Abstract
The exocyst is an evolutionarily conserved octameric protein complex that tethers post-Golgi secretory vesicles at the plasma membrane for exocytosis. To elucidate the mechanism of vesicle tethering, it is important to understand how the exocyst physically associates with the plasma membrane (PM). In this study, we report that the mammalian exocyst subunit Exo70 associates with the PM through its direct interaction with phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2). Furthermore, we have identified key conserved residues at the C-terminus of Exo70 that are crucial for the interaction of Exo70 with PI(4,5)P2. Disrupting Exo70-PI(4,5)P2 interaction abolished the membrane association of Exo70. We have also found that wild-type Exo70 but not the PI(4,5)P2-binding–deficient Exo70 mutant is capable of recruiting other exocyst components to the PM. Using the ts045 vesicular stomatitis virus glycoprotein trafficking assay, we demonstrate that Exo70-PI(4,5)P2 interaction is critical for the docking and fusion of post-Golgi secretory vesicles, but not for their transport to the PM.
INTRODUCTION
Exocytosis is important for a variety of cellular functions, ranging from the release of hormones to the incorporation of membrane proteins for cell growth and morphogenesis. The late stage of exocytosis is a multistep process that includes directional transport, tethering, docking, and fusion of post-Golgi secretory vesicles with the plasma membrane (PM). The tethering step, defined as the initial contact of secretory vesicles with the PM before SNARE-mediated docking and fusion (Pfeffer, 1999; Guo et al., 2000; Waters and Hughson, 2000; Whyte and Munro, 2002), is mediated by the exocyst, an evolutionarily conserved octameric complex composed of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84 (for review, see Guo et al., 2000; Hsu et al., 2004; Munson and Novick, 2006; Wang and Hsu, 2006). In budding yeast, the exocyst components localize to the growing end of the daughter cell (“bud”), where active exocytosis and membrane addition take place (TerBush and Novick, 1995; Finger et al., 1998, Guo et al., 1999). This localization pattern contrasts that of the membrane fusion machine, the t-SNAREs, which are evenly distributed along both the mother and daughter cell membrane (Brennwald et al., 1994). In mammalian cells, the exocyst components were found in the cytosol, recycling endosomes and trans-Golgi network (Yeaman et al., 2001; Fölsch et al., 2003; Ang et al., 2004; Langevin et al., 2005). However, they are recruited to the PM during a number of cellular processes. For example, in epithelial cells, the exocyst is recruited to the adherens junction region upon cell–cell contact, where it mediates protein and membrane addition at the basolateral domain (Grindstaff et al., 1998; Yeaman et al., 2001); in developing neurons, the exocyst is localized to the growing neurites, where it mediates membrane expansion (Hazuka et al., 1999; Vega and Hsu, 2001); during cell migration, the exocyst is recruited to the leading edges of the PM (Rosse et al., 2006; Zuo et al., 2006).
The mechanism by which the exocyst mediates vesicle tethering to the PM is unclear. One key question yet to be resolved is how the exocyst itself associates with the PM. Using fluorescence recovery after photobleaching (FRAP) analyses and immunoelectron microscopy, Boyd et al. (2004) have shown that Exo70 is stably localized to the yeast bud tip membrane and remains polarized even when the actin cables are disrupted, suggesting that Exo70 is a candidate in this complex involved in membrane targeting of the exocyst. In Madin-Darby canine kidney (MDCK) cells, extragenically expressed GFP-tagged Exo70 is localized to the PM near cell–cell contacts, suggesting that Exo70 may mediate PM association independent of the rest exocyst components in these cells (Matern et al., 2001). Recent structural studies have revealed that Exo70 contains a number of conserved basic residues that cluster on a surface patch at the C-terminal end of the tertiary structure that may directly bind to the PM (Dong et al., 2005; Hamburger et al., 2006; Moore et al., 2007). In fact, the C-terminal sequence of Exo70 is the most evolutionarily conserved region of this protein.
Here, we report that mammalian Exo70 directly interacts with PI(4,5)P2 in the PM via the positively charged residues at its C-terminus. We have also identified key residues in Exo70 that are important for this interaction. Finally, using the ts045 vesicular stomatitis virus glycoprotein (VSV-G) trafficking assay, we found that the Exo70-lipid interaction is critical for PM stages of exocytosis, but not for the trafficking steps through endoplasmic reticulum (ER) and Golgi. Our study revealed a molecular mechanism by which the exocyst directly interacts with the PM that is critical for vesicle tethering and exocytosis.
MATERIALS AND METHODS
DNA Plasmid Construction
Wild-type rat Exo70 (rExo70) cDNA and various truncates of Exo70 were cloned in-frame into pEGFP-C1 for expression as green fluorescent protein (GFP) fusions or in pEBG for expression as glutathione S-transferase (GST) fusion in mammalian cells. rExo70 was also cloned into pGEX-KG (a modified form of pGEX-2T, from Amersham Biosciences, Piscataway, NJ) for expression as GST fusion in bacteria. The Exo70 mutants were generated using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). All the constructs were confirmed by nucleotide sequencing.
Cell Culture and RNA Interference Experiments
HeLa cells were cultured at 37°C in DMEM supplemented with 10% fetal bovine serum and 100 U/ml penicillin and 100 μg/ml streptomycin in a 5% CO2 incubator. For RNA interference (RNAi) experiments, cells were grown to 50% confluence and transfected with small interfering RNA (siRNA) duplexes using Oligofectamine (Invitrogen, Carlsbad, CA). The human Exo70 siRNA target sequence is 5′-GGTTAAAGGTGACTGATTA-3′. The control Luciferase GL2 siRNA target sequence is 5′-AACGTACGCGGAATACTTCGA-3′. The efficiency of Exo70 knockdown was determined by Western blot.
Confocal Microscopy
Transfected HeLa cells were grown on coverslips, washed with phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde at room temperature for 12 min, washed, permeabilized for 5 min with PBST (PBS-Tween), and blocked for 10 min with 2% bovine serum albumin in PBST. The coverslips were incubated sequentially with primary and secondary antibodies for fluorescence observation using the Leica TCS SL laser-scanning confocal microscope (63× objective; Deerfield, IL). Images were processed with Adobe Photoshop (Adobe Systems, San Jose, CA; version 7.0).
Large Unilamellar Vesicle Sedimentation Assay
Large unilamellar vesicle (LUV) sedimentation assay was performed as previously described (Hokanson and Ostap, 2006). Phospholipids were purchased from Avanti Polar Lipids (Alabaster, AL). LUVs with a 100-nm diameter were prepared by size extrusion. Various lipids were mixed at different molar ratios, dried with nitrogen stream, and resuspended at a concentration of 2 mM in a buffer containing 12 mM HEPES, pH 7.0, and 176 mM sucrose. The mixed lipids were subjected to five cycles of freeze-thaw and a 1-min bath sonication before being passed through 100-nm filters using a mini-extruder. LUVs were dialyzed overnight in the HNa100 buffer (10 mM HEPES, pH 7.0, 100 mM NaCl, 1 mM EGTA, and 1 mM dithiothreitol [DTT]). The percentages of phosphotidylserine (PS), PI(3)P, PI(4)P, PI(3,5)P2, PI(4,5)P2, and PI(3,4,5)P3 indicated in the text are the molar percentages of total PS and PIPs with the remainder being phosphatidylcholine (PC). Lipid concentrations are given as total lipid. The binding of Exo70 to LUVs was determined by sedimentation assays in 200 μl total volume using TLA-100 rotor (Beckman Coulter, Fullerton, CA). Sucrose-loaded LUVs were precipitated at 150,000 × g for 30 min at 25°C. The supernatants and pellets were subjected to 10% SDS-PAGE and stained with SYPRORed (Invitrogen) for quantification of free and bound materials with the Image Quant software (Molecular Dynamics, Sunnyvale, CA).
