Abstract
The lens is an avascular tissue, separated from the aqueous and vitreous humors by its own extracellular matrix, the lens capsule. Here we demonstrate that the lens capsule is a source of essential survival factors for lens epithelial cells. Primary and immortalized lens epithelial cells survive in low levels of serum and are resistant to staurosporine-induced apoptosis when they remain in contact with the lens capsule. Physical contact with the capsule is required for maximal resistance to stress. The lens capsule is also a source of soluble factors including fibroblast growth factor 2 (FGF-2) and perlecan, an extracellular matrix component that enhances FGF-2 activity. Matrix metalloproteinase 2 (MMP-2) inhibition as well as MMP-2 pretreatment of lens capsules greatly reduced the protective effect of the lens capsule, although this could be largely reversed by the addition of either conditioned medium or recombinant FGF-2. These data suggest that FGF-2 release from the lens capsule by MMP-2 is essential to lens epithelial cell viability and survival.
INTRODUCTION
Cell survival requires the continued exposure to specific survival factors. In a classic study (Ishizaki et al., 1993), lens epithelial cells were shown to survive in culture, in the absence of cell–cell contact, as long as the right growth factor cues were available. These factors could be provided exogenously either in the form of conditioned media or in serum supplement (Ishizaki et al., 1993), leading to the widely accepted general hypothesis that cells are programmed to die unless the appropriate signals are received.
The recent proposal of the extracellular matrix (ECM) reservoir hypothesis suggests that the ECM itself can act as a reservoir for growth and survival factors (Bergers et al., 2000; Mott and Werb, 2004) that are released via the action of various matrix metalloproteinases (MMPs; reviewed in McCawley and Matrisian, 2001; Mott and Werb, 2004). MMPs have been shown to release fibroblast growth factor (FGF)-2 (MMP-1, -3; Whitelock et al., 1996) and fibroblast growth factor receptor (FGFR)-1 (MMP-2; Levi et al., 1996), activate transforming growth factor (TGF)-β1/2 (Yu and Stamenkovic, 2000), and to both activate and release insulin-like growth factor (IGF)-1 (MMP-1, -2, -3, and -9; Fowlkes et al., 1994; and MMP-2, -3, and -7; Imai et al., 1997). MMPs are therefore an essential component in this proposed role for the ECM in cell survival and cell proliferation (Ishizuya-Oka et al., 2000; Wiseman et al., 2003). From such data, we can see that the ECM reservoir hypothesis requires three essential components: an ECM, growth factors sequestered by the ECM, and the presence of MMPs to release the growth factors from the ECM. This hypothesis changes our perception of the ECM as it can no longer be considered to solely provide a physical support or appropriate cues for integrin signaling (Hynes, 1992; Adams and Watt, 1993), but is also a source of cell proliferation and survival factors (Klagsbrun, 1990; Taipale and Keski-Oja, 1997; Bergers et al., 2000; Mott and Werb, 2004; Tran et al., 2004).
This reservoir hypothesis was proposed on the basis of studies on angiogenesis and tumor progression. For instance, vascular endothelial growth factor (VEGF) release is necessary for tumor progression by driving the activation of quiescent cells in the vasculature (Bergers et al., 2000), and MMPs were shown to increase the bioavailability of VEGFs needed for this (Hashimoto et al., 2002). The ECM reservoir hypothesis clearly has implications for a broad range of other cell biological situations, including tumor metastasis (Fidler, 2002) and the establishment of stem cell niches (Roberts et al., 1988; Carter et al., 2004; Leone et al., 2005).
In the experimental systems used to propose the ECM reservoir hypothesis, the specific origin and site of growth factor sequestration or even which cells produce the MMPs (Bergers et al., 2000; Wiseman et al., 2003) are hard to identify. In many cases it is activities derived from other cells that release growth factors from the ECM (Bergers et al., 2000; Mott and Werb, 2004). We decided, therefore, to examine the reservoir hypothesis of the ECM in a defined system, namely the eye lens epithelium and its associated ECM, the lens capsule, because the lens can be isolated intact and free from other contaminating cells and tissues.
In this study, we show that the lens capsule maintains lens epithelial cell viability even in the presence of apoptogens. We confirm that lens epithelial cells produce several MMPs including MMP-2, which releases FGF-2 from the lens capsule as proof of principle for the ECM-reservoir hypothesis in this system. MMP-2 is the most important MMP for epithelial cell viability in this system, and our data show that this activity, in combination with FGF-2 release, are key aspects to the survival of lens epithelial cells on the lens capsule.
MATERIALS AND METHODS
Bovine Lens Capsule Processing
Whole bovine eyes were obtained from Northern Counties Meat Group (Sunderland, United Kingdom). The lenses were dissected out of the eyes within <12 h after culling and left overnight in DMEM (Sigma-Aldrich, Poole, United Kingdom) supplemented with 0.1% (vol/vol) fetal calf serum (Sigma-Aldrich), 2 mM l-glutamine, 200 U/l penicillin, and 0.2 mg/l streptomycin in a 37°C, 5% (vol/vol) CO2 incubator. The next morning, the lens capsules were dissected by placing the anterior capsule face-down and by cross-cutting and pinning down the posterior capsule onto Sylgard-covered (Dow Corning, Barry, United Kingdom) wells of a 12-well plate, with the inside of the capsular bag facing upward, after which the fiber mass was removed. When these capsules were to be seeded with cells, the capsules were then trypsinized for 1 h (trypsin-EDTA provided by Sigma-Aldrich), washed once in phosphate-buffered saline (PBS), once in 70% (vol/vol) ethanol, and twice in PBS, after which they were left in PBS overnight in an incubator maintained at 37°C and 5% (vol/vol) CO2.
Cells and Cell Lines
The cell line H36CE2 was established from the central epithelium of a lens from a 3-y-old postmortem donor who died in 1994. These cells were immortalized with SV40 Large-T antigen, expressed in adenovirus, as described previously (Andley et al., 1994; Kleinjan et al., 2001). Karyotype characterization was undertaken (Rooney and Czepulkowski, 1992) and was in broad agreement with previously published data for a SV40 Large-T antigen immortalized lens epithelial cell line, B3 (Andley et al., 1994). RT-PCR confirmed that the H36CE2 cells retained a lens epithelial cell phenotype (αA-crystallin, αB-crystallin, and FoxE3 positive). RNA was extracted from the cells using the GenElute Mammalian Total RNA kit (Sigma-Aldrich), and cDNA was generated according to the manufacturer's instructions (Superscript First strand synthesis system, Invitrogen, Paisley, United Kingdom). Primary bovine lens epithelial cells were removed from lens capsules by trypsinization, seeded onto plastic dishes, and grown in DMEM supplemented with glutamine, penicillin, streptomycin, and 10% (vol/vol) FBS.
Cell Viability Studies
Cells were seeded at a concentration of 50,000 cells/ml in 12-well plates with or without a lens capsule covering the bottom of the well. Cells were cultured in high serum (10% [vol/vol] fetal calf serum) or low serum (0.1% [vol/vol] fetal calf serum) supplemented media. Viable cell number was determined at various time points after seeding, by the one-step CellTiter 96 Aqueous NonRadioactive Cell proliferation Assay kit (Promega, Southampton, United Kingdom) following manufacturer's instructions. Briefly, after a change of media, 100 μl of reagent was added to 500 μl of media in the presence of the cells and left for 90 min after which media samples were read on an ANTHOS LUCY1 plate reader (Anthos Labtec Instruments, Leighton Buzzard, United Kingdom) at 490 nm and absorbance values recorded. Staurosporine was added 48 h after seeding to allow cells to adjust to their respective substrates. MMP inhibitors were added at the time of seeding the cells to prevent all MMP-ECM interactions from the start of the experiment. Staurosporine (Tamaoki et al., 1986; Oncogene Research Products, Nottingham, United Kingdom) was used at 0.5 μM (protein kinase A [PKA] IC50 = 7 nM, PKC IC50 = 0.7 nM, PKG IC50 = 8.5 nM), OA-Hy (Emonard et al., 1999; Calbiochem, Nottingham, United Kingdom) at 100 μM (Ki = 1.7 μM) and MMP inhibitor II (Pikul et al., 1998; Calbiochem) at 5 nM (MMP-1 IC50 = 24 nM, MMP-3 IC50 = 18.4 nM, MMP-7 IC50 = 30 nm, and MMP-9 IC50 = 2.7 nM). The concentration chosen for the MMP inhibitor II is selective for MMP-9 (see IC50 values above), whereas the concentration chosen for the OA-Hy has been shown to be selective for MMP-2 inhibition (Polette et al., 1999), which we have confirmed independently by zymography (data not shown).
TUNEL Studies
Cells were cultured in high serum (10% [vol/vol] fetal calf serum) supplemented media with or without 0.5 μM staurosporine on either glass coverslips or bovine lens capsules cleaned of all primary cells. After 48 h, cell apoptosis was assessed by TUNEL staining (terminal dUTP nicked-end labeling) and propidium iodide counterstaining (Boehringer-Mannheim, Lewes, United Kingdom) following the manufacturer's protocol.
