. The Golgi association of endothelial nitric oxide synthase is necessary for the efficient synthesis of nitric oxide. J. Biol. Chem. 1995;270:17641–17644. doi: 10.1074/jbc.270.30.17641. [DOI] [PubMed] [Google Scholar]
- Shalaby F., Rossant J., Yamaguchi T. P., Gertsenstein M., Wu X. F., Breitman M. L., Schuh A. C. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Sun J., Liao J. K. Induction of angiogenesis by heat shock protein 90 mediated by protein kinase Akt and endothelial nitric oxide synthase. Arterioscler. Thromb. Vasc. Biol. 2004;24:2238–2244. doi: 10.1161/01.ATV.0000147894.22300.4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Mendelsohn M. E. Synergistic activation of endothelial nitric-oxide synthase (eNOS) by HSP90 and Akt: calcium-independent eNOS activation involves formation of an HSP90-Akt-CaM-bound eNOS complex. J. Biol. Chem. 2003;278:30821–30827. doi: 10.1074/jbc.M304471200. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Shibuya M. The 230 kDa mature form of KDR/Flk-1 (VEGF receptor-2) activates the PLC-gamma pathway and partially induces mitotic signals in NIH3T3 fibroblasts. Oncogene. 1997;14:2079–2089. doi: 10.1038/sj.onc.1201047. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Yamaguchi S., Chida K., Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbe S., Mills I. G., Stenmark H., Kitamura N., Clague M. J. Endosomal localization and receptor dynamics determine tyrosine phosphorylation of hepatocyte growth factor-regulated tyrosine kinase substrate. Mol. Cell. Biol. 2000;20:7685–7692. doi: 10.1128/mcb.20.20.7685-7692.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema V. J., Marrero M. B., Venema R. C. Bradykinin-stimulated protein tyrosine phosphorylation promotes endothelial nitric oxide synthase translocation to the cytoskeleton. Biochem. Biophys. Res. Commun. 1996;226:703–710. doi: 10.1006/bbrc.1996.1417. [DOI] [PubMed] [Google Scholar]
- Weis S., Cui J., Barnes L., Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J. Cell Biol. 2004;167:223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell L., Lindquist S. L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- Xu W., Mimnaugh E., Rosser M. F., Nicchitta C., Marcu M., Yarden Y., Neckers L. Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J. Biol. Chem. 2001;276:3702–3708. doi: 10.1074/jbc.M006864200. [DOI] [PubMed] [Google Scholar]
- Xu Y., Lindquist S. Heat-shock protein hsp90 governs the activity of pp60v-src kinase. Proc. Natl. Acad. Sci. USA. 1993;90:7074–7078. doi: 10.1073/pnas.90.15.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Singer M. A., Lindquist S. Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc. Natl. Acad. Sci. USA. 1999;96:109–114. doi: 10.1073/pnas.96.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. G., Gilmore R., Leone G., Coffey M. C., Weber B., Lee P. W. Hsp90 phosphorylation is linked to its chaperoning function. Assembly of the reovirus cell attachment protein. J. Biol. Chem. 2001;276:32822–32827. doi: 10.1074/jbc.M105562200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.