Membrane Fractionation
HeLa cells were plated in 10-cm dishes at 1.5 × 106 cells per dish. The next day cells were transfected with DNA by FuGene6 reagent and incubated at 37°C overnight. Homogenization and subcellular fractionation of the cells to isolate the PM fraction, cytosol, the low-density microsomal fraction (LDM), and the high-density microsomal fraction (HDM) were performed basically as previously described (Weber et al., 1988). All steps were performed at 4°C in the presence of a protease inhibitor cocktail. The distribution of Exo70 and Sec8 were detected by monoclonal antibodies (kind gifts of Dr. Shu-Chan Hsu, Rutgers University).
GST Pulldown Assay
HeLa cells were transfected with GFP-tagged Exo70 or exo70-1, and the cells were lysed in a buffer containing 20 mM Tris-HCl, pH 7.5, 25 mM KCl, 1 mM MgCl2, 0.5 mM EGTA, 1 mM DTT, 0.5% Triton X-100, and protease inhibitors. Cell lysates were incubated overnight with glutathione-Sepharose conjugated with GST or GST-TC10 (Q75L) at 4°C. After incubation, the beads were washed five times with the lysis buffer, and the bound proteins were analyzed by Western blot using an anti-GFP antibody. To detect the interaction of Exo70 and Sec8 in the cell, HeLa cells were transfected with GST-tagged Exo70 or exo70-1, and cell lysates were incubated overnight with glutathione-Sepharose beads at 4°C. After incubation, the beads were washed, and the bound proteins were detected by Western blot using anti-GST or anti-Sec8 monoclonal antibodies.
VSV-G Trafficking Assay
HeLa cells were transfected with EXO70 siRNA. luciferase siRNA was used as the negative control. After 24 h of the siRNA treatment, HeLa cells were transfected with VSV-G-45ts-GFP mutant and immediately placed at 40°C. After overnight growth, the cells were shifted to 32°C for 0, 15, 30, 60, and 90 min in the presence of cycloheximide (100 μg/ml). The cells were then fixed for GFP observation or immunofluorescence. The 8G5 mAb against the extracellular domain of VSV-G was kindly provided by Dr. Douglas Lyles (Wake Forest University). No detergent was used in the immunofluorescence procedure. Cells with surface VSV-Gs were quantified, and statistical analyses were performed using Student's t test. In some cases, HeLa cells were transfected with VSV-G-myc and GST-Exo70 or GST-exo70-1 after 24 h of the EXO70 siRNA treatment. The cells were divided into two sets based on their treatments. For Set I, the cells were fixed, permeabilized, and stained with anti-myc mAb (9E10) and anti-GST polyclonal antibody to test the intracellular traffic of VSV-G and to detect the expression of Exo70 or exo70-1 at 0-, 30-, 60-, and 90-min points. For Set II, cells of the 90-min point group were first stained with the 8G5 antibody, then permeabilized, and stained with anti-GST polyclonal antibody. Anti-mouse Alexa488 and anti-rabbit Alexa594 were used as secondary antibodies for the above experiments. For the quantification of surface VSV-G signals at various points, boundary of the cell surface was outlined, and average fluorescence intensity of surface VSV-G signal was quantified using ImageJ 1.73v software and then divided by the perimeter of the cell surface. For the quantification of VSV-G in different membrane compartments, boundaries of the whole cell, the Golgi, and the cell periphery were outlined, and VSV-G fluorescence in these areas was then quantified using ImageJ 1.73v software after subtraction of background outside the cell using the following equations:
RESULTS
Association of Exo70 with the Plasma Membrane in HeLa Cells
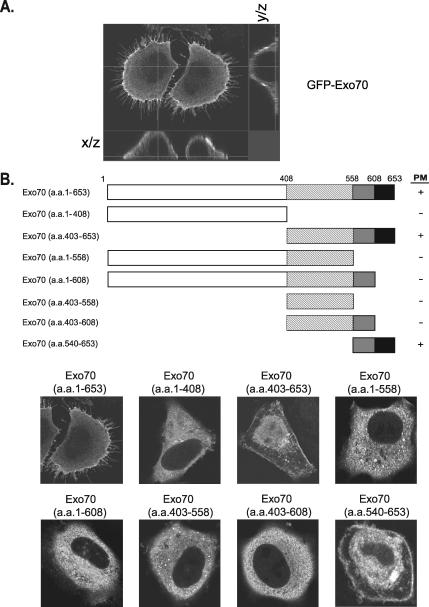
We have examined the localization of GFP-tagged rat Exo70 in HeLa cells. As shown in Figure 1A, GFP-Exo70 was clearly detected at the PM as revealed by serial optical sectioning from different axis (Figure 1A). In addition, cells expressing GFP-Exo70 displayed filopodia-like structures as previously reported (Wang et al., 2004; Xu et al., 2005; Zuo et al., 2006). We next mapped the region of Exo70 that is required for its association with the PM by examining the localization of a series of GFP-tagged Exo70 truncates in HeLa cells. As shown in Figure 1B, Exo70 with its C-terminal domain deleted (amino acids 1-408; Exo70-NT) was cytosolic, whereas the C-terminus of Exo70 (amino acids 403-653; Exo70-CT) was enriched at the PM (Figure 1B), suggesting a critical role of Exo70 C-terminus in membrane association. The crystal structure of yeast Exo70 revealed that this protein is a long rod composed mainly of α-helices that fold into four domains (named domains A, B, C, and D; Dong et al., 2005; Hamburger et al., 2006). Domain D (the C-terminal 114 amino acids) is the most evolutionarily conserved domain in Exo70 that contains a number of basic residues that cluster into an electro-positive patch on the surface of the C-terminus. Because in many cases the association of proteins with the PM involves interactions with the negatively charged phospholipids in membrane via clusters of basic residues (for reviews, see McLaughlin et al., 2002; Balla, 2005), it is likely that those positively charged residues at the C-terminus of mammalian Exo70 are directly involved in membrane association. We sought to examine whether domain D is required for the PM localization of Exo70. We generated truncations of Exo70, in which the last 95 amino acids (aa 559-653) or the last 45 amino acids (aa 609-653) were deleted. The resulting Exo70 fragments, Exo70 (aa 1-558) and Exo70 (aa 1-608), were both cytosolic (Figure 1B), indicating that domain D of mammalian Exo70, especially the last 45 amino acids, is required for the association of Exo70 with the PM. We have also tested the localization of Exo70 (aa 403-558) and Exo70 (aa 403-608), which are C-terminal fragments of Exo70 with the last 95 amino acids (aa 559-653) or the last 45 amino acids (aa 609-653) deleted, and found that both of the fragments were cytosolic (Figure 1B). We next investigated whether the C-terminal fragments of Exo70 containing aa 403-653 or aa 540-653 (domain D) were able to associate with the PM. As shown in Figure 1B, both fragments were localized to the PM, suggesting that domain D of Exo70 is sufficient for its PM localization. A portion of these fragments was also detected in the nucleus, probably resulting from nonspecific retention of the GFP fusion. Collectively, these data indicate that the C-terminus of Exo70 is both necessary and sufficient for the PM targeting of Exo70.