Apoptotic Morphology Quantification
Cells were cultured on either glass coverslips or bovine lens capsules cleaned of all primary cells. Cell culture conditions were high serum (10% [vol/vol] fetal calf serum) supplemented medium with or without 0.5 μM staurosporine. After 48-h exposure to staurosporine, cells were fixed in 4% wt/vol paraformaldehyde and stained with DAPI (1 μg/ml in PBS) in order to visualize the nuclei. They were then mounted in Citifluor and examined with fluorescence microscopy using a Zeiss Axioplan microscope (Welwyn Garden City, United Kingdom). The capsules were mounted by being placed, cells-up, onto a microscope slide. The coverslips or capsules were examined, and cells were counted under 40× magnification until either a total of 1000 cells had been counted or until 100 fields had been examined, whichever came first. Each cell counted was scored as either normal or apoptotic (with compressed and/or fragmented chromatin; i.e., a pyknotic nucleus). The number of apoptotic nuclei was then expressed as a percentage of the total number of cells counted. Three independents repeats were made for each data point, and the significance of the results gathered was assayed by χ2 test.
Coculture and Conditioned-Media Experiments
Isolated bovine lens capsules were cleaned of cells as described above. For the coculture experiments, a capsule was placed in the bottom chamber of the well in a 12-well plate as required. A semipermeable membrane insert (Falcon/Becton Dickinson, Plymouth, United Kingdom) was added to each well, giving a top chamber, which contained a coverslip seeded with 12,500 cells. This cell number was calculated to reflect the difference between the surface area of the coculture insert compared with the area of the lower well to ensure that the starting cell density remained constant for both chambers. For the conditioned-media experiment, lens capsules were kept in low serum (0.1% [vol/vol] fetal calf serum) supplemented media for 72 h. In some instances, the lens capsules were pretreated with 80 ng of human recombinant MMP-2 in low serum media (R&D Systems, Abingdon, United Kingdom). These conditioned media were sometimes added back to fresh lens capsules. Cell viability was monitored by the one-step CellTiter 96 Aqueous NonRadioactive Cell proliferation Assay kit (Promega) as described above.
Cell Cycle Analysis
H36CE2 cells were cultured in 10% (vol/vol) fetal calf serum-supplemented cell culture medium on either uncoated plastic dishes or bovine lens capsules that had been cleaned of all primary cells, at a seeding density of 50,000 cells per well. After 48 h, the cells were processed for cell cycle analysis following the CyStain DNA 2step (PARTEC, Münster, Germany) protocol. Briefly, cells were detached with trypsin, spun down for 5 min at 1000 rpm, and then resuspended in nuclear extraction buffer and left at room temperature for 20 min, after which cells were spun down for 10 min at 1000 rpm, fixed in cold 70% ethanol, and left at −20°C until batch analysis. Immediately before analysis, cells were spun down and resuspended in nuclear extraction buffer and then left at room temperature for 10 min, before addition of the staining solution. Samples were run on a FACSAria flow cytometer (Becton Dickinson) and analyzed using a 60 mW UV laser. Doublets were discriminated against using Area versus Width of the 450-nm signal from DAPI. Data were analyzed by FlowJo software (Tree Star, Ashland, OR), and the G1, S, and G2 ratio was determined by the Watson (pragmatic) method provided within the FlowJo software.
Real-Time Analysis Using TaqMan Low-Density Arrays
RNA was extracted from the cells using the GenElute Mammalian Total RNA kit (Sigma-Aldrich) and cDNA generated according to the manufacturer's instructions (Superscript First-strand Synthesis System, Invitrogen). TaqMan low-density array (LDA) enables quantitative real-time PCR in a 384-well low-density array. Pre-designed TaqMan primers and probes sets for 48 target genes were chosen from an on-line catalogue (http://www.appliedbiosystems.com/) and manufactured by Applied Biosystems (Foster City, CA). Each array contained eight sample ports, with each feeding 48 reaction wells. LDAs were run according to the manufacturer's protocol. Briefly, each sample port was loaded with 100 μl PCR mix (100 ng of total RNA converted to cDNA and 2× TaqMan Universal PCR mix; Applied Biosystems). Thermal cycling conditions were as follows: 50°C, 2 min; 94.5°C, 10 min; 40 cycles of 97°C, 30 s; and 59.7°C for 1 min and were performed on the ABI Prism 7900HT sequence detection system (Applied Biosystems, Warrington, United Kingdom). Expression levels of target genes were normalized to GAPDH. Data were analyzed using 7900HT system and SDS software. The cycle threshold (Ct) value reflects the cycle number at which the fluorescence generated within a reaction crosses the threshold. Gene expression values were calculated based on the ΔCt method where ΔCt represents the Ct of the target minus that of GAPDH. Due to the exponential nature of PCR, ΔCt was converted to linear form using the equation: 2−(ΔCt), to give the relative quantity of the target gene expression. The amplification efficiencies associated with TaqMan gene expression assays designed by ABI are equal and thus allow expression levels of target genes to be compared with values obtained for cells grown on plastic being used as the baseline.
Immunoblotting
Immunoblotting was used to confirm the presence of gelatinases in conditioned medium from H36CE2 cells grown on plastic and on the lens capsule. Conditioned media samples were collected after 96-h culture and concentrated 25-fold using an Amicon Ultra-4 centrifuge filter with a 30K nominal molecular weight limit (Millipore, Molsheim, France) as described elsewhere (Fuchshofer et al., 2003). For MMP-2 detection the culture medium used was serum-free to prevent bovine serum albumin (BSA) obscuring the MMP-2 signal. For MMP-9 detection, the culture medium used was supplemented with 0.1% (vol/vol) serum in accordance with the rest of our experiments. Samples were separated for 1 h at 200 V on a 12% (wt/vol) acrylamide gel and blotted onto a nitrocellulose membrane (Whatman, Dassel, Germany) for 2 h. The membrane was washed in blocking buffer (5% [wt/vol] milk in TTBS) for 1 h at room temperature, after which the membrane was incubated in the primary antibody solution overnight at 4°C, rinsed twice, and incubated with the secondary antibody solution for 1 h at 37°C. After further rinsing, the membrane was incubated for 5 min in the dark in enhanced chemiluminescence (ECL) solution, and the image was collected using a cooled CCD camera system (Fujifilm Intelligent Dark Box II, Tokyo, Japan). Images were processed using the companion computer program LAS-1000 and Adobe Photoshop CS (San Jose, CA). Mouse monoclonal antibodies to MMP-2 and MMP-9 (clone 101724 and 4H3, respectively; R&D Systems) were used that detect both latent and active forms of these MMPs. Human recombinant latent MMP-2 and MMP-9 (R&D Systems) were used as positive controls.
Immunofluorescence Confocal Microscopy and Scanning Electron Microscopy
Bovine lens capsules that had been cleaned of all primary cells as described above were treated with 80 ng of recombinant human MMP-2 (R&D Systems) per capsule for 72 h in substrate buffer (5 mM CaCl2, 50 mM Tris, pH 8.0). For immunofluorescence microscopy analysis, 10-μm frozen sections were made using a Leica CM3050S cryostat (Milton Keynes, United Kingdom), and sections were fixed in 4% paraformaldehyde for 10 min, permeabilized in 1% NP40 in PBS for 15 min, and blocked in 10% goat serum in PBS for 20 min. Primary polyclonal anti-FGF-2 (clone PC16, Calbiochem) and monoclonal anti-perlecan (clone A7L6, Chemicon) antibodies were used. Secondary antibodies used were TRICT anti-rabbit IgG (Sigma-Aldrich) and fluorescein isothiocyanate (FITC) anti-mouse IgG (Scottish Antibody Production Unit, Carluke, Scotland). Pictures were taken using a Bio-Rad Microradiance confocal microscope coupled to Lasersharp 2000 software (Bio-Rad Laboratories, Ltd., Hemel Hampstead, United Kingdom). For scanning electron microscopy analysis, whole lens capsules were fixed in 2% gluteraldehyde in PBS overnight, washed two times for 10 min in 0.2 M sodium cacodylate, pH 7.4, incubated in 1% osmium tetroxide in 0.2 M sodium cacodylate, pH 7.4 for 1 h, washed 10 min in water, dehydrated through ethanol series (50, 75, 90, 95, 100, and 100%, 5 min each), critical point-dried with CO2, and coated with 5 nm platinum in a Cressington 308 coater. They were then viewed in a Hitachi S-5200 feSEM at 10 kV accelerating voltage.
ELISA Measurement of Growth Factor Levels
FGF-2 ELISA (R&D Systems) was conducted according to manufacturer's instructions. The assay measured both free and bound FGF-2 forms. Samples consisted either of unconcentrated medium taken from BLEC and H36CE2 cells grown on lens capsules in 0.1% (vol/vol) serum for 48 h or of unconcentrated substrate buffer (5 mM CaCl2, 50 mM Tris, pH 8.0) taken from lens capsules that had been cleaned of all cells and exposed to human recombinant MMP-2 for 72 h.
