Abstract
The activity and trafficking of the Na+,K+-ATPase are regulated by several hormones, including dopamine, vasopressin, and adrenergic hormones through the action of G-protein–coupled receptors (GPCRs). Arrestins, GPCR kinases (GRKs), 14-3-3 proteins, and spinophilin interact with GPCRs and modulate the duration and magnitude of receptor signaling. We have found that arrestin 2 and 3, GRK 2 and 3, 14-3-3 ε, and spinophilin directly associate with the Na+,K+-ATPase and that the associations with arrestins, GRKs, or 14-3-3 ε are blocked in the presence of spinophilin. In COS cells that overexpressed arrestin, the Na+,K+-ATPase was redistributed to intracellular compartments. This effect was not seen in mock-transfected cells or in cells expressing spinophilin. Furthermore, expression of spinophilin appeared to slow, whereas overexpression of β-arrestins accelerated internalization of the Na+,K+-ATPase endocytosis. We also find that GRKs phosphorylate the Na+,K+-ATPase in vitro on its large cytoplasmic loop. Taken together, it appears that association with arrestins, GRKs, 14-3-3 ε, and spinophilin may be important modulators of Na+,K+-ATPase trafficking.
INTRODUCTION
The Na+,K+-ATPase, or sodium pump, is expressed ubiquitously in virtually all tissues and plays a key role in the maintenance of intracellular electrolyte homeostasis (Skou and Esmann, 1992). The Na+,K+-ATPase consists of two subunits. The catalytic α-subunit contains 10 transmembrane domains as well as sites for ion recognition, inhibitor binding, and protein kinase A and C (PKA and PKC, respectively) phosphorylation (Therien and Blostein, 2000). The glycoprotein β-subunit has a single transmembrane domain. It is also essential for the functional expression of Na+,K+-ATPase and is involved in the pump's structural and functional maturation (Scheiner-Bobis and Farley, 1994). Structural and biochemical studies demonstrate that the domain from TM4 to TM5 of the Na+,K+-ATPase α-subunit forms a large intracellular loop that is important for the pump's catalytic cycle. It contains both the ATP-binding site and the pump's site for catalytic phosphorylation (Ohtsubo et al., 1990). The ATP hydrolysis accomplished by this domain provides the energy that the pump invests in Na+ and K+ transport.
G-protein–coupled receptors (GPCRs) constitute a very large family that mediate the binding and response to hormones such as dopamine (Narkar et al., 2002), parathyroid hormone (PTH; Khundmiri and Lederer, 2002), adrenalin (Mallick et al., 2000; Brismar et al., 2002), and arginine vasopressin (Djelidi et al., 2001), which are known to control the activity and trafficking of the Na+,K+-ATPase. It has been demonstrated that the trafficking and signaling of GPCRs are regulated by arrestins (Wang and Limbird, 2002; Shenoy and Lefkowitz, 2003; Tan et al., 2004; Wang et al., 2004a), spinophilin (Smith et al., 1999; Brady et al., 2003; Wang et al., 2004a), and 14-3-3 proteins (Prezeau et al., 1999; Couve et al., 2001; Tazawa et al., 2003) through direct associations. Among four isoforms of arrestins, arrestin 2 (also called β-arrestin 1) and arrestin 3 (also called β-arrestin 2) regulate the trafficking and signaling of GPCRs (Attramadal et al., 1992; Parruti et al., 1993). Arrestin induces endocytosis and down-regulation of activated receptors that are phosphorylated by G-protein–coupled receptor kinases (GRKs). Spinophilin antagonizes arrestin binding through inhibition of GRK phosphorylation of GPCRs and prolongs signaling by preventing receptor internalization. Recent studies indicate that a membrane protein unrelated to the GPCRs, Na+/H+ exchanger NHE5 isoform, associates with arrestins, and this association leads to a decrease in the cell surface expression of NHE5 (Szabo et al., 2005). Moreover, the NHE3 Na+/H+ exchanger isoform, which is closely related to NHE5, is also regulated by hormones, including dopamine (Gomes and Soares-da-Silva, 2004; Pedrosa et al., 2004), PTH (Wang et al., 2004b), and adrenalin (Liu et al., 1997; Liu and Gesek, 2001) through the action of GPCRs. It has been shown that the Na+,K+-ATPase interacts with ε-isoform of 14-3-3 during endocytosis after dopamine stimulation (Efendiev et al., 2005). Here we show that the Na+,K+-ATPase associates with arrestin, spinophilin, GRK, and 14-3-3 ε and that these proteins modulate the trafficking of the Na+,K+-ATPase.
MATERIALS AND METHODS
Antibodies
Anti-Na+,K+-ATPase monoclonal antibodies 6H and α5 are directed against the amino terminus of the rat Na+,K+-ATPase α-subunit (Pietrini et al., 1992) and an epitope between residues 338–724 of the chicken Na+,K+-ATPase (Ishii et al., 1994), respectively. Anti-H+,K+-ATPase polyclonal antibody HK9 is directed against the amino terminus of the H+,K+-ATPase α-subunit (Gottardi and Caplan, 1993). Anti-HA antibody was obtained from Covance (Berkeley, CA) and anti-flag antibody was from Sigma (St. Louis, MO). Anti-arrestin 2 and 3 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
Plasmid Construction
The A domain of the rat Na,K-ATPase α-subunit was amplified by PCR (Pagel et al., 2003). This construct was subcloned as a BamHI/EcoRI fragment into the pGEX-4T-3 vector (Amersham Pharmacia, Piscataway, NJ) to produce a cDNAs encoding a glutathione S-transferase (GST)-fusion protein. The large cytoplasmic loop connecting the TM4+TM5 of the Na+,K+-ATPase α-subunit was amplified by PCR with primers that included EcoRI and Not I restriction sites. The PCR fragment was subcloned into pGEX-4T-3 vector, in which the insert was fused to the carboxy terminus of GST. To generate deletions, BspEI, ClaI, MfeI, and HindIII sites were introduced in the pGEX-4T3 construct by creating silent mutations. Mutated constructs were digested with NotI plus BspEI, NarI, ClaI, MfeI, or HindIII for C-terminal deletions or EcoRI and ClaI for the N-terminal deletion. Small fragments were removed by agarose gel electrophoresis, blunt ends were introduced with pfu DNA polymerase, and the modified constructs were recircularized by ligation. The H85N chimera, which is composed of the H+,K+-ATPase (from amino acid 1-85) and the Na+,K+-ATPase (from amino acid 86 to the C-terminus), was subcloned into the mammalian expression vector pCB6 as previously described (Dunbar et al., 2000). Spinophilin was myc-tagged at the amino terminus by generating a PCR fragment in which the initiating codon was replaced by a SalI site. This fragment was cloned in frame along with the 3′ remainder of the spinophilin cDNA into the eukaryotic expression vector pMyc-RK5. Flag-tagged spinophilin was amplified by PCR with primers that included KpnI and XbaI restriction sites and a sequence encoding a flag epitope tag. Arrestin 2 and 3, GRK 2 and 3, and 14-3-3 ε and ζ were cloned by PCR from a human kidney cDNA library. PCR was performed with primers that included KpnI and XbaI restriction sites and sequences encoding flag or hemagglutinin (HA) epitope tags. The flag or HA tags were fused to the amino termini for arrestins and 14-3-3 proteins and to the carboxyl termini for GRK 2 and 3. The PCR fragments were subcloned into the mammalian expression vector pcDNA 3.1(+). All PCR primer sequences are available on request.