Figure 1.
Association of Exo70 with the plasma membrane (PM) in HeLa cells. (A) GFP-Exo70 was detected at the PM. HeLa cells were transfected with GFP-tagged Exo70, fixed, and observed using a confocal microscope. Serial optical sections in the x-z and y-z planes were taken. (B) Various truncates of Exo70 were tested for their localization at the PM. HeLa cells were transfected with GFP-tagged Exo70 and Exo70 truncates as diagramed. The cells were fixed, stained, and observed under microscope. Full-length and the C-terminal fragments of Exo70 containing domain D (aa 558-653) are associated with the PM. +, membrane association.
Exo70 Directly Interacts with Phospholipids through Its C-Terminus
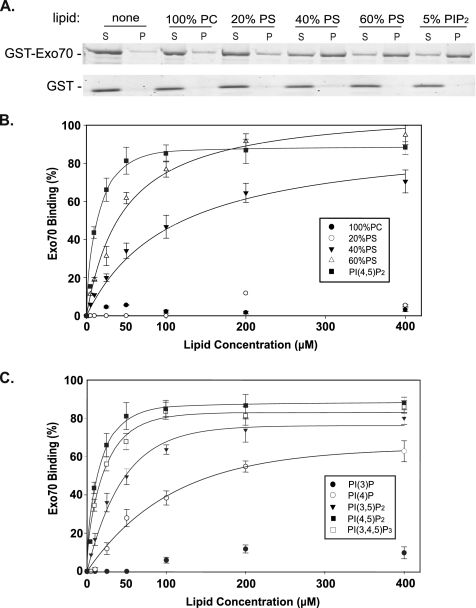
Based on sequence analysis, domain D of Exo70 contains a number of basic residues that are well conserved in yeast. Moreover, based on the crystal structure, the basic residues cluster onto a surface patch in the folded Exo70 protein. Therefore, it is likely that Exo70 directly interact with phospholipids through this basic patch. To test this possibility, we examined the binding between recombinant GST-Exo70 and various phospholipids by cosedimentation assay using the methods as previously described (Papayannopoulos et al., 2005; Hokanson and Ostap, 2006). PI(4,5)P2 is the major phosphatidylinositol species, and constitutes 1–5% of the total lipids in the PM (for review, see McLaughlin et al., 2002). Binding experiments were performed with LUVs composed of the neutral PC and PI(4,5)P2 at 5% or the acidic phospholipid PS in molar percentages of 20, 40, and 60%. Because the recombinant Exo70 protein could only access the outer leaflet of the reconstituted LUV vesicles, the effective PI(4,5)P2 and PS in the reaction were only half of the total. As shown in Figure 2A, Exo70 bound to LUVs containing 5% PI(4,5)P2, but not to LUVs containing 100% neutral PC. Exo70 also bound to PS; however, the binding was weak unless the molar ratio of PS in the LUVs was raised to 60%. As a control, GST did not bind to LUVs with any lipid composition.
Figure 2.
The interaction between Exo70 and phospholipids in vitro. (A) GST-Exo70 purified from bacteria (0.15 μM) was incubated with liposomes (200 μM) containing 100% PC, 5% PI(4,5)P2, 20% PS, 40% PS, and 60% PS. After centrifugation at 150,000 × g for 30 min, the proteins in supernatant (S) and pellet (P) were subjected to SDS-PAGE and visualized by SYPRORed staining. Exo70 bound to vesicles containing 5% PI(4,5)P2 or 60% PS. It also bound weakly to vesicles containing 40% PS. GST did not bind to liposomes with any lipid composition in the cosedimentation assay. (B and C) The interaction of Exo70 with phospholipids. Exo70, 0.15 μM, was incubated with increasing concentrations of LUVs composed of (B) 100% PC, 5% PI(4,5)P2, 20% PS, 40% PS, and 60% PS or (C) PI(3)P, PI(4)P, PI(3,5)P2, PI(4,5)P2, and PI(3,4,5)P3. The percentage of bound Exo70 was plotted with the increasing liposome concentration with a single rectangular hyperbola equation (B = BmaxX/[Kd + X]) using SigmaPlot. Each point is the average of three measurements. Error bars, SD.
We have also measured the affinity of Exo70 for various lipids (Figure 2B). The bound Exo70 was quantified and plotted against the lipid concentration with the equation: B = BmaxX/[Kd + X], where Kd is the dissociation constant and X and B represent the concentrations of the free Exo70 and the bound Exo70, respectively. The Kd was obtained by nonlinear regression. As shown in Table 1, Exo70 bound to LUVs composed of 5% PI(4,5)P2 with a Kd of 13.9 ± 3.0 μM and bound to LUVs composed of 60% PS with a Kd of 46.3 ± 9.7 μM. The Kd value for PI(4,5)P2 would be much smaller if expressed in terms of pure PI(4,5)P2, because the molar ratio of PI(4,5)P2 is only 5% of the total lipids in the reconstituted LUVs. Overall, these results suggest that Exo70 may associate with the PM through its direct interaction with PI(4,5)P2.
Table 1.
Comparison of the affinities of Exo70 and exo70-1 for phospholipids
| LUV composition | Kd (μM) for Exo70 | Kd (μM) for exo70-1 |
|---|---|---|
| 100% PC | ≫400 | ≫400 |
| 20% PS | ≫400 | ≫400 |
| 60% PS | 46.30 ± 9.7 | 146.23 ± 14.6 |
| 5% PI(4,5)P2 | 13.92 ± 3.0 | ≫400 |
The Kd was obtained by rectangular hyperbola equation using the SigmaPlot software. For some of the bindings, the Kd was ≫400 because the binding never reached saturation.
To determine the selectivity of Exo70-PI(4,5)P2 interaction, we have tested the binding of Exo70 to four additional phosphoinositides: PI(3)P, PI(4)P, PI(3,5)P2, and PI(3,4,5)P3. In mammalian cells, PI(4)P and PI(4,5)P2 are the two most abundant phosphoinositides concentrated on the Golgi and PM, respectively (Di Paolo and De Camilli, 2006). PI(4,5)P2 comprises more than 95% of the bis-phosphoinositides in mammals (Bonangelino et al., 2002). PI(3)P and PI(3,5)P2 are mainly localized to the endosomal compartments (for reviews, see Behnia and Munro, 2005; Di Paolo and De Camilli, 2006). PI(3,4,5)P3 is present in negligible amount in mammalian cells at the “resting” state, but is up-regulated at specific domains of the PM in response to certain stimuli (Insall and Weiner, 2001). Recently, it was found that PI(3,4,5)P3 is also localized to the basolateral domain of MDCK cells (Gassama-Diagne et al., 2006). As shown in Figure 2C, Exo70 barely binds to PI(3)P; the affinity of Exo70 for PI(4,5)P2 (Kd = 13.92 ± 3.0 μM) is ∼4–5-fold higher than PI(3,5)P2 (Kd = 58.34 ± 9.2 μM) and more than 10-fold higher than PI(4)P (Kd = 173.12 ± 15.5 μM). The affinity of Exo70 for PI(3,4,5)P3 (Kd = 15.55 ± 4.6 μM) is comparable to that of PI(4,5)P2.