Data Analysis
The absorbance values recorded using the one-step CellTiter 96 aqueous nonradioactive cell proliferation assay kit (Promega) and the ELISA kit (R&D Systems) were converted into viable cells number and growth factor level, respectively, using standard curves for each cell line and growth factor, respectively. Six wells were used for each treatment per experiment. The data from three independent experiments were pooled, and the mean and SE of the mean calculated for each treatment. Significance between treatments was determined by an independent-sample, two-tailed t test, and pairwise comparisons were made, with a significance level set at 0.05.
RESULTS
The Lens Capsule Protects Lens Epithelial Cells against Serum Deprivation and Staurosporine-induced Apoptosis
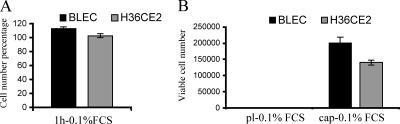
Previous experiments conducted with human lens explants have shown that lens epithelial cells kept within the capsular bag can proliferate in the total absence of serum (Wormstone et al., 1997), whereas lens epithelial cells grown on a plastic substrate undergo apoptosis when deprived of serum (Wang et al., 1999). In low levels of serum (0.1% [vol/vol] serum), primary bovine lens epithelial cells, BLEC, and immortalized human lens epithelial cells, H36CE2, attached with equal efficiency to both plastic dishes and bovine lens capsules, as shown by the measurement of cell number 1 h after plating (Figure 1A). After 96-h exposure to 0.1% (vol/vol) serum, however, no BLEC and H36CE2 cells survived when plated onto plastic dishes (Figure 1B, pl-0.1% FCS). In contrast, cells plated onto bovine lens capsules were readily detected (Figure 1B, cap-0.1% FCS).
Figure 1.
Lens epithelial cells are protected against serum deprivation when cultured on the lens capsule. BLEC and H36CE2 cells were seeded onto either plastic dishes (pl) or lens capsules (cap) in 0.1% (vol/vol) fetal cal serum (FCS). After 1 h in culture (A), there was no statistical difference (p > 0.05 for both BLEC and H36CE2) between the numbers of cells attached to the lens capsule or to the plastic dishes (bars show the average number of cells attached to the lens capsules as a percentage of the average number of cells attached to the plastic dishes). After 96 h in culture (B), viable cells were only detected on the lens capsules (cap). Those on plastic (pl) appeared not to have survived.
These data suggested that the lens capsule protected the lens epithelial cells against cell death in low levels of serum. To test this hypothesis, we examined the ability of the bovine lens capsule to protect lens epithelial cells against staurosporine-induced apoptosis in the presence of 10% (vol/vol) serum. In this experiment, the ability of staurosporine to kill cells grown on the plastic culture dishes (Figure 2, A and B, pl-c, pl-s) was compared with those cells grown on lens capsules (Figure 2, A and B, cap-c, cap-s). On plastic, there was a dramatic decrease in cell number in both cell lines after 48 h of staurosporine treatment (Figure 2, A and B, pl-c and pl-s). When cells were grown on the lens capsule substrate, however, significantly less cell loss was observed (Figure 2, A and B, cap-c and cap-s). In fact the BLEC cells even appeared to be insensitive to staurosporine treatment (Figure 2A, cap-c and cap-s), perhaps reflecting the primary origin of these cells compared with the large T antigen–immortalized H36CE2 cells. In agreement with these cell viability results, when BLEC cells were TUNEL stained, those treated for 48 h with staurosporine in 10% (vol/vol) medium resulted in the detection of apoptotic DNA damage for cells grown on glass coverslips (Figure 2, C and D), but not for cells grown on the lens capsule (Figure 2, E and F). Quantification of apoptotic damage, by counting the percentage of pyknotic nuclei in BLEC cells grown in high serum (10% [vol/vol]) medium in the presence of staurosporine for 48 h, showed a significantly greater number of apoptotic nuclei in cells cultured on coverslips, rather than on lens capsules (Figure 2G). These results strongly suggest that the lens capsule is capable of preventing staurosporine-induced apoptosis in lens epithelial cells.
Figure 2.
Lens epithelial cells are protected against staurosporine-induced apoptosis when cultured on the lens capsule. (A and B) BLEC cells (A) and H36CE2 cells (B) were grown on either uncoated plastic dishes (pl-c) or on bovine lens capsules (cap-c) in 10% (vol/vol) serum and then challenged with 0.5 μM staurosporine (pl-s; cap-s). After 48-h exposure to staurosporine, most of the BLEC (A, pl-s) and H36CE2 (B, pl-s) grown on the plastic dishes did not survive compared with controls (A, B; pl-c and pl-s; p < 0.05 for both BLEC and H36CE2). When grown on the lens capsules (A and B, cap-s), there was, however, a smaller decrease in cell number (A and B; cf. cap-c and cap-s; p > 0.05 and p < 0.05 for BLEC and H36CE2, respectively). (C–F) Immunofluorescence microscopy of BLEC cells labeled with both propidium iodide (red) and TUNEL staining (green) and grown on either glass coverslips (C and D) or on bovine lens capsules (E and F) in the absence (C and E) or presence (D and F) of 0.5 μM staurosporine in media containing 10% (vol/vol) serum. After 48 h in the presence of staurosporine, chromatin reorganization was frequently observed in the nuclei of cells grown on glass as detected by propidium iodide staining. These propidium iodide–positive nuclei were also largely TUNEL-positive, indicating that significant DNA damage has also occurred, as indicated by the yellow color of their nuclei (arrows). In contrast, cells grown on the bovine lens capsule were mostly not TUNEL positive and chromatin reorganization was also not usually seen (F). Scale bars, 10 μm. (G) BLEC cells grown on either glass coverslips (gl) or on bovine lens capsules (cap) in the absence (-c) or presence (-s) of 0.5 μM staurosporine in media containing 10% (vol/vol) serum were labeled with DAPI and then scored for the presence of apoptotic nuclei. The number of apoptotic cells after 48-h exposure to staurosporine was then expressed as percentage of total cell number as described in Materials and Methods. In the absence of staurosporine, the percentage of apoptotic cells was low for cells grown on both glass (gl-c) and lens capsule substrates (cap-c). With the addition of staurosporine, cells grown on the coverslips exhibited a very large increase in the number of apoptotic cells (gl-p and gl-s; p < 0.05). In contrast, cells grown on the lens capsule exhibited a much smaller increase in number of apoptotic cells (cap-s).
To test whether the lens capsule had a solely antiapoptotic effect, rather than a mixture of proproliferative and antiapoptotic activities, we subjected H36CE2 cells grown on either uncoated plastic dishes or bovine lens capsules to cell cycle analysis. After 48 h culture in 10% (vol/vol) serum-supplemented cell culture medium, only a small, nonsignificant increase in the G2 population was observed in H36CE2 cells grown on the lens capsules, compared with H36CE2 cells grown on plastic (Table 1), suggesting that the presence of the lens capsule per se does not strongly affect the cell cycle of lens epithelial cells.
Table 1.
Cell cycle analysis of H36CE2 cells
| Cell treatment | % G1 population | % S population | % G2 population |
|---|---|---|---|
| H36CE2 pl | 44.6 ± 6.5 | 38.6 ± 4.9 | 11.6 ± 2.4 |
| H36CE2 cap | 44.9 ± 8.1 | 34.0 ± 7.9 | 17.1 ± 3.9 |
H36CE2 cells were grown either on uncoated plastic dishes (pl) or on bovine lens capsules (cap) in 10% (vol/vol) serum supplemented medium for 48 h before being collected and processed for cell cycle analysis. The percentages of cells within the G1 and S populations were similar for both substrates, with a small, nonsignificant (χ2 test; p > 0.10) increase in the G2 population when H36CE2 cells were grown on bovine lens capsules. Numbers shown are the average ± SD of three independent repeats.