Cell Culture and Transfection
COS cells and LLC-PK1 cells were cultured in a humidified incubator under 5% CO2 in α-MEM (GIBCO, Carlsbad, CA) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin. DNA transfection in COS cells was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions, and assays were performed 30 h after transfection. LLC-PK1 cells were used for stable transfection. LLC-PK1 cells were transfected by the calcium phosphate method and selected in 0.5 mg/ml hygromycin B. Expression of arrestins and spinophilin was confirmed by Western blot and immunofluorescence.
Immunohistochemistry
Mice were anesthetized and the internal organs were fixed as described by Biemesderfer et al. (1992). The brains were cut at 2-μm thickness on a CM 3050S cryostat. Tissue was incubated with anti-arrestin 2 or spinophilin polyclonal antibodies and anti-Na+,K+-ATPase mAb, 6H, followed by anti-mouse Alexa Fluor 594–and anti-rabbit Alexa Fluor 488–conjugated IgG (Molecular Probes, Eugene, OR). For transient expression in COS cells, COS cells were washed with phosphate-buffered saline (PBS) containing 1 mM MgCl2, and 0.1 mM CaCl2 (PBS2+) fixed in cold methanol for 7 min and washed three times with PBS2+. Fixed cells were permeabilized in permeabilization buffer (0.1% bovine serum albumin [BSA], 1% Triton X-100 in PBS2+) for 15 min and blocked in goat serum dilution buffer (GSDB; 10% goat serum, 1% Triton X-100, and 10 mM glycine in PBS2+) for 30 min. Cells were incubated with primary antibodies diluted in GSDB buffer for 1 h at room temperature and washed three times with permeabilization buffer and then incubated with fluorescein isothiocyanate–conjugated goat anti-mouse IgG (Sigma) and rhodamine-conjugated goat anti-rabbit IgG (Chemicon, Temecula, CA) diluted in GSDB buffer for 1 h, after which they were washed three times in PBS2+ and once in water. Cells were mounted in Vectashield (Vector Laboratories, Burlingame, CA). Fluorescence was visualized with a Zeiss LSM 410 laser confocal microscope (Thornwood, NY). Images are the product of eightfold line averaging. Contrast and brightness settings were chosen so that all pixels were within the linear range.
In Vitro Transcription/Translation and GST Pulldown Assay
In vitro translation was performed with the TNT-coupled reticulocyte lysate system (Promega, Madison, WI) according to the product manual. Flag-tagged arrestin 2 or flag-tagged spinophilin in pcDNA3.1, which has a T7 promoter, was used as a template. A pGEX construct including the large cytoplasmic loop of the Na+,K+-ATPase α-subunit was transformed into Escherichia coli BL-21. The expression of GST fusion protein was induced with 0.1 mM IPTG (isopropyl-1-thio-β-d-galactopyranoside), and a protein extract was prepared with 1% Triton X-100 in PBS. The extract was incubated with glutathione-Sepharose 4B beads (Amersham Pharmacia, Piscataway, NJ) for 6 h at 4°C. Nonspecific binding was blocked with 0.1% BSA in PBS for 1 h and beads were incubated with translated products. After incubation, these beads were washed four times with washing buffer containing 1% Tween 20, 1% NP-40, 500 mM NaCl, and 10 mM Tris-HCl, pH 8, and one time with PBS. Specifically adherent polypeptides were eluted in SDS-PAGE sample buffer and analyzed by SDS-PAGE and Western blotting.
Immunoprecipitation
Transfected cells were incubated with 1 ml of lysis buffer containing 1% Triton X-100, 150 mM NaCl, 5 mM MgCl2, and 25 mM Tris-HCl, pH 7.4, for 30 min at 4°C. Insoluble material was removed through centrifugation at 10,000 × g for 30 min at 4°C. After centrifugation, 20 μl of lysate was saved for the determination of protein expression. The rest of lysate was incubated with antibody and protein A- or protein G- agarose beads (Pierce, Rockford, IL). The bead complexes were washed four times with washing buffer containing 0.1% NP-40, 0.1% Tween 20, 500 mM NaCl, and 10 mM Tris-HCl, pH 8.0, and once with PBS. Proteins were eluted in SDS-PAGE sample buffer. The samples were separated by SDS-PAGE and analyzed by Western blotting.
Cell Fractionation by Continuous Sucrose Gradient Centrifugation
Transfected COS cells were washed with cold PBS and incubated for 10 min on ice in hypotonic buffer containing 10 mM Tris-HCl, pH 7.4, and 0.5 mM MgCl2. Cells were homogenized with 25 strokes in a close-fitting dounce homogenizer. An equivalent volume of sucrose solution containing 0.5 M sucrose, 5 mM MgCl2, 25 mM KCl, and 50 mM Tris-HCl, pH 7.4, was added and the mixture was homogenized again with another 25 strokes. The homogenate was layered onto a 1.02 to 0.25 M sucrose gradient and centrifuged at 100,000 × g for 2 h. Twenty fractions were collected from the top to the bottom. These fractions were analyzed by Western blotting.