Mutations in Domain D Disrupt the Plasma Membrane Association of Exo70
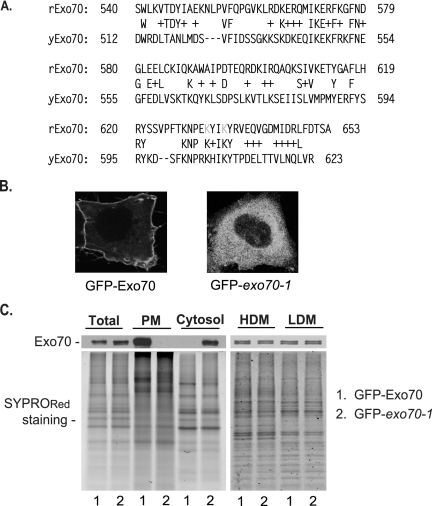
The direct binding of Exo70 with PI(4,5)P2 suggests a critical role for the domain D basic residues in the PM association of Exo70. To identify the responsible residues that are essential for Exo70 to bind to the PM, the conserved basic residues in domain D of Exo70 (Figure 3A) were targeted for mutagenesis and the resulting mutants were tested for their cellular localization (summarized in Supplementary Table 1). Among the 10 mutants we tested, 6 failed to associate with the PM. The other four mutations had no effect on Exo70 localization, suggesting that not all of the basic residues are involved in PI(4,5)P2 binding. We focused on one of the mutants, named exo70-1, in which residues K632 and K635 have been mutated to alanine. As shown in Figure 3B, GFP-tagged wild-type Exo70 (GFP-Exo70) was enriched at the PM, whereas GFP-tagged exo70-1 was distributed diffusely throughout the intracellular regions. These results indicate that K632 and K635 are required for the PM localization of Exo70.
Figure 3.
Mutations at Exo70 C-terminus abolish the association between Exo70 and the PM. (A) Sequence alignment between the C-termini (domain D) of yeast Exo70 and rat Exo70. The mutated residues in the exo70-1 mutant were marked in grey. (B) The localization of the exo70 mutant, exo70-1, in HeLa cells. HeLa cells were transfected with GFP-tagged wild-type Exo70 as a control (left) and exo70-1 (right). The exo70-1 mutant failed to associate with the PM. (C) Membrane fractionation was performed to examine the localization of GFP-Exo70 and GFP-exo70-1 in HeLa cells. Equal amounts of proteins from each fraction of the cells expressing Exo70 and exo70-1 were loaded on SDS-PAGE. The total proteins in each lane were detected by SYPRORed staining. Exo70 was detected by Western blot. LDM, low-density microsomal membranes, mostly the Golgi fraction; HDM, high-density microsomal membranes, mostly the ER fraction.
To directly test whether K632 and K635 are essential for the physical association of Exo70 with the PM, we performed subcellular membrane fractionation assay using HeLa cells transfected with GFP-tagged wild-type Exo70 or exo70-1. Cell lysates were fractionated, and the presence of Exo70 or exo70-1 in the PM, cytosol, and other fractions was examined. As shown in Figure 3C, wild-type Exo70 was found in the PM fraction, whereas exo70-1 was mostly present in the cytosol. The amount of Exo70 and exo70-1 in the HDM (mostly ER membrane) and LDM (mostly Golgi and endosomal compartments) fractions was almost the same. During our studies, we have noticed that GFP-tagging of Exo70 may facilitate its translocation from cytosol to the PM. It is likely that GFP-tagging causes conformational changes on Exo70, exposing the lipid-binding site on Exo70; and mutations on the C-terminus of Exo70 may abolish its membrane-association (see below). That may explain the observed clear PM versus cytosol distribution of GFP-Exo70 versus GFP-exo70-1 in Figure 3. The membrane fractionation data, together with the fluorescence localization results, suggest that K632 and K635 are critical residues in Exo70 that are involved in the physical association of Exo70 with the PM.
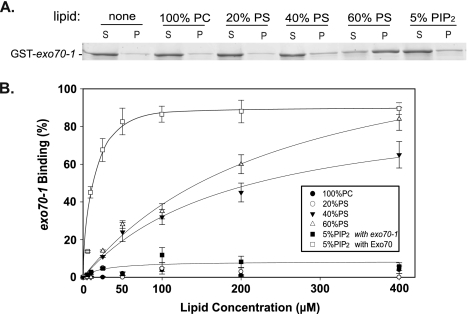
Next, we examined the interaction of the mutant exo70-1 protein with phospholipids in the cosedimentation assay. As shown in Figure 4 and Table 1, the interaction between exo70-1 and 5% PI(4,5)P2 was almost abolished, indicating that K632 and K635 are critical residues for the binding of Exo70 to PI(4,5)P2. Interestingly, the binding of exo70-1 for PS was only partially affected, suggesting that these two residues confer certain degree of specificity for PI(4,5)P2.
Figure 4.
Mutations at the C-terminus of Exo70 affect its interaction with phospholipids. (A) GST-tagged Exo70 and exo70-1 (0.15 μM) were used in the lipid cosedimentation assay. The binding of exo70-1 to 5% PI(4,5)P2 was abolished, whereas its interaction with 60% PS was reduced. (B) Binding curves of Exo70 and exo70-1 to LUVs containing various phospholipids.
It was previously shown that Exo70 interacts with the constitutively activated form of TC10 (Q67L; Inoue et al., 2003). We then tested whether mutations in exo70-1 affect this interaction. Cell lysates expressing GFP-tagged Exo70 or exo70-1 were incubated with GST-TC10 (Q67L) conjugated to the glutathione-Sepharose for binding reaction. Exo70 or the exo70 mutant bound to the Sepharose was detected by immunoblotting using an anti-GFP antibody. The amount of exo70-1 bound to the TC10 beads was comparable to that of the wild-type Exo70 (Supplementary Figure 1A). As a control, neither the wild-type Exo70 nor exo70-1 bound to the GST beads. The result indicates that mutating the two key basic residues in exo70-1 does not affect its interaction with TC10. Therefore, the loss of PM association of the exo70 mutant is unlikely resulted from impaired interaction with TC10. The binding results strongly suggest that Exo70 associates with the PM via its direct interaction with PI(4,5)P2. In addition to TC10, we have also tested the binding of exo70-1 with another exocyst component Sec8. We found that mutations on exo70-1 did not affect its interaction with Sec8 in the cell (Supplementary Figure 1B). This result is consistent with the previous reports in mammalian and yeast cells that the interaction of Exo70 with the other exocyst components for complex assembly is mediated by the N-terminal domains of Exo70 (Inoue et al., 2003; Dong et al., 2005).