MMPs Are Expressed by Lens Cells
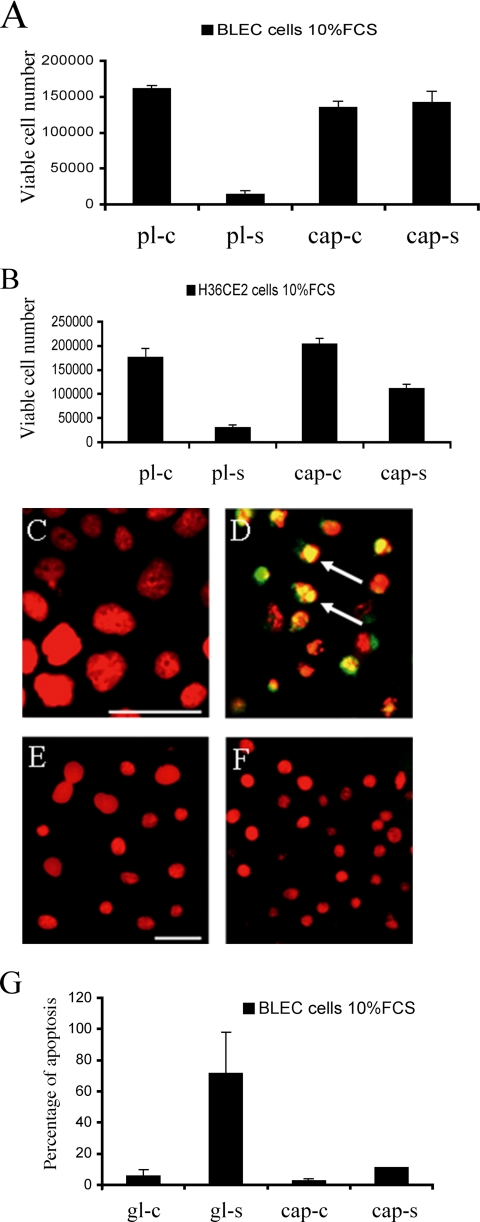
Others have reported that lens epithelial cells grown on the lens capsule produce matrix metalloproteinases, and specifically MMP-2 and -9, when exposed to stress (Tamiya et al., 2000). To investigate whether this expression pattern would be conserved in our system, RNA was isolated from the human H36CE2 cells grown for 48 h in 0.1% (vol/vol) serum on bovine lens capsules and uncoated plastic dishes, and the expression profile for ADAMTS-1 to -20, MMP-1 to -28 and TIMP-1 to -4 were monitored by a TaqMan gene expression assays (representative result shown in Supplementary Figure 1). GAPDH was used to normalize the expression levels for the two values, and then the relative change in RNA levels for the cells grown on the lens capsule versus the plastic were plotted on a log scale (Figure 3A). These data confirmed the presence of MMP-2 and also identified MMP-1, -3, -14, and -15 as expressed by H36CE2 cells grown on both substrates. Only ADAMTS-4 and MMP-2, however, were consistently up-regulated in cells grown on the lens capsules compared with cells grown on plastic, and MMP-9 was not detected for cells grown on either substrate (Figure 3A). The expression of the inhibitors TIMP-1 and -4 (Brew et al., 2000) were also consistently down-regulated in cells grown on lens capsules compared with those grown on plastic (Figure 3A). The presence and activation status of MMP-2 was checked by immunoblotting of lens cell conditioned media after 48 h of culture in serum- free medium (Figure 3B). The pro-MMP-2 form (72 kDa) and to a lesser extent its active cleaved (57 kDa) form were present in the media from H36CE2 cells grown on plastic dishes. Conditioned media from cells grown on the capsule contained predominantly the cleaved 57-kDa active form of MMP2. Again, MMP-9 was not detected (Figure 3C) confirming the RNA expression data. Collectively, these data show that culture of lens epithelial cells on the lens capsule correlates with higher levels of MMP-2 expression and activation.
Figure 3.
MMP-2 expression and activation are increased in H36CE2 cells grown on the lens capsule compared with H36CE2 cells grown on plastic. (A) Analysis of the TaqMan gene expression array data plotted as relative change (ΔΔCt) in transcript levels between extracts from an equal number of cells cultured for 48 h on the lens capsule (bars) and the plastic substrate (baseline). These data show that H36CE2 cells grown on the lens capsule consistently express more ADAMTS-4 and MMP-2 and less TIMP-1 and - 4 than H36CE2 grown on plastic. MMP-9 mRNA expression was not detected. (B and C) Conditioned medium from H36CE2 cells grown on the plastic (pl) and the lens capsule (cap) for 48 h were concentrated by centrifugation and analyzed by immunoblotting for the presence of MMP-2 (B) and MMP-9 (C). Human recombinant latent gelatinases (hrMMP-2 and -9; asterisks) were included as standards. MMP-2 was detected in the medium from cells grown on both substrates in serum-free medium. Serum-free medium was used to prevent BSA from obscuring the MMP-2 protein signal after sample concentration. In medium from the lens capsule cultures, MMP-2 was predominantly detected as the cleaved active form (upper arrowhead; B) with a further cleaved minor form (lower arrowhead), whereas in medium from plastic cultures MMP-2 was mainly detected in the latent form (asterisk). MMP-9 was not detected in medium from cells grown on either substrate in 0.1% (vol/vol) serum conditions.
Soluble Survival Factors Are Released from the Lens Capsule
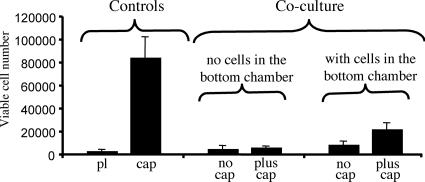
The presence of active MMP-2 suggested that ECM degradation products could be released from the lens capsule to enable the survival of the lens epithelial cells. To address this question, we cultured H36CE2 with lens capsules using a coculture chamber system containing 0.1% (vol/vol) serum supplemented media. Here, the H36CE2 cells were grown in an upper chamber, separated by a semipermeable membrane from a lower chamber. Various additions were made to the lower chamber, such as H36CE2 cells only (Figure 4; Coculture; with cells in bottom chamber; no cap), a lens capsule alone (Figure 4; Coculture; no cells in bottom chamber; plus cap) or H36CE2 cells grown on a lens capsule (Figure 4; Coculture; with cells in bottom chamber; plus cap). After 48 h in culture, comparing the treatment cell viability values to those for the controls (Figure 4; Controls; pl, cap) showed H36CE2 cell number to be highest when both lens capsule and cells were present in the bottom chamber (Figure 4; Coculture; with cells in bottom chamber; plus cap). These data suggest that soluble factors can be released by attached cells from the lens capsule to maintain the viability of H36CE2 cells in the upper chamber in low serum conditions. This improved survival was, however, still significantly less than that of H36CE2 cells grown in direct contact with the lens capsule (Figure 4; Controls; cap). Therefore, maximal lens cell viability in low-serum conditions requires both physical attachment to the lens capsule as well as the release of soluble factors from the matrix.
Figure 4.
Both attachment and soluble factors are involved in H36CE2 cells viability on the lens capsule. Controls, the maximum cell viability potential of H36CE2 cells grown directly on both plastic (pl) and the lens capsule (cap) was measured after 48 h in 0.1% (vol/vol) serum conditions. Coculture experiments, the effect of the lens capsule upon H36CE2 cell viability was measured in a coculture system in 0.1% (vol/vol) serum. In this coculture system, H36CE2 viability was measured for cells grown on an insert contained within a plastic dish well. This allowed for different combinations of lens capsule and H36CE2 cells to be added to the bottom chamber and for the effect of soluble factors upon the viability of the H36CE2 cells grown on the well insert to be determined. After 48 h in culture, in the absence of both cells and lens capsule in the bottom chamber, H36CE2 viability was very similar to that seen for the plastic control (cf. Coculture; ‘no cells in bottom chamber; no cap’ with Controls; pl; p > 0.05). Adding a lens capsule to the bottom chamber in the absence of any additional cells in this compartment was also unable to increase significantly the base level of cell viability for H36CE2 cells in the upper chamber (cf. Coculture, “no cells in bottom chamber, plus cap” with Controls, pl; p > 0.05). In contrast, adding both a lens capsule and H36CE2 cells to the bottom chamber significantly increased H36CE2 cell viability in the upper chamber (cf. Coculture, “with cells in bottom chamber; plus cap” with Controls; pl; p < 0.05). The addition of H36CE2 cells alone to the bottom chamber had no significant effect upon the viability of H36CE2 cells in the upper chamber (cf. Coculture, “with cells in bottom chamber; no cap” with Controls, pl; p > 0.05).