Biotinylation and Internalization Assays
LLC-PK1 cells which were stably transfected with cDNAs encoding arrestins, spinophilin, or empty vector (mock) were grown to confluence on 24-mm diameter Transwell filter inserts (Corning Costar, Acton, MA). Cells were washed in ice-cold PBS2+ and incubated with 2.5 mM Sulfo-NHS-SS-biotin (Pierce) in biotinylation buffer (10 mM triethanolamine, 2 mM CaCl2, 125 mM NaCl2, pH 7.4) for two times for 20 min. Excess biotin was quenched 3 × 5 min with 100 mM glycine in PBS2+. For internalization assays, cells were placed in media heated to 37°C and allowed to incubate at 37°C for 30 min. For MesNa stripping, cells were removed from the incubator, washed with ice-cold PBS2+, and incubated 3 × 20 min at 4°C in MesNa solution (100 mM sodium 2-mercaptoethanesulfonate, 100 mM NaCl, 1 mM EDTA, 0.2% BSA, and 50 mM Tris, pH 8.6). Excess MesNa was quenched by incubating the cells in 120 mM iodoacetic acid in PBS2+ for three times for 5 min. Samples were incubated in lysis buffer for 30 min at 4°C, and insoluble material was removed by centrifugation at 10,000 × g for 30 min at 4°C. The supernatant was rotated overnight at 4°C with streptavidin-conjugated agarose beads (Pierce). The bead complexes were washed four times with lysis buffer and one time with PBS. Proteins were eluted in SDS-PAGE sample buffer. The samples were separated by SDS-PAGE and analyzed by Western blotting.
Purification of the Na+,K+-ATPase from Rabbit Kidney
A rabbit was anesthetized with pentobarbital, and the kidneys were removed. The kidneys were washed with cold His-Sucrose buffer containing 30 mM histidine and 250 mM sucrose, pH 7.2, and homogenized. The homogenate was centrifuged at 6000 × g for 30 min, and the supernatant was retained. The pellet was resuspended in His-sucrose buffer, homogenized, and centrifuged again. The supernatants were combined and centrifuged at 50,000 × g for 30 min. The pellet was resuspended in imidazole-EDTA buffer containing 25 mM imidazole and 1 mM EDTA, pH 7.2. The microsome pellet was incubated with 10 mg/ml BSA and 0.75 mg/ml SDS for 5 min at 22°C. To this mixture, one-fifth volume of 10 mg/ml BSA was added and centrifuged at 50,000 × g for 60 min. The pellet was resuspended in imidazole-EDTA buffer. The ouabain-sensitive specific activity of this preparation purified of Na+,K+-ATPase was 46.5 μmol Pi/mg/h.
In Vitro Phosphorylation of the Na+,K+-ATPase
Purified Na+,K+-ATPase was prepared from rabbit kidney as described above. GST fusion proteins, including the large cytoplasmic loop of the Na+,K+-ATPase α-subunit, were prepared as described above in reference to the GST pulldown assay. HA-tagged GRK 2 and 3 were expressed in COS cells, and cells were lysed in buffer containing 2% CHAPS, 150 mM NaCl, 5 mM MgCl2, and 25 mM Tris-HCl, pH 7.4. GRKs were purified by immunoprecipitation with HA antibody. Immunocomplexes were washed three times with lysis buffer and two times with phosphorylation buffer containing 1.2 mM CaCl2, 10 mM MgCl2, and 50 mM HEPES, pH 7.5. Purified Na+,K+-ATPase or GST fusion protein (2 μg) were mixed with the GRK immunoprecipitates in phosphorylation buffer. Phosphorylation was initiated by addition of 100 μM ATP containing [γ-32P]ATP (10 μCi) and terminated after 30 min of incubation at 22°C through the addition of SDS-PAGE sample buffer. Samples were subjected to SDS-PAGE, and the gels were dried and analyzed by autoradiography.
RESULTS
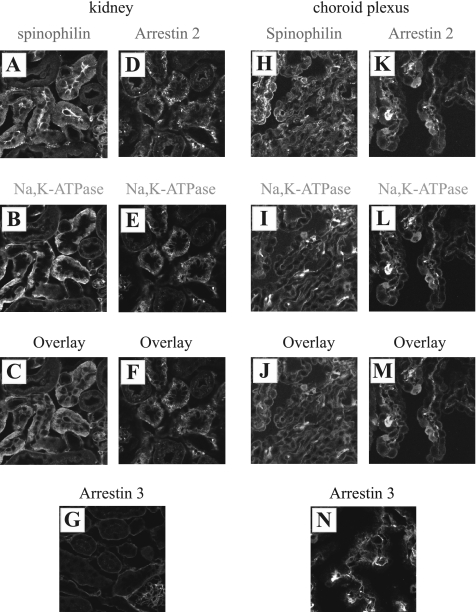
Localization of Na+,K+-ATPase, Spinophilin, and Arrestin 2 and 3 in Mouse Kidney and Choroid Plexus
To confirm that the Na+,K+-ATPase, spinophilin, and arrestins localize to the same subcellular structures in physiologically relevant tissues, immunocytochemistry was performed on sections prepared from mouse kidney (Figure 1, A–G) and brain (Figure 1, H–N). Mouse sections were labeled with an anti-Na+,K+-ATPase antibody (Figure 1, B, E, I, and L), with an antibody directed against spinophilin (Figure 1, A and H), against arrestin 2 (Figure 1, D and K), and against arrestin 3 (Figure 1, G and N). Na+,K+-ATPase was detected at the basolateral membrane of renal tubule epithelial cells. The Na+,K+-ATPase and spinophilin were partially colocalized along the basolateral infoldings of epithelial cells in the proximal tubules (C). Spinophilin was also expressed at apical membrane of proximal tubule cells (A). There are four isoforms of arrestin, and it has been shown that arrestin 2 (Parruti et al., 1993) and arrestin 3 (Attramadal et al., 1992) interact with GPCRs. Arrestin 2 was expressed at apical and basal membranes and in intracellular compartments in renal tubule cells (D), and the Na+,K+-ATPase and arrestin 2 were partially colocalized primarily at intracellular structures (F). Arrestin 3 was expressed at the basolateral membrane in renal tubule cells (G). We were not able to perform double staining with arrestin 3 and the Na+,K+-ATPase because both antigens were detected with mouse antibodies, but comparison of their individual distributions suggest that they were colocalized. Because spinophilin is highly expressed in brain, brain sections were also examined. Choroid plexus forms the blood-cerebrospinal fluid barrier, and through its activity it plays a key role in the governing composition of the cerebrospinal fluid. The Na+,K+-ATPase is expressed at the apical membrane in epithelial cells of the choroid plexus (Figure 1, I and L; Masuzawa et al., 1984; Siegel et al., 1984). Spinophilin was also expressed at or close to the apical membrane (Figure 1H). The Na,K-ATPase and arrestin are colocalized at the apical membrane and in intracellular compartments of choroid plexus epithelial cells (Figure 1, K–M). Unlike the Na+,K+-ATPase, arrestin 3 was expressed at the basolateral surfaces of choroid plexus epithelial cells (Figure 1N).