Exo70 Recruits Sec8 to the Plasma Membrane
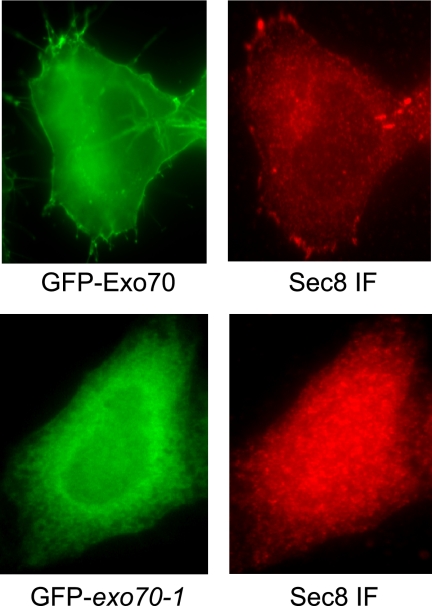
We next asked whether Exo70 is involved in recruiting other members of the exocyst to the membrane. It has been shown that the exocyst components were mostly located in the cytoplasm in cultured HeLa cells (Zuo et al., 2006). We then examined whether an increase of Exo70 at the PM would recruit Sec8 to the membrane. GFP-Exo70 and GFP-exo70-1 were expressed in HeLa cells, and Sec8 in these cells was detected by immunofluorescence staining using the anti-Sec8 mAb 2E12. As shown in Figure 5, both GFP-Exo70 and Sec8 were detected at the PM. On the contrary, in cells expressing GFP-exo70-1 that is deficient in binding PI(4,5)P2, Sec8 was found in the cytoplasm and intracellular membrane structures. This result suggests that the wild type, but not the mutant Exo70, is able to recruit the other exocyst components to the PM. Because exo70-1 maintains its ability to interact with Sec8, the loss of PM association of Sec8 in cells expressing GFP-exo70-1 is unlikely resulted from a defect in exocyst complex assembly.
Figure 5.
Exo70 is required for the PM localization of Sec8. GFP-Exo70 and GFP-exo70-1 were transfected into HeLa cells. Sec8 in the transfected cells was detected by the anti-Sec8 monoclonal antibody 2E12. Sec8 was detected at the PM in cells expressing GFP-Exo70 (top panel), but not in cells expressing GFP-exo70-1 (bottom panel).
Exo70 Is Essential for Exocytosis of VSV-G ts045 at the Plasma Membrane
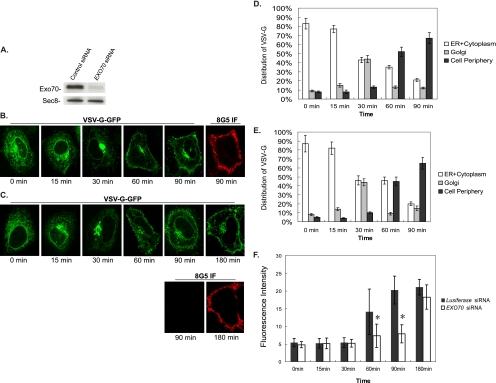
Because the exocyst functions to tether post-Golgi secretory vesicles at the PM, the physical interaction between Exo70 and phospholipids could be important for exocytosis. Here we examined the effect of Exo70 knockdown on the trafficking of a temperature-sensitive VSV-G mutant (ts045) to the PM. At the restrictive temperature (40°C), the VSV-G ts045 mutant is misfolded and retained in the ER. Shifting cells to 32°C allows correct folding and trafficking of the protein through the Golgi apparatus to the PM. We treated HeLa cells with EXO70 siRNA specific for human Exo70 (hExo70), and the luciferase siRNA was used as a control. The treatment reduced the level of Exo70 by more than 90% without affecting the level of other exocyst components such as Sec8 (Figure 6A). Cells treated with EXO70 siRNA or luciferase siRNA were transfected with GFP-tagged VSV-G mutant ts045 (VSV-G-GFP). Cells were then kept at 40°C overnight and shifted to 32°C for various times before being examined by fluorescence microscopy. The translocation of VSV-G-GFP inside the cells was detected by GFP fluorescence, whereas the incorporation of VSV-G-GFP to the PM (at the 90-min point) was evaluated by immunostaining using the 8G5 mAb against the extracellular domain of VSV-G (Lefrancois and Lyles, 1982). As shown in Figure 6B, in luciferase siRNA-treated cells, VSV-G-GFP was retained in the ER before the cells were shifted from 40 to 32°C (0 min). After the shift, the protein was translocated from ER to the Golgi apparatus around 30 min and then to the cell surface at 90 min. The exocytosis of VSV-G-GFP at the PM at 90 min was clearly detected by immunostaining of nonpermeabilized cells with the 8G5 antibody (Figure 6B). In EXO70 siRNA-treated cells, the translocation of VSV-G-GFP from the ER to the Golgi apparatus and from the Golgi to the PM followed time courses similar to that in control siRNA-treated cells. However, at 90 min, the extracellular exposure of VSV-G-GFP was barely detectable in nonpermeabilized cells; instead, it could only be detected after 180 min (Figure 6C), suggesting that the fusion of the exocytic vesicles with the PM was severely delayed. Quantification of the GFP signal in different intracellular compartments indicates that the cellular distributions of VSV-G-GFP at various time points after the temperature shift were similar in EXO70 siRNA and luciferase siRNA-treated cells (Figure 6, D and E): at 0 min, a majority of VSV-G-GFP was located at the ER (88% for luciferase siRNA treatment and 84% for EXO70 siRNA treatment); at 30 min, most of the VSV-G-GFP was located at the Golgi apparatus (91% for luciferase siRNA and 92% for EXO70 siRNA treatment); and at 90 min, VSV-G-GFP was located at the cell periphery (88% for luciferase siRNA and 74% for EXO70 siRNA treatment). In contrast, quantification of the surface VSV-G signal revealed a different pattern in EXO70 siRNA and luciferase siRNA-treated cells. As shown in Figure 6F, at 0-, 15-, and 30-min points, fluorescence intensity of exposed VSV-G was negligible both in control and EXO70 siRNA-treated cells, which suggests that VSV-G-GFP was translocating in the intracellular compartments; at 60 and 90 min, fluorescence intensity of exposed VSV-G was much less in EXO70 siRNA-treated cells than in control siRNA-treated cells; at 180 min, fluorescence intensity of surface VSV-G in EXO70 siRNA-treated cells began to appear at the cell surface. These results suggest that the fusion of the exocytic VSV-G vesicles with the PM was delayed in cells treated with EXO70 siRNA. These results demonstrated that Exo70 is probably not required for the trafficking of VSV-G from the intracellular compartments to the cell periphery, but is essential for the efficient tethering or subsequent docking/fusion of VSV-G–containing exocytic vesicles with the PM.
Figure 6.
Exocytosis of VSV-G ts045 is blocked in EXO70 siRNA knockdown cells. (A) HeLa cells were treated with EXO70 siRNA and Luciferase siRNA (as control). Exo70 was knocked down as detected by Western blot. The amount of Sec8 in these cells was not affected. The anti-Exo70 monoclonal antibody (13F3) and anti-Sec8 monoclonal antibody (2E12) were used in the Western blot analysis. (B) Luciferase siRNA-treated HeLa cells were transfected with VSV-G-GFP, kept at 40°C overnight, and shifted to 32°C for 0, 15, 30, 60, and 90 min in the presence of cycloheximide (100 μg/ml). The cells were then fixed and stained as described in Materials and Methods. VSV-G was transported from ER, to the Golgi, and to the PM. (C) In EXO70 knockdown cells, VSV-G transport to the PM through the endoplasmic reticulum and Golgi complex was normal. However, the incorporation of VSV-G (stained by the 8G5 monoclonal antibody recognizing the extracellular domain of VSV-G) was considerably delayed. Instead of being detected at the surface at 90 min as in control cells, VSV-G was detectable after 180 min from temperature arrest. (D and E) VSV-G association with various intracellular compartments was quantified in cells expressing luciferase siRNA (D) or EXO70 siRNA (E). For the quantification, boundaries of the whole cell, Golgi region and cell periphery region were outlined, and VSV-G fluorescence in these areas was then quantified using ImageJ 1.73v software after subtraction of background outside the cell. (F) Quantification of fluorescence intensity of surface VSV-G signal stained by the 8G5 monoclonal antibody. Boundary of the cell surface was outlined, and average fluorescence intensity of surface VSV-G signal was quantified using ImageJ 1.73v software and then divided by the perimeter of the cell surface. Three independent experiments (20 cells each) were carried out. Error bars, SD. p < 0.01.