FGF-2 Is Released from the Lens Capsule by MMP-2
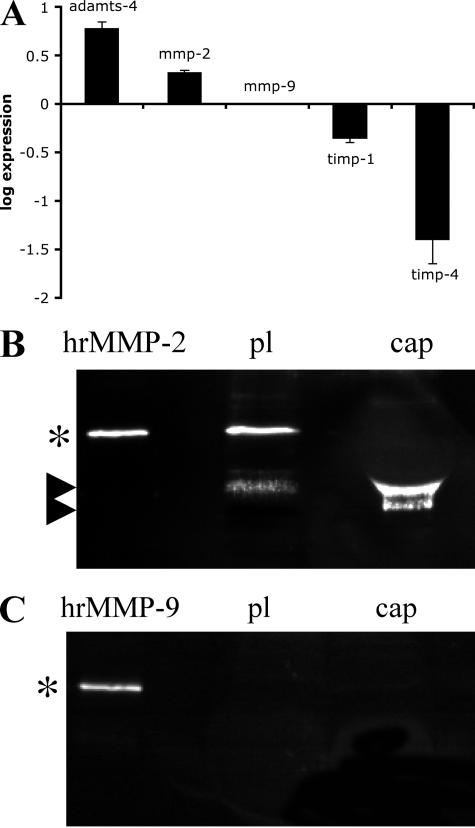
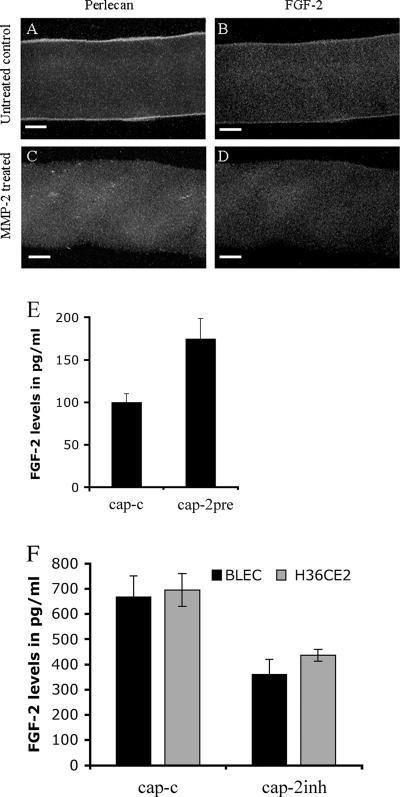
To test whether MMP-2 could release growth factors from lens capsules, human recombinant MMP-2 (R&D Systems, Minneapolis, MN) was added to bovine lens capsules which had been cleaned of cells beforehand and the presence of growth factor in MMP-2-treated capsules (Figure 5, A–D) was investigated. FGF-2 (McAvoy and Chamberlain, 1989; Le and Musil, 2001), IGF-1 (Piatigorsky et al., 1973; Shirke et al., 2001), and TGF-β2 (Luetteke et al., 1993; Weng et al., 1997) have all been demonstrated as important for lens cell growth and differentiation. As visualized by immunofluorescence microscopy (Figure 5, A–D), even after 72 h of exposure in substrate buffer, FGF-2 and its binding partner perlecan (Roghani et al., 1994; Venkataraman et al., 1994; Padera et al., 1999) were concentrated at both inner and outer faces of the anterior lens capsule (Figure 5, A and B) and could be released with MMP-2 treatment (Figure 5, C and D). Levels of released FGF-2 were significantly increased in the presence of human recombinant MMP-2, as detected by ELISA (Figure 5E). In contrast, no significant IGF-1 or TGF-β2 release by MMP-2 were detected by ELISA measurements of conditioned buffer (data not shown). Also after 72-h exposure, scanning electron microscopy of the inner surface of the anterior lens capsule showed that the main morphological features of the lens capsule were retained after MMP-2 treatment, although the finer filamentous organization was lost (Figure 6, A and B). These data suggest that the lens capsule contains a reservoir of FGF-2 at the cell-ECM interface that can be released as a result of MMP-2 activity. Subsequently, MMP inhibitors, and specifically MMP-2 inhibitors, should reduce the levels of FGF-2 released from the capsule by lens cells. A selective MMP-2 inhibitor was used to test this hypothesis. Inhibition of MMP-2 reduced the levels of FGF-2 released into the medium for BLEC and H36CE2 cells grown on lens capsules in 0.1% (vol/vol) serum for 48 h (Figure 5F; cap-c and cap-2inh). Taken together, these data show that there are significant levels of FGF-2 sequestered in the lens capsule, which can be released by MMP-2.
Figure 5.
MMP-2 can release lens capsule-bound FGF-2 and perlecan and affect the amount of FGF-2 accessible to cells grown on the lens capsule. MMP-2 treatment of the lens capsule depletes capsule-bound perlecan and FGF-2 stores. (A–D) Bovine lens capsules cleaned of all primary cells were exposed to human recombinant MMP-2 for 72 h in substrate buffer (5 mM CaCl2, 50 mM Tris-HCl, pH 8.0) and then processed for immunofluorescence microscopy. In the untreated lens capsules, perlecan (green; A) and FGF-2 (red; B) were still concentrated at the outer and inner faces of the anterior lens capsule. In MMP-2–treated lens capsules, both layers were largely depleted [perlecan (green; C) and FGF-2 (red; D)]. Scale bars, 20 μm. (E) Bovine lens capsules cleaned of all primary cells were exposed to human recombinant MMP-2 for 72 h in substrate buffer (5 mM CaCl2, 50 mM Tris, pH 8.0), and levels of FGF-2 released into the medium were measured by ELISA. In the treated samples, levels of FGF-2 released into the medium were significantly increased with the addition of human recombinant MMP-2 (p < 0.05). (F) FGF-2 release from the lens capsule by H36CE2 and BLEC cells is reduced by MMP-2 inhibition. H36CE2 cells and BLEC cells were seeded onto lens capsules in 0.1% (vol/vol) FCS-supplemented medium and challenged with 100 mM OA-Hy as a selective inhibitor of MMP-2. After 48 h, MMP-2 (cap-c: cap-2inh) inhibition resulted in significant decreases in FGF-2 levels released into the medium as measured by ELISA (p < 0.05 for both BLEC and H36CE2).
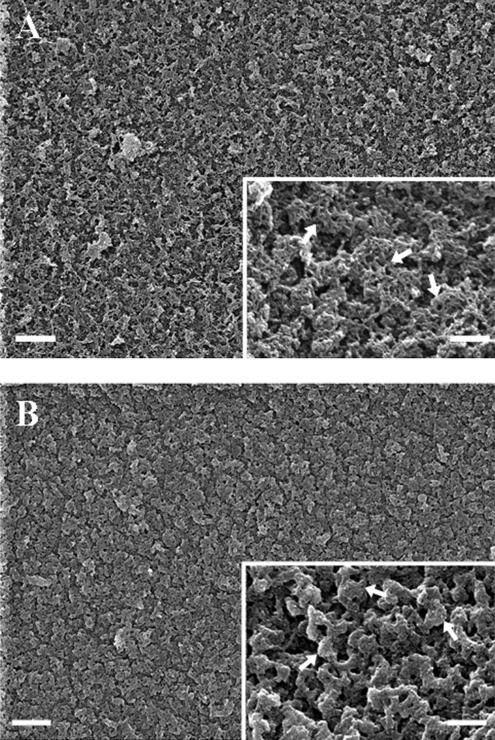
Figure 6.
MMP-2 treatment affects the surface architecture of the lens capsule. Bovine lens capsules cleaned of all primary cells were exposed to human recombinant MMP-2 for 72 h in substrate buffer (5 mM CaCl2, 50 mM Tris, pH 8.0) and prepared for SEM analysis as described in Materials and Methods. (A) In the untreated lens capsules, at low magnification the inner surface of the anterior capsule had a homogenous appearance with an intricate meshwork of surface material. At higher magnification (A, insert), this meshwork of fine filaments was seen to be locally arranged in a honeycomb pattern (A, inset, arrows). (B) In MMP-2–treated lens capsules, at low magnification the ECM surface had a similar general appearance but had lost the fine detail. The honeycomb surface structures were removed and condensed remnants appeared in their place (B, inset, arrows). Scale bars, 1 μm; Scale bars, (insets) 0.2 μm.
MMP-2 Inhibition Decreases Lens Epithelial Cells Viability
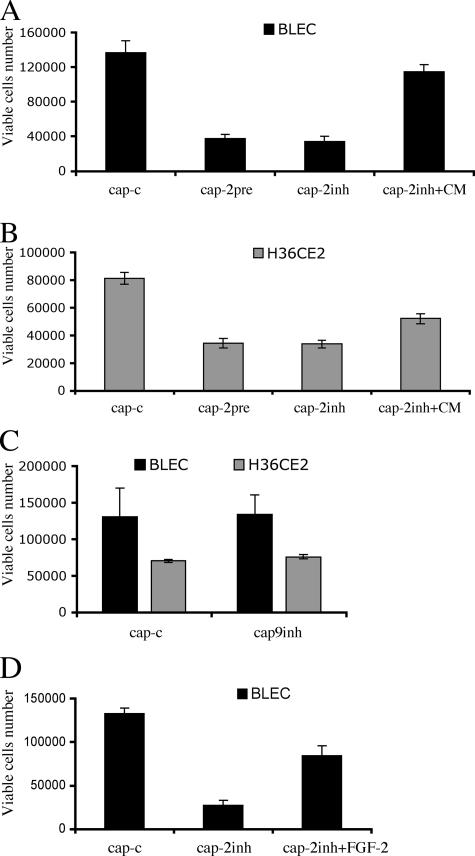
The effect of selectively inhibiting MMP-2 upon epithelial cell viability was determined (Figure 7). After 48 h in 0.1% (vol/vol) serum conditions, MMP-2 inhibition (Figure 7, A and B, cap-2inh) significantly reduced the viability of both H36CE2 cells and BLEC cells. MMP-2 inhibition, however, did not prevent the initial attachment of H36CE2 cells to the lens capsule (data not shown). To test whether the lens capsule store of growth factors could be fully depleted by MMP-2, we pretreated lens capsules with human recombinant MMP-2 in 0.1% (vol/vol) serum for 72 h before cell seeding (Figure 7, A and B, cap-2pre). This treatment very effectively inhibited the viability of both primary bovine lens epithelial cells (Figure 7A) and H36CE2 cells (Figure 7B). Again, initial attachment of H36CE2 cells to MMP-2 pretreated lens capsules was not severely affected (data not shown). The conditioned medium collected from the MMP-2–treated capsules could partially prevent cell loss due to MMP-2 inhibition (Figure 7, A and B, cap-2inh+CM). MMP-9 inhibition was also tested and was shown to have no effect on either H36CE2 or BLEC cell viability after 48 h in 0.1% (vol/vol) serum conditions (Figure 7C). Taken together, these results suggest that MMP-2 is the most important MMP for sustaining lens cell viability and that its effects are mediated by soluble factors (e.g., FGF-2) released from the lens capsule.