Figure 1.
Immunolocalization of arrestins, spinophilin and the Na+,K+-ATPase in situ. Mouse kidney (A–G) and choriod plexus (H–N) sections were stained with spinophilin (A and H), arrestin 2 (D and K), arrestin 3 (G and N), and Na+,K+-ATPase (B, E, I, and L) antibodies. Merged images are shown in C, F, K, and M (×63 magnification). Spinophilin and arrestin 2 and 3 were partially colocalized with the Na+,K+-ATPase at basolateral membranes in the renal tubule cells or at apical membranes in the choroid plexus. Typical results from one of three experiments are shown.
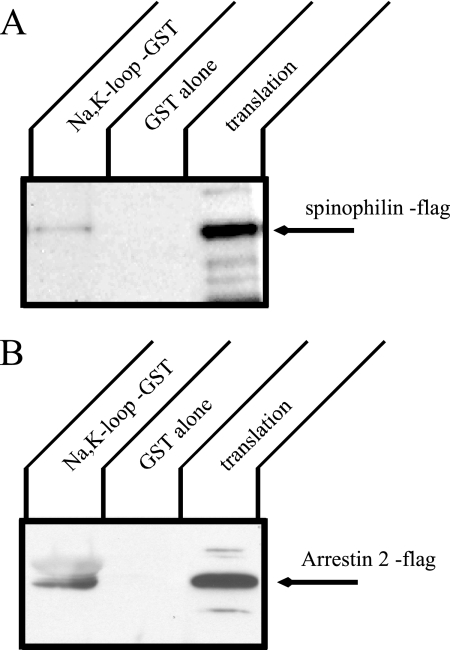
GST Pulldown Using In Vitro–translated Products
To determine whether arrestin and spinophilin bind directly to the Na+,K+-ATPase α-subunit, we performed a GST pulldown assay (Figure 2). Flag-tagged spinophilin and arrestin 2 were prepared by in vitro translation in reticulocyte lysates and subjected to pulldown assays with a GST fusion protein incorporating the large cytoplasmic loop of the Na+,K+-ATPase. Bound spinophilin or arrestin was detected by Western blot with anti-flag antibody. There was no binding of spinophilin or arrestin 2 with GST alone. In contrast, the large cytoplasmic loop of the Na+,K+-ATPase α-subunit pulled down both spinophilin and arrestin. These results indicate that the Na+,K+-ATPase is capable of binding to both arrestin and spinophilin.
Figure 2.
In vitro translation of spinophilin or arrestin and pulldown with a GST construct incorporating the large cytoplasmic loop of the Na,K-ATPase α-subunit. Flag-tagged spinophilin and arrestin proteins were prepared by in vitro translation, and GST pulldown was performed with GST alone or GST plus the large cytoplasmic loop of the Na+,K+-ATPase. Spinophilin (A) and arrestin (B) were detected by Western blot with anti-flag antibody. Both spinophilin and arrestin directly associate with the large cytoplasmic loop of the Na,K-ATPase α-subunit. Typical results from one of three experiments are shown.
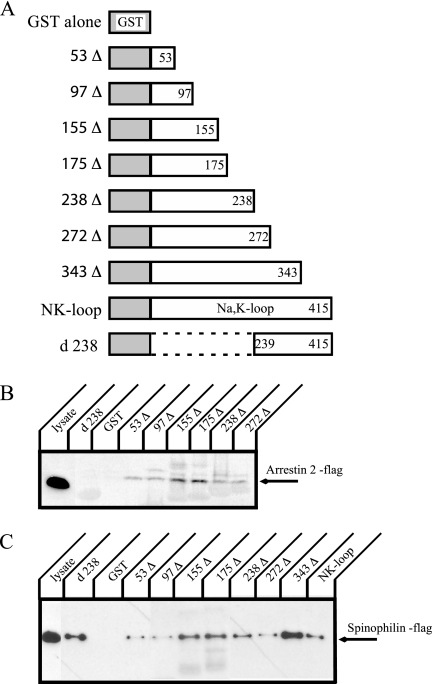
Characterization of the Interaction of the Large Cytoplasmic Loop of the Na+,K+-ATPase with Arrestin and Spinophilin
GST pulldown studies revealed that arrestin and spinophilin associate with the large cytoplasmic loop of the Na+,K+-ATPase α-subunit (Na+,K+-loop). To narrow down the domain that constitutes the interaction site, deletion constructs were used in the GST pulldown assay. The Na+,K+-loop is comprised of 415 amino acids. We generated constructs in which the C-terminal side of the GST-Na+,K+-loop fusion protein was truncated stepwise (Figure 3A). A truncation at the N-terminal side of the Na+,K+-loop was also generated. The GST fusion proteins were expressed in E. coli and purified. Protein levels were measured by analysis of Coomassie- stained gels (data not shown). GST pulldown with cell lysates that transiently expressed flag-tagged arrestin 2 (Figure 3B) or spinophilin (Figure 3C) was performed. Arrestin 2 was pulled down by all deletion constructs except for d238. This result indicates that 53 amino acids at the N-terminal of the Na+,K+-loop are sufficient for binding with arrestin 2. On the other hand, all constructs including the complementary constructs d238 and 238Δ pulled down spinophilin. This result shows that there must be at least two interacting sites for spinophilin in the Na+,K+-loop. The 343Δ fusion protein always showed much better binding than the full-length Na+,K+-loop. It is possible therefore, that sequences located in the 72 amino acids at the C-terminus in the Na+,K+-loop exert an inhibitory effect on spinophilin association.