The Interaction of Exo70 with PI(4,5)P2 Is Important for the Exocytosis of VSV-G ts045
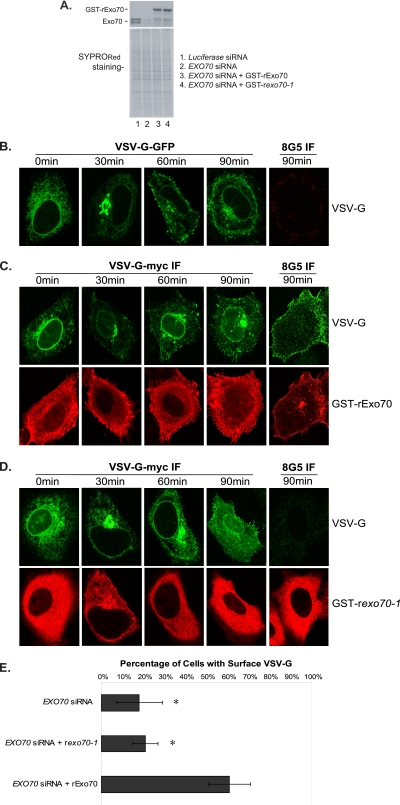
To investigate the role of the Exo70-PI(4,5)P2 binding in exocytosis, it is necessary to specifically disrupt the interaction between Exo70 and phospholipids in vivo. We therefore examined the transport of VSV-G in EXO70 siRNA knockdown cells transfected with the rat exo70 mutant, exo70-1, that is defective in the PI(4,5)P2 binding. EXO70 siRNA-treated cells expressing wild-type rat Exo70 (rExo70 WT) were used as control. Rat Exo70 is over 90% identical in protein sequence to human Exo70, yet on the nucleotide level, it cannot be targeted by the EXO70 siRNA oligos used in this study. The level of Exo70 knockdown and the expression of GST-rExo70 and GST-rexo70-1 are shown in Figure 7A.
Figure 7.
VSV-G ts045 exocytosis defect in EXO70 siRNA knockdown cells expressing the exo70-1 mutant. (A) EXO70 siRNA knockdown cells were transfected with GST-tagged rat Exo70 (GST-rExo70) or rat exo70 mutant (GST-rexo70-1). The amount of endogenous Exo70 in HeLa cells, and the amount of extragenically expressed GST-tagged rat Exo70 were detected by anti-Exo70 monoclonal antibody (top panel). The total proteins in the cell lysates were detected by SYPRORed staining (bottom panel). (B) VSV-G-GFP trafficking in HeLa cells transfected with EXO70 siRNA. The cells were grown at 40°C overnight after transfection. The cells were then shifted to 32°C for 0, 30, 60, and 90 min in the presence of cycloheximide, fixed, and stained as described in Materials and Methods. The 8G5 monoclonal antibody was used to detect the surface-incorporated VSV-G. (C and D) VSV-G-myc trafficking was examined in EXO70 siRNA knockdown cells expressing GST-rExo70 (C) or GST-rexo70-1 (D). VSV-G-myc and GST-tagged wild-type rExo70 (C) or exo70-1 (D) were cotransfected into EXO70 knockdown HeLa cells. VSV-G transport to the PM was rescued in the EXO70 siRNA knockdown cells expressing wild-type rExo70, whereas VSV-G transport was not rescued in the EXO70 siRNA knockdown cells expressing exo70-1. (E) Quantification of the percentage of cells with surface VSV-G staining.
In EXO70 siRNA-treated cells, only a diminutive amount of VSV-G-GFP was detected on the cell surface after 90 min of growth at the permissive temperature (Figure 7B). Expression of wild-type rat Exo70 restored normal surface incorporation of VSV-G (VSV-G-myc) in EXO70 siRNA knockdown cells (Figure 7C); therefore the rat Exo70 can serve as a rescue reagent for the RNAi experiment in HeLa cells. In contrast, in EXO70 siRNA-treated cells expressing the exo70-1 mutant that is defective in PI(4,5)P2 binding, the surface exposure of VSV-G-myc was diminished, whereas the translocation of VSV-G-myc from the intracellular compartments to the cell periphery was not affected (Figure 7D). VSV-G-myc rather than VSV-G-GFP was used here so that different pairs of proteins in the cells could be stained (GST-rExo70 and myc vs. GST-rExo70 and 8G5). Quantification of cells with surface VSV-G indicates that expressing wild-type rat Exo70 in EXO70 siRNA-treated cells restores VSV-G surface exposure in the majority of the cells (from 18 to 61%), whereas expressing rat exo70-1 does not have an obvious effect (Figure 7E). These results suggest that the interaction of Exo70 with membrane lipids is essential for the exocytosis of VSV-G at the PM.
DISCUSSION
To understand the molecular basis of vesicle tethering at the PM, it is important to elucidate how the tethering complex itself, the exocyst, is targeted to the PM. Here we identified a direct interaction between the exocyst component Exo70 and PI(4,5)P2; and demonstrated that this interaction is important for the recruitment of the exocyst to the PM. Furthermore, disruption of this interaction blocked later stages of exocytosis of post-Golgi secretory vesicles at the PM.
PI(4,5)P2 and PS are the major negatively charged lipids in the PM. The fact that Exo70 binds to both 5% PI(4,5)P2 and 60% PS indicates that the interaction of Exo70 with the phospholipids is electrostatic in nature. This type of interaction has been found in a number of proteins, such as N-WASP and MARCKS (McLaughlin and Murray, 2005). Comparing the charges of PI(4,5)P2 versus PS at the physiological pH, LUVs composed of 5% PI(4,5)P2 have approximately the same amount of effective charges as LUVs composed of 15–20% PS. However, the interaction of Exo70 with LUVs composed of 20% PS is much weaker. Mutations on exo70-1 that eliminate some of the positive charges in the Exo70 C-terminus nearly abolished the ability of Exo70 to bind PI(4,5)P2; however, the ability of this mutant to bind PS at high concentrations (≥60%) was only partially affected. These data suggest that Exo70 has significant binding specificity for PI(4,5)P2 over PS. We have also examined the interaction of Exo70 with other phosphoinositides and observed its selectivity for PI(4,5)P2 over the stereoisomeric PI(3,5)P2 and other monophosphorylated phosphoinositides. We have also found that Exo70 binds PI(3,4,5)P3 with an affinity that is comparable to that for PI(4,5)P2. PI(3,4,5)P3 has recently been found to be localized to the basolateral domain in MDCK cells (Gassama-Diagne et al., 2006), and the exocyst has been implicated in basolateral vesicle targeting (Grindstaff et al., 1998). In other types of mammalian cells, although the concentration of PI(3,4,5)P3 is low at the PM in resting cells, it can be rapidly up-regulated in response to extracellular stimuli. It is therefore possible that Exo70 binds to PI(3,4,5)P3 under certain physiological circumstances or in certain cell types.