Figure 7.
MMP-2 mediates lens epithelial cell viability via the release of soluble factors from the lens capsule. (A and B) BLEC (A) and H36CE2 (B) cells were grown in 0.1% (vol/vol) serum on lens capsules that had been exposed previously to human recombinant MMP-2 for 72 h (cap-2pre). After 48 h in culture, this pretreatment caused a significant decrease in cell viability (A and B: cap-c and cap-pre; p < 0.05 for both BLEC and H36CE2). This decrease was not significantly different from the decrease in cell viability obtained when either BLEC or H36CE2 cells were exposed to the MMP-2 selective inhibitor OA-Hy for the 48-h culture time (A and B: cap-c and cap-2inh; p < 0.05 for both BLEC and H36CE2 and A and B: cap-2pre and cap-2inh; p > 0.05 for both BLEC and H36CE2). The addition of conditioned medium from MMP-2 pretreated lens capsules (CM) to either BLEC or H36CE2 cells grown on lens capsules in the presence of MMP-2 selective inhibitor OA-Hy (A and B: cap-2inh+CM) partially reversed the decrease in lens epithelial cell viability induced by the addition of selective MMP-2 inhibitor (A and B: cap-2inh and cap-2inh+CM; p < 0.05 for both BLEC and H36CE2, and A and B: cap-c and cap-2inh+CM; p > 0.05 and p < 0.05 for BLEC and H36CE2, respectively). (C) Exposure to MMP inhibitor II as a selective MMP-9 inhibitor failed to decrease the cell viability of either BLEC or H36CE2 cells grown on lens capsules in 0.1% (vol/vol) serum (cap-c and cap-9inh; p > 0.05 for both BLEC and H36CE2). (D) BLEC grown on the lens capsules were exposed to 100 μM MMP2-selective inhibitor OA-Hy (cap-2inh) in 0.1% (vol/vol) serum-supplemented medium. The subsequent decrease in cell viability (cap-c and cap-2inh) could be significantly, but not completely, reversed by the single addition of recombinant FGF-2 (200 pg/ml; cap-2inh and cap-2inh+FGF, p < 0.05, and cap-c and cap-2inh+FGF, p < 0.05).
Readdition of FGF-2 Counteracts the Inhibition of Epithelial Cell Proliferation by MMP-2
These data suggest that it should be possible to reverse the effects of MMP-2 inhibition by simply providing media supplemented with FGF-2, if this growth factor were key to lens epithelial cell survival (McAvoy and Chamberlain, 1989; Richardson et al., 1993; Le and Musil, 2001; Lovicu and McAvoy, 2001). Supplementing the growth media of primary bovine lens epithelial cells with exogenous recombinant FGF-2 in the presence of the selective MMP-2 inhibitor OA-Hy, restored ∼70% of the proliferation level after 48 h in 0.1% (vol/vol) serum conditions (Figure 7D). These data confirm that MMP-2 and FGF-2 are key components in promoting lens epithelial cell stress resistance on the lens capsule.
DISCUSSION
The Lens Capsule Generates a Microenvironment Conducive to Epithelial Cell Stress Resistance
In this study, we describe the role for the lens capsule as an ECM store of FGF-2, a growth factor required for lens epithelial cell proliferation, migration and differentiation (Robinson, 2006), most likely via MAPK signaling (Lovicu and McAvoy, 2001; Iyengar et al., 2007). The data presented here provide evidence of the protective role of this ECM store of FGF-2 in a simple, well-defined experimental system that mimics the microenvironment of the lens after surgery or injury (Wormstone et al., 1997; Tamiya et al., 2000). We have identified MMP-2 as a major activity required to support lens cell viability, via the release of FGF-2 from the lens capsule.
It has long been recognized that the ECM determines cell proliferation and survival via integrin attachment and activation (Matter and Ruoslahti, 2001; Hynes, 2002). Indeed the cross-talk between integrin signaling and growth factor receptors (Schwartz and Ginsberg, 2002) is a clear indication of the importance of both pathways to cell proliferation, survival, and differentiation. The three-dimensional features of the ECM and its stiffness provide additional cues to the microenvironment that influences cell proliferation (Lo et al., 2000; Bissell et al., 2002; Zahir and Weaver, 2004). Our data show that maximal cell number is achieved only when the cells are in direct contact with the lens capsule (Figure 4). These are essential features of the ECM role in cell proliferation and differentiation, but growth/survival factors also play an equally important role as previously shown using the lens epithelial cell paradigm (Ishizaki et al., 1993). Our data extend these seminal studies by identifying the lens capsule as a major source of the growth/survival factors and demonstrating the additional cues that are required for lens epithelial cell stress resistance and viability are provided by the lens capsule.
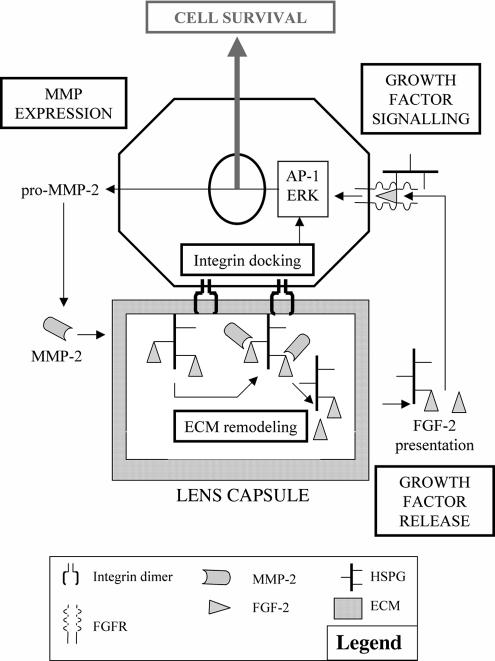
We have proposed a model (Figure 8) to link the physical attachment to the lens capsule, MMP activity, and the subsequent release of growth factors such as FGF-2 from the lens capsule. In other systems, FGF-2 is able to induce gelatinase expression (Kohn et al., 1995; El Ramy et al., 2005; Wang et al., 2005), suggestive of a positive feedback loop between growth factor release and the expression/activation of MMPs to support lens epithelial cell survival and maintain proliferation. Our data have also demonstrated how important the physical contact with the lens capsule is to enhance the effects of the released growth factors (Figure 4). In other systems, recent studies have suggested that MMP-2 gene expression could be induced via AP-1 after ILK activation after integrin engagement (Troussard et al., 1999; Lee et al., 2005). It is the combination of these physical cues, the availability of growth factors from the ECM and the production of MMPs that support lens cell proliferation. We propose therefore the MEG cycle (MMP-ECM-Growth factor) to account for our observations.
Figure 8.
The MMP-ECM-Growth factor (MEG) cycle for cell viability. The presence and activity of MMP-2 in the lens cells' environment, likely after integrin-mediated gene transcription as described for other systems (Troussard et al., 1999; Lee et al., 2005), leads to the degradation of HSPGs in the ECM, allowing soluble FGF-2 release into the media. FGF-2 then binds its receptor and activates downstream signaling pathways (Lovicu and McAvoy, 2001; Chandrasekher and Sailaja, 2003; Iyengar et al., 2007) to promote cell survival. This represents the simplest form of the cycle and does not exclude contributions from other proteinases, cryptogenic ECM sites, and IGF-1 and TGF-β2 release that can all influence epithelial cell survival. In other systems, FGF-2 can feedback to increase gelatinase expression (El Ramy et al., 2005; Wang et al., 2005).
Lens epithelial cell stress resistance requires MMP-2
Others have reported the presence of MMP-2 in lens cells grown on the lens capsule (Wormstone et al., 2002), with the induction of both MMP-2 and -9 being conditional on the addition of exogenous growth factors (Wormstone et al., 2002) or oxidative stress (Tamiya et al., 2000). Both MMPs are implicated in the TGF-β–induced epithelial-mesenchyme transition (EMT) of lens epithelial cells (Dwivedi et al., 2006). In our model, we have demonstrated that the presence of MMP-2, but not MMP-9, is essential for lens epithelial cell resistance to serum deprivation on the lens capsule. The MMP-2 selective inhibitor OA-Hy significantly inhibited epithelial cell viability, whereas a selective MMP-9 inhibitor had no effect (Figure 7). Also, pretreatment of bovine lens capsules with human recombinant MMP-2 for 72 h was sufficient to reduce cell viability very effectively (Figure 7). Neither MMP-2 inhibition nor MMP-2 pretreatment prevented cell attachment to the lens capsule, although cell loss was detected as early as 6 h after the start of both treatments (Figure 7). This suggests that attachment to the substrate per se is not sufficient to guarantee the long-term survival of lens cells on their ECM. MMP-2 processing of the lens capsule and the subsequent release of soluble factors is also needed.