Figure 3.
Truncation constructs of the large cytoplasmic loop of the Na+,K+-ATPase α-subunit and GST pulldown of arrestin 2 and spinophilin. (A) Schematic representations of the GST fusion protein constructs incorporating various deletions in the large cytoplasmic loop of the Na+,K+-ATPase α-subunit. Δs correspond to the C-terminal deletions, whereas d238 is the N-terminal deletion of the large cytoplasmic loop. The numbers refer to the residues that correspond to the C-terminal ends or, in the case of the d238 construct, the N-terminal end, of the pump sequence in the GST construct. (B) Flag-tagged arrestin 2 was expressed in COS cells, and cell lysate was incubated with GST fusion proteins. Arrestin 2 was detected by Western blot with anti-flag antibody. The 53 amino acids at the extreme N-terminal end of the large cytoplasmic loop of the Na+,K+-ATPase α-subunit appear to be sufficient for binding with arrestin 2. (C) Flag-tagged spinophilin was expressed in COS cells, and cell lysates were incubated with GST fusion proteins. Spinophilin was detected by Western blot with anti-flag antibody. Spinophilin appears to interact with at least two binding sites in the large cytoplasmic loop. Typical results from one of four experiments are shown.
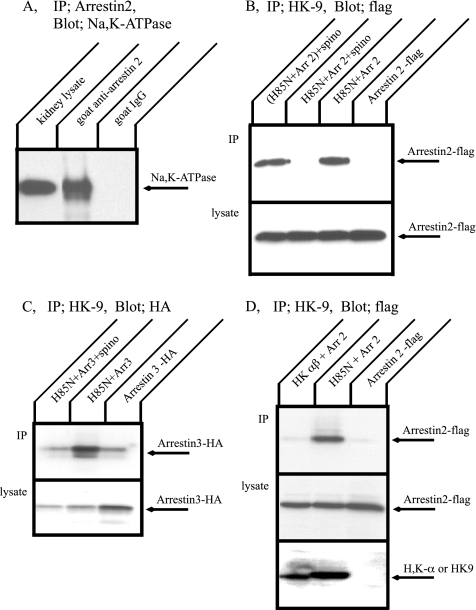
Coimmunoprecipitation of the Na+,K+-ATPase α-Subunit and Arrestin 2
Immunoprecipitation was performed from kidney tissue to determine whether the Na+,K+-ATPase associates with arrestin 2 in situ using goat anti-arrestin 2 antibody or goat serum as a negative control (Figure 4A). The Na+,K+-ATPase α-subunit that associated and coprecipitated with arrestin 2 was detected by Western blotting with anti-Na+,K+- ATPase α-subunit antibody. Arrestin 2 antibody clearly coprecipitated the Na+,K+-ATPase α-subunit. This result supports the conclusion that the Na+,K+-ATPase associates with arrestin 2 and forms a stable complex in situ.
Figure 4.
Immunoprecipitation of the Na+,K+-ATPase and arrestin. (A) Mouse kidney lysate was incubated with a goat antibody directed against arrestin 2 or with nonimmune goat serum (control) followed by protein A beads. Immune complexes were separated by SDS-PAGE, and Western blot was performed with anti Na+,K+-ATPase antibody, α5. The Na+,K+-ATPase was coprecipitated with arrestin 2. (B) COS cells were transfected with flag-arrestin 2 alone, H85N plus flag-arrestin 2, or H85N, flag-arrestin 2, and spinophilin. Immunoprecipitation was performed with HK9 antibody directed against the N terminus of H85N, and arrestin 2 was detected by Western blotting with anti-flag antibody. Arrestin 2 was pulled down with the Na+,K+-ATPase α-subunit. This association, however, was inhibited by coexpression of spinophilin. (C) COS cells were transfected with HA-arrestin 3 alone, H85N plus HA-arrestin 3, or H85N, HA-arrestin 3 and spinophilin. Immunoprecipitation was performed with HK9 antibody and arrestin 3 was detected by Western blotting with anti-HA antibody. Arrestin 3 was also coimmunoprecipitated with the Na+,K+-ATPase, and this association was blocked by coexpression of spinophilin. (D) COS cells were transfected with flag-arrestin 2 alone or H+,K+-ATPase α- and β-subunits plus flag-arrestin 2. Immunoprecipitation was performed with HK9 antibody and arrestin 2 was detected by Western blotting with anti-flag antibody. Arrestin 2 was not immunoprecipitated with the H+,K+-ATPase. Typical results from one of five experiments are shown.