In yeast, we have found that the amount of the exocyst complex associated with the PM was much lower in the temperature-sensitive mss4 mutant cells, in which the PI(4,5)P2 level in the PM was reduced (data not shown), indicating that PI(4,5)P2 mediates the membrane targeting of the exocyst. Moreover, the structural analysis of Exo70 provided important insights into the potential mechanism of membrane association of Exo70 (Dong et al., 2005; Hamburger et al., 2006). On the basis of the crystal structure information of yeast Exo70, we have made mutations on the rat Exo70 residues K632 and K635 (exo70-1), which are positively charged amino acids well conserved in the yeast Exo70 sequence. Our in vitro vesicle sedimentation experiments demonstrated that these mutations disrupted the PI(4,5)P2-binding. Furthermore, the VSV-G trafficking analysis further demonstrated its functional importance in exocytosis at the PM. While we were preparing this article, Moore et al. (2007) resolved the crystal structure of mouse Exo70. Analysis of the structure indicates that the point mutations on exo70-1 are localized on the loop between Helix 18 and 19 on the surface of the mouse Exo70, which is almost identical to that of the yeast Exo70.
Taking advantage of the VSV-G trafficking assay, we were able to analyze the role of Exo70 in various stages of membrane traffic. More importantly, using the exo70-1 mutant in the EXO70 RNAi knockdown cells, we were able to specifically examine the functional significance of Exo70- PI(4,5)P2 interaction in VSV-G exocytosis. The exocyst has been found in various cellular compartments, including Golgi and recycling endosomes, in addition to the PM (Yeaman et al., 2001; Fölsch et al., 2003; Ang et al., 2004; Langevin et al., 2005). Here we found that the Exo70-PI(4,5)P2 interaction is not involved in the early stages of VSV-G trafficking through the endoplasmic reticulum and Golgi. Rather, it is critical for the PM events such as vesicle tethering and fusion. When the Exo70-PI(4,5)P2 interaction was disrupted, the transport of VSV-G to the PM was barely changed. However, the incorporation of VSV-G into the PM was significantly affected, as revealed by the 8G5 antibody specifically recognizing the extracellular domain of VSV-G. Similarly the exocyst has been implicated in tethering and fusion of Glut4-containing vesicles in 3T3-L1 adipocytes (Inoue et al., 2003; Ewart et al., 2005; Tsuboi et al., 2005). It is possible that other exocyst components also interact with phospholipids (Moskalenko et al., 2003). However, specific disruption of Exo70-PM interaction is sufficient to block exocytosis in mammalian cells.
The association of the exocyst with the PM is an important step in vesicle tethering. When and where this interaction takes place may regulate the kinetics and location of exocytosis. In the budding yeast S. cerevisiae, the exocyst complex is specifically localized to the growing tip of the daughter cell (the “bud tip”), which is the site of active exocytosis and cell surface expansion. Moreover, Exo70 primarily functions at the early stages of the yeast cell cycle, suggesting a temporal control of Exo70 function (He et al., 2007). In mammalian cells, growth factor signaling involving small GTPases may mediate the subunits assembly, translocation of the exocyst from intracellular compartments to, or activation of the exocyst at, the PM (Sugihara et al., 2002; Moskalenko et al., 2002, 2003; Inoue et al., 2003; Takaya et al., 2004; Zuo et al., 2006). Future work will be focused on the identification and characterization of proteins that temporally and/or spatially regulate Exo70 and other exocyst components using different eukaryotic systems.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Drs. Margaret Chou, Michael Marks (University of Pennsylvania), and Zhaohui Xu (University of Michigan) for their constructive discussions and Dr. Michael Ostap (University of Pennsylvania) for advice on lipid binding experiments. We thank Drs. Douglas Lyles (Wake Forest University) and Shu-Chan Hsu (Rutgers University) for the valuable antibodies used in the experiments. This work is supported by grants from National Institutes of Health (RO1-GM64690), American Cancer Society, and the Pew Scholars Program to W.G.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-05-0461) on August 29, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Ang A. L., Taguchi T., Francis S., Fölsch H., Murrells L. J., Pypaert M., Warren G., Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J. Cell Biol. 2004;167(3):531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla T. Inositol-lipid binding motifs: signal integrators through protein-lipid and protein-protein interactions. J. Cell Sci. 2005;118:2093–2104. doi: 10.1242/jcs.02387. [DOI] [PubMed] [Google Scholar]
- Behnia R., Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. doi: 10.1038/nature04397. [DOI] [PubMed] [Google Scholar]
- Bonangelino C. J., Nau J. J., Duex J. E., Brinkman M., Wurmser A. E., Gary J. D., Emr S. D., Weisman L. S. Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. J. Cell Biol. 2002;156(6):1015–1028. doi: 10.1083/jcb.200201002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C., Hughes T., Pypaert M., Novick P. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J. Cell Biol. 2004;167(5):889–901. doi: 10.1083/jcb.200408124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennwald P., Kearns B., Champion K., Keranen S., Bankaitis V., Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79(2):245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Di Paolo G. P., De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443(7112):651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Dong G., Hutagalung A. H., Fu C., Novick P., Reinisch K. M. The structures of exocyst subunit Exo70p and the Exo84p C-terminal domains reveal a common motif. Nat. Struct. Mol. Biol. 2005;12:1094–1100. doi: 10.1038/nsmb1017. [DOI] [PubMed] [Google Scholar]
- Finger F. P., Hughes T. E., Novick P. Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell. 1998;92(4):559–571. doi: 10.1016/s0092-8674(00)80948-4. [DOI] [PubMed] [Google Scholar]
- Ewart M. A., Clarke M., Kane S., Chamberlain L. H., Gould G. W. Evidence for a role of the exocyst in insulin-stimulated Glut4 trafficking in 3T3–L1 adipocytes. J. Biol. Chem. 2005;280(5):3812–3816. doi: 10.1074/jbc.M409928200. [DOI] [PubMed] [Google Scholar]
- Fölsch H., Pypaert M., Maday S., Pelletier L., Mellman I. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J. Cell Biol. 2003;163:351–362. doi: 10.1083/jcb.200309020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassama-Diagne A., Yu W., ter Beest M., Martin-Belmonte F., Kierbel A., Engel J., Mostov K. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat. Cell Biol. 2006;8(9):963–970. doi: 10.1038/ncb1461. [DOI] [PubMed] [Google Scholar]
- Grindstaff K. K., Yeaman C., Anandasabapathy N., Hsu S. C., Rodriguez-Boulan E., Scheller R. H., Nelson W. J. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93(5):731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- Guo W., Roth D., Walch-Solimena C., Novick P. The exocyst is an effector for Sec4p targeting secretory vesicles to sites of exocytosis. EMBO J. 1999;18(4):1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Sacher M., Barrowman J., Ferro-Novick S., Novick P. Protein complexes in transport vesicle targeting. Trends Cell Biol. 2000;10(6):251–255. doi: 10.1016/s0962-8924(00)01754-2. [DOI] [PubMed] [Google Scholar]