MMP-2 inhibition significantly reduced the levels of FGF-2 in conditioned media (Figure 7), but the decrease in primary lens cell number was largely reversed by adding recombinant FGF-2 (Figure 7). These data demonstrate the importance of FGF-2 to lens cell survival, but they also suggest that MMP-2 has other targets and activities in this system because FGF-2 readdition did not completely reverse OA-Hy inhibition. At this stage, we cannot exclude contributions from other MMPs expressed by lens cells (Figure 3 and Supplementary Data). Also, the capsule could contain low levels of other, as yet uncharacterized, proteinases with the potential to release these growth factors, which in combination would influence lens cell survival, proliferation, and differentiation (Liu et al., 1996). The bio-context of FGF-2 availability could also influence its effect, because heparan sulfate proteoglycans (HSPGs) that facilitate FGF-2 binding to its receptor (Roghani et al., 1994; Venkataraman et al., 1994; Padera et al., 1999) are also potential substrates for MMP-2 (Fowlkes and Winkler, 2002). HSPs are important components of the lens capsule (Azuma and Hara, 1998) and altering their biosynthesis can cause both lens hypoplasia and anophthalmia (Pan et al., 2006). Indeed, MMP-2 is also known to activate latent cytokines, such as IL1-B and TNF-α (Fowlkes and Winkler, 2002) and most recently been shown to proteolyse and release E-cadherin, promoting EMT in lens epithelial cells exposed to TGF-β (Dwivedi et al., 2006). It can also alter the ECM microenvironment to reveal cryptic proliferative sites in laminin and collagen (Pirila et al., 2003) and generate “matrikines” from processed ECM molecules (Tran et al., 2004). Although we have not identified which of these possibilities are most relevant here, we have shown that FGF-2 is released from the lens capsule by MMP-2, and both are required to protect lens epithelial cell from stress on the lens capsule. These data also support the ECM-reservoir hypothesis (Bergers et al., 2000; Mott and Werb, 2004) and confirm that the lens capsule contains a store of accessible FGF-2.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Carol English (Northern Region Genetics Service, Institute of Human Genetics, University of Newcastle, Newcastle upon Tyne, NE1 3BZ, United Kingdom) for the cytogenetic analyses of the human lens epithelial cell line, Ian Dimmick (North East England Stem Cell Institute, International Center for Life, Newcastle upon Tyne, NE1 3BZ, United Kingdom) for the cell cycle analyses of the human lens epithelial cell line, and Northern Counties Meat Group (Gateshead, NE10 0QH) for the supply of bovine material. The support of Fight for Sight (F.M.D.T., C.G.) and British Eye Research Foundation (F.M.D.T.) are gratefully acknowledged.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-05-0416) on August 15, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Adams J. C., Watt F. M. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- Andley U. P., Rhim J. S., Chylack L. T., Jr, Fleming T. P. Propagation and immortalization of human lens epithelial cells in culture. Invest. Ophthalmol. Vis. Sci. 1994;35:3094–3102. [PubMed] [Google Scholar]
- Azuma N., Hara T. Extracellular matrix of opacified anterior capsule after endocapsular cataract surgery. Graefes Arch. Clin. Exp. Ophthalmol. 1998;236:531–536. doi: 10.1007/s004170050117. [DOI] [PubMed] [Google Scholar]
- Bergers G., Brekken R., McMahon G., Vu T. H., Itoh T., Tamaki K., Tanzawa K., Thorpe P., Itohara S., Werb Z., Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J., Radisky D. C., Rizki A., Weaver V. M., Petersen O. W. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–546. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K., Dinakarpandian D., Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim. Biophys. Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- Carter L. A., MacDonald J. L., Roskams A. J. Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J. Neurosci. 2004;24:5670–5683. doi: 10.1523/JNEUROSCI.0330-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekher G., Sailaja D. Differential activation of phosphatidylinositol 3-kinase signaling during proliferation and differentiation of lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 2003;44:4400–4411. doi: 10.1167/iovs.03-0136. [DOI] [PubMed] [Google Scholar]
- Dwivedi D. J., Pino G., Banh A., Nathu Z., Howchin D., Margetts P., Sivak J. G., West-Mays J. A. Matrix metalloproteinase inhibitors suppress transforming growth factor-beta-induced subcapsular cataract formation. Am. J. Pathol. 2006;168:69–79. doi: 10.2353/ajpath.2006.041089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ramy R., Verot A., Mazaud S., Odet F., Magre S., Le Magueresse-Battistoni B. Fibroblast growth factor (FGF) 2 and FGF9 mediate mesenchymal-epithelial interactions of peritubular and Sertoli cells in the rat testis. J. Endocrinol. 2005;187:135–147. doi: 10.1677/joe.1.06146. [DOI] [PubMed] [Google Scholar]
- Emonard H., Marcq V., Mirand C., Hornebeck W. Inhibition of gelatinase A by oleic acid. Ann. NY Acad. Sci. 1999;878:647–649. doi: 10.1111/j.1749-6632.1999.tb07751.x. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. The organ microenvironment and cancer metastasis. Differentiation. 2002;70:498–505. doi: 10.1046/j.1432-0436.2002.700904.x. [DOI] [PubMed] [Google Scholar]
- Fowlkes J. L., Enghild J. J., Suzuki K., Nagase H. Matrix metalloproteinases degrade insulin-like growth factor-binding protein-3 in dermal fibroblast cultures. J. Biol. Chem. 1994;269:25742–25746. [PubMed] [Google Scholar]
- Fowlkes J. L., Winkler M. K. Exploring the interface between metallo-proteinase activity and growth factor and cytokine bioavailability. Cytokine Growth Factor Rev. 2002;13:277–287. doi: 10.1016/s1359-6101(02)00005-9. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R., Welge-Lussen U., Lutjen-Drecoll E. The effect of TGF-beta2 on human trabecular meshwork extracellular proteolytic system. Exp. Eye Res. 2003;77:757–765. doi: 10.1016/s0014-4835(03)00220-3. [DOI] [PubMed] [Google Scholar]
- Hashimoto G., Inoki I., Fujii Y., Aoki T., Ikeda E., Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J. Biol. Chem. 2002;277:36288–36295. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Specificity of cell adhesion in development: the cadherin superfamily. Curr. Opin. Genet. Dev. 1992;2:621–624. doi: 10.1016/s0959-437x(05)80182-0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Imai K., Hiramatsu A., Fukushima D., Pierschbacher M. D., Okada Y. Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor-beta1 release. Biochem. J. 1997;322(Pt 3):809–814. doi: 10.1042/bj3220809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki Y., Voyvodic J. T., Burne J. F., Raff M. C. Control of lens epithelial cell survival. J. Cell Biol. 1993;121:899–908. doi: 10.1083/jcb.121.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuya-Oka A., Li Q., Amano T., Damjanovski S., Ueda S., Shi Y. B. Requirement for matrix metalloproteinase stromelysin-3 in cell migration and apoptosis during tissue remodeling in Xenopus laevis. J. Cell Biol. 2000;150:1177–1188. doi: 10.1083/jcb.150.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar L., Wang Q., Rasko J. E., McAvoy J. W., Lovicu F. J. Duration of ERK1/2 phosphorylation induced by FGF or ocular media determines lens cell fate. Differentiation. 2007;75:662–668. doi: 10.1111/j.1432-0436.2007.00167.x. [DOI] [PubMed] [Google Scholar]
- Lovicu F. J., McAvoy J. W. FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signalling. Development. 2001;128:5075–5084. doi: 10.1242/dev.128.24.5075. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M. The affinity of fibroblast growth factors (FGFs) for heparin; FGF-heparan sulfate interactions in cells and extracellular matrix. Curr. Opin. Cell Biol. 1990;2:857–863. doi: 10.1016/0955-0674(90)90084-r. [DOI] [PubMed] [Google Scholar]
- Kleinjan D. A., Seawright A., Schedl A., Quinlan R. A., Danes S., van Heyningen V. Aniridia-associated translocations, DNase hypersensitivity, sequence comparison and transgenic analysis redefine the functional domain of PAX6. Hum. Mol. Genet. 2001;10:2049–2059. doi: 10.1093/hmg/10.19.2049. [DOI] [PubMed] [Google Scholar]
- Le A. C., Musil L. S. FGF signaling in chick lens development. Dev. Biol. 2001;233:394–411. doi: 10.1006/dbio.2001.0194. [DOI] [PubMed] [Google Scholar]