Next, we investigated the dependence of the interaction between the Na+,K+-ATPase and the arrestin 2 upon the expression of spinophilin. For these experiments, COS cells were cotransfected with cDNAs encoding arrestin 2 as well as a cDNA encoding the H85N chimera α-subunit construct. H85N is a chimera in which the first 85 residues of the Na+,K+-ATPase α-subunit are replaced by those of the gastric H+,K+-ATPase. This chimera manifests functional properties identical to those of the Na+,K+-ATPase and is recognized by the HK9 antibody directed against the N-terminus of the H+,K+-ATPase α-subunit (Dunbar et al., 2000). Figure 4B shows Western blot patterns of transfected COS cell lysates subjected to immunoprecipitation with the HK9 antibody and then detected with the anti-flag antibody, which recognizes the exogenous arrestin 2. As expected, when cells were transfected only with arrestin 2, no arrestin was observed in the HK9 immunoprecipitate. In contrast, we found that arrestin 2 was immunoprecipitated when H85N was coexpressed with arrestin 2. Coexpression of spinophilin, however, blocked association of H85N and arrestin 2. Furthermore, spinophilin must be expressed in the same cells as H85N and arrestin to inhibit interaction, because mixing lysate from cells expressing spinophilin with lysate from cells expressing H85N and arrestin 2 did not affect the interaction between H85N and arrestin. When expressed in the same cells, arrestin and spinophilin did not coimmunoprecipitate (data not shown). As shown in Figure 4C, arrestin 3 was also coimmunoprecipitated with the H85N α-subunit. Arrestin 3 binding with H85N α-subunit was diminished by coexpressing spinophilin, consistent with the results obtained with arrestin 2. These results suggest that, like GPCRs, the Na+,K+-ATPase may be regulated by arrestin and spinophilin.
Gastric H+,K+-ATPase is a member of the P-type ATPase family and a very close relative of the Na+,K+-ATPase. The H+,K+-ATPase is composed of two subunits and has the same topology as the Na+,K+-ATPase. H+,K+-ATPase α- and β-subunits and flag-tagged arrestin 2 were transiently coexpressed in COS cells, and immunoprecipitation was performed with HK9 antibody (Figure 4D). Arrestin 2 was not pulled down with the H+,K+-ATPase. We confirmed that the H+,K+-ATPase β-subunit was precipitated with the H+,K+-ATPase α-subunit under these conditions (data not shown). This result demonstrates that there is specificity in arrestin binding among similar P-type ATPases.
Effect of Arrestin and Spinophilin on the Localization of the Na+,K+-ATPase
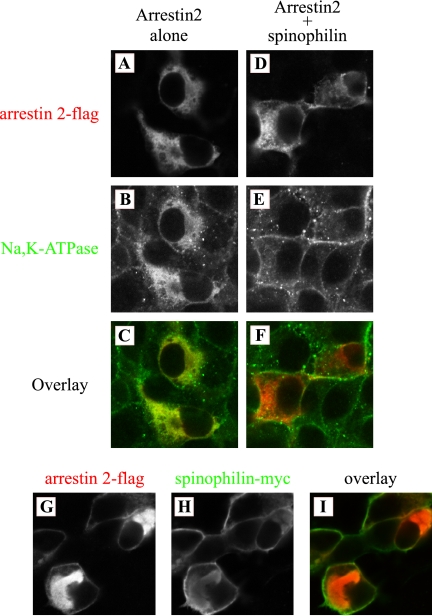
It has been shown that arrestins and spinophilin regulate the trafficking of GPCRs. Recent studies also showed that cell surface expression of the Na+/H+ exchanger NHE 5 was decreased by overexpression of arrestin. Therefore, we investigated the effect of arrestin and spinophilin on the localization of the Na+,K+-ATPase (Figure 5). COS cells were transfected with flag-arrestin 2 alone or flag-arrestin 2 plus myc-spinophilin, and cells were stained with flag antibody for arrestin 2 (Figure 5, A, D, and G), the Na+,K+-ATPase antibody for endogenous Na+,K+-ATPase (Figure 5, B and E), and anti-myc antibody for spinophilin (Figure 5H). When cells were transfected arrestin 2 alone, arrestin 2 was expressed predominantly in association with intracellular structures (Figure 5A). The Na+,K+-ATPase was also redistributed to intracellular structures (Figure 5B) and was colocalized with arrestin 2 (Figure 5C). In the presence of spinophilin, however, the effect of arrestin 2 was abolished. The Na+,K+-ATPase did not colocalize with arrestin 2 (Figure 5F) and was expressed primarily at the cell surface (Figure 5E), as is the Na+,K+-ATPase in untransfected cells (data not shown). Spinophilin was expressed mainly at plasma membrane either in the presence or absence of arrestin 2 (Figure 5H). The expression of arrestin 2 was also altered by spinophilin expression. In the presence of spinophilin, arrestin was found at the plasma membrane (Figure 5G). Arrestin 3 had an effect similar to that of arrestin 2 (data not shown). These results are consistent with the immunoprecipitation results and indicate that arrestin and spinophilin regulate Na+,K+-ATPase trafficking through direct associations between the Na+,K+-ATPase and arrestin 2.
Figure 5.
Immunolocalization of the Na+,K+-ATPase, arrestin, and spinophilin in COS cells. COS cells were transfected with flag-arrestin alone (A–C) or with flag-arrestin plus myc-spinophilin (D–I), and cells were stained with anti-flag for arrestin (A, D, and G) and anti-Na+,K+-ATPase (B and E) or anti-c-myc for spinophilin (H) antibodies. Overlay patterns are shown in C, F, and I (×200 magnification). The Na+,K+-ATPase is located in intracellular structures when cells express arrestin alone. This effect is not observed when arrestin is expressed together with spinophilin. Arrestin is distributed at least in part at the plasma membrane when spinophilin is coexpressed. Typical results from one of three experiments are shown.
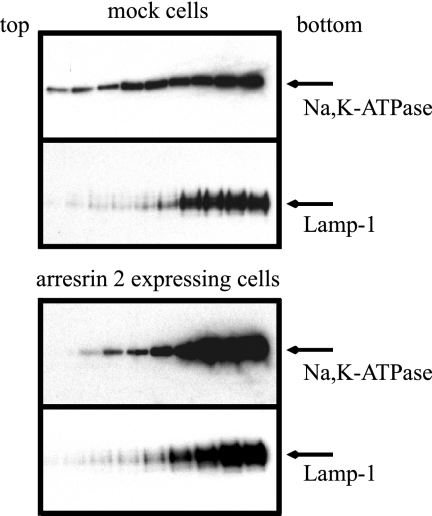
It appeared that arrestin overexpression led to relocalization of the Na+,K+-ATPase to intracellular compartments. This phenomenon was confirmed by sucrose gradient centrifugation (Figure 6). Na+,K+-ATPase was not detected in fractions 1–11, the lightest gradient fractions, in mock-transfected or arrestin-expressing cells (data not shown). In the mock-transfected cells, the Na+,K+-ATPase was broadly distributed in all of the fractions from numbers 12–20. On the other hand, in transfected cells expressing arrestin, the distribution of the Na+,K+-ATPase in the gradient changed dramatically. The fractionation behavior of the Na+,K+-ATPase in these cells closely resembled that of lamp-1, which is a lysosomal marker protein. All of the fractions between numbers 12 and 20 had almost equal amounts of arrestin (data not shown). These results indicate that arrestin expression induces the redistribution of the Na+,K+-ATPase to a dense intracellular compartment that cofractionates, at least in part, with lysosomes.