- Hamburger Z. A., Hamburger A. E., West A. P., Jr, Weis W. I. Crystal structure of the S. cerevisiae exocyst component Exo70p. J. Mol. Biol. 2006;356(1):9–21. doi: 10.1016/j.jmb.2005.09.099. [DOI] [PubMed] [Google Scholar]
- He B., Xi F., Zhang J., TerBush D., Zhang X., Guo W. Exo70p mediates the secretion of specific exocytic vesicles at early stages of the cell cycle for polarized cell growth. J. Cell Biol. 2007;176(6):771–777. doi: 10.1083/jcb.200606134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokanson D., Ostap M. Myo1c binds tightly and specifically to phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate. Proc. Natl. Acad. Sci. USA. 2006;103(9):3118–3123. doi: 10.1073/pnas.0505685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazuka C. D., Foletti D. L., Hsu S. C., Kee Y., Hopf F. W., Scheller R. H. The sec6/8 complex is located at neurite outgrowth and axonal synapse-assembly domains. J. Neurosci. 1999;19(4):1324–1334. doi: 10.1523/JNEUROSCI.19-04-01324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. C., TerBush D., Abraham M., Guo W. The exocyst complex in polarized exocytosis. Int. Rev. Cytol. 2004;233:243–265. doi: 10.1016/S0074-7696(04)33006-8. [DOI] [PubMed] [Google Scholar]
- Inoue M., Chang L., Hwang J., Chiang S. H., Saltiel A. R. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422(6932):629–633. doi: 10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- Insall R. H., Weiner O. D. PIP3, PIP2, and cell movement—similar messages, different meanings? Dev. Cell. 2001;1(6):743–747. doi: 10.1016/s1534-5807(01)00086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin J, Morgan M. J., Sibarita J. B., Aresta S., Murthy M., Schwarz T., Camonis J., Bellaiche Y. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev. Cell. 2005;9(3):355–376. doi: 10.1016/j.devcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Lefrancois L., Lyles D. S. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. II. Monoclonal antibodies of nonneutralizing and cross-reactive epitopes of Indiana and New Jersey serotypes. Virology. 1982;121(1):168–174. doi: 10.1016/0042-6822(82)90126-x. [DOI] [PubMed] [Google Scholar]
- Matern H. T., Yeaman C., Nelson W. J., Scheller R. H. The Sec6/8 complex in mammalian cells: characterization of mammalian Sec3, subunit interactions, and expression of subunits in polarized cells. Proc. Natl. Acad. Sci. USA. 2001;17:9648–9653. doi: 10.1073/pnas.171317898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S., Wang J., Gambhir A., Murray D. PIP2 and proteins: interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- McLaughlin S., Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438(7068):605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- Moore B. A., Robinson H. H., Xu Z. The crystal structure of mouse Exo70 reveals unique features of the mammalian exocyst. J. Mol. Biol. 2007;371(2):410–421. doi: 10.1016/j.jmb.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalenko S., Henry D. O., Rosse C., Mirey G., Camonis J. H., White M. A. The exocyst is a Ral effector complex. Nat. Cell Biol. 2002;1:66–72. doi: 10.1038/ncb728. [DOI] [PubMed] [Google Scholar]
- Moskalenko S., Tong C., Rosse C., Mirey G, Formstecher E., Daviet L., Camonis J., White M. A. Ral GTPases regulate exocyst assembly through dual subunit interactions. J. Biol. Chem. 2003;278(51):51743–51748. doi: 10.1074/jbc.M308702200. [DOI] [PubMed] [Google Scholar]
- Munson M., Novick P. The exocyst defrocked, a framework of rods revealed. Nat. Struct. Mol. Biol. 2006;13(7):577–581. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- Papayannopoulos V., Co C., Prehoda K. E., Snapper S., Taunton J., Lim W. A. A polybasic motif allows N-WASP to act as a sensor of PIP(2) density. Mol. Cell. 2005;17(2):181–191. doi: 10.1016/j.molcel.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R. Transport-vesicle targeting: tethers before SNAREs. Nat. Cell Biol. 1999;1(1):E17–E22. doi: 10.1038/8967. [DOI] [PubMed] [Google Scholar]
- Rosse C., Hatzoglou A., Parrini M. C., White M. A., Chavrier P., Camonis J. RalB mobilizes the exocyst to drive cell migration. Mol. Cell Biol. 2006;26:727–734. doi: 10.1128/MCB.26.2.727-734.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara K., Asano S., Tanaka K., Iwamatsu A., Okawa K., Ohta Y. The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nat. Cell Biol. 2002;4:73–78. doi: 10.1038/ncb720. [DOI] [PubMed] [Google Scholar]
- Takaya A., Ohba Y., Kurokawa K., Matsuda M. RalA activation at nascent lamellipodia of epidermal growth factor-stimulated Cos7 cells and migrating Madin-Darby canine kidney cells. Mol. Biol. Cell. 2004;15:2549–2557. doi: 10.1091/mbc.E03-11-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush D. R., Novick P. Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J. Cell Biol. 1995;130(2):299–312. doi: 10.1083/jcb.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T., Ravier M. A., Xie H., Ewart M. A., Gould G. W., Baldwin S. A., Rutter G. A. Mammalian exocyst complex is required for the docking step of insulin vesicle exocytosis. J. Biol. Chem. 2005;280(27):25565–25570. doi: 10.1074/jbc.M501674200. [DOI] [PubMed] [Google Scholar]
- Vega I. E., Hsu S. C. The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth. J. Neurosci. 2001;21(11):3839–3848. doi: 10.1523/JNEUROSCI.21-11-03839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Liu Y, Adamson C. L., Valdez G., Guo W., Hsu S.-C. The mammalian exocyst, a complex required for exocytosis, inhibits tubulin polymerization. J. Biol. Chem. 2004;279:35958–35966. doi: 10.1074/jbc.M313778200. [DOI] [PubMed] [Google Scholar]
- Wang S., Hsu S. C. The molecular mechanisms of the mammalian exocyst complex in exocytosis. Biochem. Soc. Trans. 2006;34(Pt 5):687–690. doi: 10.1042/BST0340687. [DOI] [PubMed] [Google Scholar]
- Waters M. G., Hughson F. M. Membrane tethering and fusion in the secretory and endocytic pathways. Traffic. 2000;1(8):588–597. doi: 10.1034/j.1600-0854.2000.010802.x. [DOI] [PubMed] [Google Scholar]
- Weber T. M., Joost H., Simpson I. A., Cushman S. W. Receptor Biochemistry and Methodology. In: Kahn R. C., Harrison L. C., editors. New York: Alan R. Liss; 1988. pp. 171–187. [Google Scholar]
- Whyte J. R., Munro S. Vesicle tethering complexes in membrane traffic. J. Cell Sci. 2002;115(Pt 13):2627–2637. doi: 10.1242/jcs.115.13.2627. [DOI] [PubMed] [Google Scholar]
- Xu K. F., Shen X., Li H., Pacheco-Rodriguez G., Moss J., Vaughan M. Interaction of BIG2, a brefeldin A-inhibited guanine nucleotide-exchange protein, with exocyst protein Exo70. Proc. Natl. Acad. Sci. USA. 2005;102:2784–2789. doi: 10.1073/pnas.0409871102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C., Grindstaff K. K., Wright J. R., Nelson W. J. Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells. J. Cell Biol. 2001;155(4):593–604. doi: 10.1083/jcb.200107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X., Zhang J., Zhang Y., Hsu S. C., Zhou D., Guo W. Exo70 interacts with the Arp2/3 complex and regulates cell migration. Nat. Cell Biol. 2006;8(12):1383–1388. doi: 10.1038/ncb1505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.