- Lee J. G., Dahi S., Mahimkar R., Tulloch N. L., Alfonso-Jaume M. A., Lovett D. H., Sarkar R. Intronic regulation of matrix metalloproteinase-2 revealed by in vivo transcriptional analysis in ischemia. Proc. Natl. Acad. Sci. USA. 2005;102:16345–16350. doi: 10.1073/pnas.0508085102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone D. P., Relvas J. B., Campos L. S., Hemmi S., Brakebusch C., Fassler R., Ffrench-Constant C., Suter U. Regulation of neural progenitor proliferation and survival by beta1 integrins. J. Cell Sci. 2005;118:2589–2599. doi: 10.1242/jcs.02396. [DOI] [PubMed] [Google Scholar]
- Levi E., Fridman R., Miao H. Q., Ma Y. S., Yayon A., Vlodavsky I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc. Natl. Acad. Sci. USA. 1996;93:7069–7074. doi: 10.1073/pnas.93.14.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Chamberlain C. G., McAvoy J. W. IGF enhancement of FGF-induced fibre differentiation and DNA synthesis in lens explants. Exp. Eye Res. 1996;63:621–629. doi: 10.1006/exer.1996.0156. [DOI] [PubMed] [Google Scholar]
- Lo C. M., Wang H. B., Dembo M., Wang Y. L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu F. J., McAvoy J. W. FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signalling. Development. 2001;128:5075–5084. doi: 10.1242/dev.128.24.5075. [DOI] [PubMed] [Google Scholar]
- Luetteke N. C., Qiu T. H., Peiffer R. L., Oliver P., Smithies O., Lee D. C. TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell. 1993;73:263–278. doi: 10.1016/0092-8674(93)90228-i. [DOI] [PubMed] [Google Scholar]
- Matter M. L., Ruoslahti E. A signaling pathway from the alpha5beta1 and alpha(v)beta3 integrins that elevates bcl-2 transcription. J. Biol. Chem. 2001;276:27757–27763. doi: 10.1074/jbc.M102014200. [DOI] [PubMed] [Google Scholar]
- McAvoy J. W., Chamberlain C. G. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development. 1989;107:221–228. doi: 10.1242/dev.107.2.221. [DOI] [PubMed] [Google Scholar]
- McCawley L. J., Matrisian L. M. Matrix metalloproteinases: they're not just for matrix anymore! Curr. Opin. Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- Mott J. D., Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padera R., Venkataraman G., Berry D., Godavarti R., Sasisekharan R. FGF-2/fibroblast growth factor receptor/heparin-like glycosaminoglycan interactions: a compensation model for FGF-2 signaling. FASEB J. 1999;13:1677–1687. doi: 10.1096/fasebj.13.13.1677. [DOI] [PubMed] [Google Scholar]
- Pan Y., Woodbury A., Esko J. D., Grobe K., Zhang X. Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development. 2006;133:4933–4944. doi: 10.1242/dev.02679. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Rothschild S. S., Wollberg M. Stimulation by insulin of cell elongation and microtubule assembly in embryonic chick-lens epithelia. Proc. Natl. Acad. Sci. USA. 1973;70:1195–1198. doi: 10.1073/pnas.70.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikul S., et al. Discovery of potent, achiral matrix metalloproteinase inhibitors. J. Med. Chem. 1998;41:3568–3571. doi: 10.1021/jm980253r. [DOI] [PubMed] [Google Scholar]
- Pirila E., Sharabi A., Salo T., Quaranta V., Tu H., Heljasvaara R., Koshikawa N., Sorsa T., Maisi P. Matrix metalloproteinases process the laminin-5 gamma 2-chain and regulate epithelial cell migration. Biochem. Biophys. Res. Commun. 2003;303:1012–1017. doi: 10.1016/s0006-291x(03)00452-2. [DOI] [PubMed] [Google Scholar]
- Polette M., Huet E., Birembaut P., Maquart F. X., Hornebeck W., Emonard H. Influence of oleic acid on the expression, activation and activity of gelatinase A produced by oncogene-transformed human bronchial epithelial cells. Int. J. Cancer. 1999;80:751–755. doi: 10.1002/(sici)1097-0215(19990301)80:5<751::aid-ijc20>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Richardson N. A., Chamberlain C. G., McAvoy J. W. IGF-1 enhancement of FGF-induced lens fiber differentiation in rats of different ages. Invest. Ophthalmol. Vis. Sci. 1993;34:3303–3312. [PubMed] [Google Scholar]
- Robinson M. L. An essential role for FGF receptor signaling in lens development. Semin. Cell Dev. Biol. 2006;17:726–740. doi: 10.1016/j.semcdb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roghani M., Mansukhani A., Dell'Era P., Bellosta P., Basilico C., Rifkin D. B., Moscatelli D. Heparin increases the affinity of basic fibroblast growth factor for its receptor but is not required for binding. J. Biol. Chem. 1994;269:3976–3984. [PubMed] [Google Scholar]
- Rooney D. E., Czepulkowski B. H. New York: Oxford University Press; 1992. Human Cytogenetics, A Practical Approach. [Google Scholar]
- Schwartz M. A., Ginsberg M. H. Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. 2002;4:E65–E68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- Shirke S., Faber S. C., Hallem E., Makarenkova H. P., Robinson M. L., Overbeek P. A., Lang R. A. Misexpression of IGF-I in the mouse lens expands the transitional zone and perturbs lens polarization. Mech. Dev. 2001;101:167–174. doi: 10.1016/s0925-4773(00)00584-0. [DOI] [PubMed] [Google Scholar]
- Taipale J., Keski-Oja J. Growth factors in the extracellular matrix. FASEB J. 1997;11:51–59. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++ dependent protein kinase. Biochem. Biophys. Res. Commun. 1986;135:397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Tamiya S., Wormstone I. M., Marcantonio J. M., Gavrilovic J., Duncan G. Induction of matrix metalloproteinases 2 and 9 following stress to the lens. Exp. Eye Res. 2000;71:591–597. doi: 10.1006/exer.2000.0916. [DOI] [PubMed] [Google Scholar]
- Tran K. T., Griffith L., Wells A. Extracellular matrix signaling through growth factor receptors during wound healing. Wound Repair Regen. 2004;12:262–268. doi: 10.1111/j.1067-1927.2004.012302.x. [DOI] [PubMed] [Google Scholar]
- Troussard A. A., Tan C., Yoganathan T. N., Dedhar S. Cell-extracellular matrix interactions stimulate the AP-1 transcription factor in an integrin-linked kinase- and glycogen synthase kinase 3-dependent manner. Mol. Cell. Biol. 1999;19:7420–7427. doi: 10.1128/mcb.19.11.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman G., Sasisekharan V., Cooney C. L., Langer R., Sasisekharan R. A stereochemical approach to pyranose ring flexibility: its implications for the conformation of dermatan sulfate. Proc. Natl. Acad. Sci. USA. 1994;91:6171–6175. doi: 10.1073/pnas.91.13.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., He H., Zigler J. S., Jr, Iwata T., Ibaraki N., Reddy V. N., Carper D. bFGF suppresses serum-deprivation-induced apoptosis in a human lens epithelial cell line. Exp. Cell Res. 1999;249:123–130. doi: 10.1006/excr.1999.4450. [DOI] [PubMed] [Google Scholar]
- Wang Y. S., Eichler W., Friedrichs U., Yafai Y., Hoffmann S., Yasukawa T., Hui Y. N., Wiedemann P. Impact of endostatin on bFGF-induced proliferation, migration, and matrix metalloproteinase-2 expression/secretion of bovine choroidal endothelial cells. Curr. Eye Res. 2005;30:479–489. doi: 10.1080/02713680590959358. [DOI] [PubMed] [Google Scholar]
- Weng J., Liang Q., Mohan R. R., Li Q., Wilson S. E. Hepatocyte growth factor, keratinocyte growth factor, and other growth factor-receptor systems in the lens. Invest. Ophthalmol. Vis. Sci. 1997;38:1543–1554. [PubMed] [Google Scholar]
- Whitelock J. M., Murdoch A. D., Iozzo R. V., Underwood P. A. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J. Biol. Chem. 1996;271:10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- Wiseman B. S., Sternlicht M. D., Lund L. R., Alexander C. M., Mott J., Bissell M. J., Soloway P., Itohara S., Werb Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J. Cell Biol. 2003;162:1123–1133. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormstone I. M., Liu C. S., Rakic J. M., Marcantonio J. M., Vrensen G. F., Duncan G. Human lens epithelial cell proliferation in a protein-free medium. Invest. Ophthalmol. Vis. Sci. 1997;38:396–404. [PubMed] [Google Scholar]
- Wormstone I. M., Tamiya S., Anderson I., Duncan G. TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Invest. Ophthalmol. Vis. Sci. 2002;43:2301–2308. [PubMed] [Google Scholar]
- Yu Q., Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- Zahir N., Weaver V. M. Death in the third dimension: apoptosis regulation and tissue architecture. Curr. Opin. Genet. Dev. 2004;14:71–80. doi: 10.1016/j.gde.2003.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.