Figure 6.
Cell fractionation of arrestin-expressing and mock-transfected cells by sucrose density gradient centrifugation. COS cells were transfected with vector alone (mock) or arrestin 2. Cells were homogenized in sucrose buffer and centrifuged at 800 × g. Supernatant was layered on a continuous 1.02 to 0.25 M sucrose gradient. After centrifugation, fractions were collected from the top and analyzed by Western blotting with antibodies directed against the Na+,K+-ATPase or the lysosome marker lamp-1. No Na+,K+-ATPase signal was detected in fractions 1 through 11 under either condition. The Western blotting results shown represent fraction numbers 12–20 (from left to right). Typical results from one of four experiments are shown.
Effect of Arrestin and Spinophilin on the Internalization of the Na+,K+-ATPase
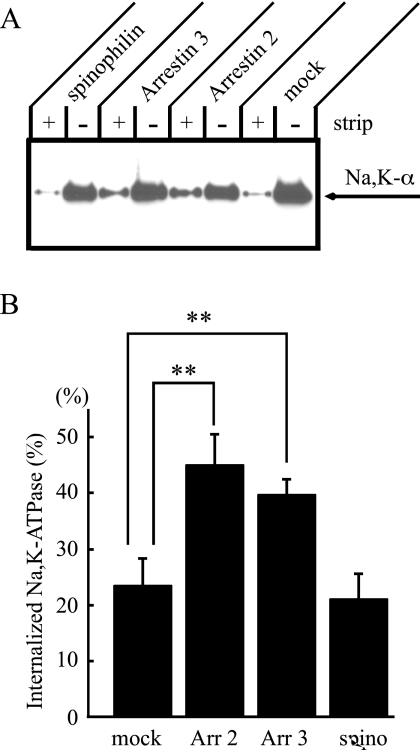
As shown in Figures 5 and 6, the Na+,K+-ATPase was associated with intracellular compartments at steady state when cells overexpressed arrestin. To quantify the extent of Na+,K+-ATPase internalization from the cell surface, we performed cell-surface biotinylation and surface stripping experiments. Stable cell lines expressing a mock plasmid, arrestin 2 or 3, or spinophilin were generated in the pig proximal tubule cell line, LLC-PK1 cells. It should be noted that these LLC-PK1 cells express spinophilin endogenously (data not shown). In the first experiment, LLC-PK1 cells were biotinylated with biotin-SS-N-hydroxysuccinimide ester at 4°C. Duplicate samples were then incubated at 37°C in the presence of PMA for 30 min to stimulate Na+,K+- ATPase endocytosis (Chibalin et al., 1999). Cells were returned to 4°C, and one plate was stripped of the remaining cell-surface biotin by exposure to the membrane-impermeant reducing agent MesNa. The cells were lysed in detergent and the lysates were incubated with streptavidin beads. Proteins that associated with the streptavidin beads were separated by SDS-PAGE, transferred to polyvinylidene fluoride (PVDF), and probed with the anti-Na+,K+-ATPase α-subunit antibody. Figure 7A shows typical Western blotting results from these internalization experiments, and Figure 7B shows the ratio of internalized (strip+) Na+,K+-ATPase, to the total pool of the biotinylated Na+,K+-ATPase (strip−). Mock-transfected cells exhibited a low level of internalization, even in the presence of PMA. When arrestin 2 or 3 were expressed, internalization of the Na+,K+-ATPase was increased by about twofold compared with mock-transfected cells. Overexpression of spinophilin had no effect on the internalization of the Na+,K+-ATPase. Endogenous spinophilin may exist in sufficient quantities to stabilize the Na+,K+-ATPase at plasma membrane. These results also support the model that arrestins modulate the trafficking of the Na+,K+-ATPase in a manner similar to their effect on GPCRs.
Figure 7.
Internalization of the Na+,K+-ATPase. (A) Stable cell lines expressing a mock plasmid, arrestin 2 (Arr 2) or arrestin 3 (Arr 3), or spinophilin (spino) were grown on Transwell plates, and cells were biotinylated at 4°C. Cells were then incubated in culture medium at 37°C for 30 min in the presence of 1 μM PMA to stimulate endocytosis. To measure the size of the internalized pool, biotin remaining at the cell surface was stripped with 2-mercaptoethanesulfonic acid, sodium salt (MesNa). Biotinylated proteins were recovered by precipitation with streptavidin-conjugated agarose beads. Total surface-biotinylated Na+,K+-ATPase (nonstrip) and internalized Na+,K+-ATPase (strip) were detected by Western blot with anti-Na,K-ATPase antibody, 6H. (B) When cells expressed arrestins, the extent of Na+,K+-ATPase internalization was about twofold greater than that detected in mock-transfected cells. In contrast, cells expressing spinophilin exhibited roughly the same extent of internalization of the Na+,K+-ATPase as mock cells. Typical results from one of eight experiments are shown. Error bars, SEM; statistically significant differences, *p < 0.05).
Coimmunoprecipitation of the Na+,K+-ATPase α-Subunit and GRKs
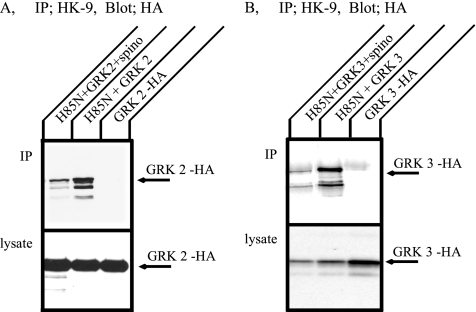
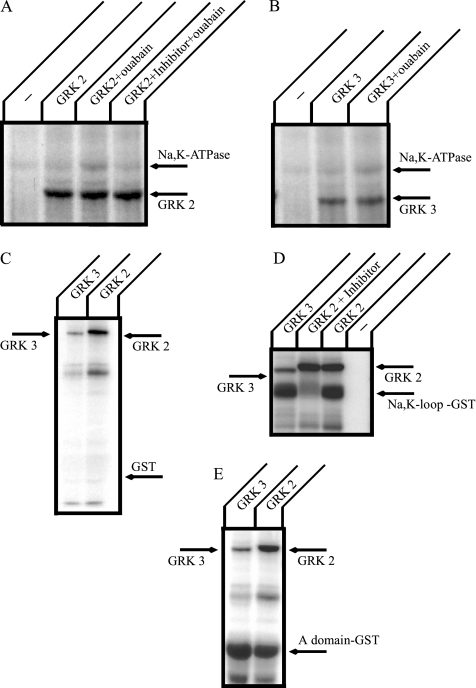
The association between GPCRs and arrestin depends on phosphorylation of the receptors by GRKs. We wondered, therefore, whether the Na+,K+-ATPase could associate with GRK 2 (also called β-adrenergic receptor kinase1) or GRK 3 (also called β-adrenergic receptor kinase 2). COS cells were transfected with HA-GRK 2 or HA-GRK 3 together with H85N in the absence or presence of spinophilin. Immunoprecipitation was performed with HK9 antibody, and GRK 2 or 3 were detected by Western blotting with anti-HA antibody (Figure 8). Both GRK 2 (Figure 8A) and GRK 3 (Figure 8B) were immunoprecipitated with the H85N α-subunit. These associations were inhibited in the presence of spinophilin. The results indicate that the Na+,K+-ATPase can associate with GRKs and may, therefore, be substrates for GRKs.
Figure 8.
Coimmunoprecipitation of the Na+,K+-ATPase and GRKs expressed in COS cells. COS cells were transfected with HA-GRK 2 or HA-GRK 3 alone, or H85N plus HA-GRK 2 or HA-GRK 3. Immunoprecipitation was performed with HK9 antibody directed against the N terminus of H85N, and GRK 2 (A) and GRK 3 (B) were detected by Western blotting with anti-HA antibody. Both GRK 2 and 3 were immunoprecipitated with the Na+,K+-ATPase α-subunit. This association, however, was inhibited in the coexpression of spinophilin. Typical results from one of five experiments are shown.
In Vitro Phosphorylation of the Na+,K+-ATPase by GRKs
Next, we investigated whether the Na+,K+-ATPase can be phosphorylated by GRK 2 or 3 in vitro (Figure 9). GRK 2 and 3 were prepared by immunoprecipitation from transiently transfected COS cells. When the Na+,K+-ATPase was incubated with material immunoprecipitated from mock-transfected cells, little or no Na+,K+-ATPase phosphorylation was observed (Figure 9, A and B). After incubation with immunoprecipitated GRK 2 or 3, the Na+,K+-ATPase was phosphorylated. In the presence of ouabain, which is a specific inhibitor of the Na+,K+-ATPase, somewhat stronger phosphorylation by both GRK 2 and 3 was observed. The ouabain-stimulated GRK 2–catalyzed phosphorylation of the Na+,K+-ATPase was prevented by the addition of GRK 2 inhibitor (Figure 9A).
Figure 9.
In vitro phosphorylation of the large cytoplasmic loop and A domain of the Na+,K+-ATPase α-subunit. Purified Na+,K+-ATPase from rabbit kidney (A and B), GST alone (C), a GST construct incorporating the large cytoplasmic loop of the Na+,K+-ATPase α-subunit (D), and a GST construct incorporating the A-domain of the Na+,K+-ATPase α-subunit (E) were incubated at 22°C for 30 min with [γ-32P]ATP, and GRKs were prepared by immunoprecipitation from COS cell lysates. Reactions were stopped by adding SDS-PAGE sample buffer, and samples were separated by SDS-PAGE. Gels were stained with Coomassie blue, dried, and analyzed by autoradiography. The purified Na+,K+-ATPase was phosphorylated by GRK 2 and 3 and this phosphorylation was enhanced in the presence of 300 μM ouabain. In addition, GRK 2 and 3 phosphorylated the large cytoplasmic loop and A-domain of the Na+,K+-ATPase α-subunit. Typical results from one of four experiments are shown.
We also analyzed the phosphorylation of GST fusion proteins incorporating the large cytoplasmic loop (Figure 9D) or the A-domain (Figure 9E) of the Na,K-ATPase α-subunit. In a control experiment, no phosphorylation of GST was observed with either GRK 2 or 3 (Figure 9C). On the other hand, the Na+,K+-loop construct was strongly phosphorylated by GRK 2 and 3, and GRK 2 phosphorylation was inhibited by GRK 2 inhibitor. The extent or apparent affinity of the interaction between the Na+,K+-loop construct and arrestin was not influenced by in vitro GRK 2 or 3 phosphorylation (data not shown).
The A-domain of the Na+,K+-ATPase consists of the N-terminal and a small cytoplasmic loop between TM 2 to TM3 of the α-subunit (Toyoshima and Mizutani, 2004). This domain includes both PKC phosphorylation (Therien and Blostein, 2000) and 14-3-3 ε binding sites (Efendiev et al., 2005). It has been shown that 14-3-3 ε binding to the Na+,K+-ATPase depends on PKC phosphorylation (Efendiev et al., 2005). It has been also demonstrated that 14-3-3 proteins bind and regulate GPCRs (Prezeau et al., 1999; Couve et al., 2001; Tazawa et al., 2003). We tested whether the A-domain could be phosphorylated by GRKs (Figure 9E). We found that both GRK 2 and 3 can phosphorylate the isolated A-domain of the Na+,K+- ATPase. These results indicate that GRK 2 and 3 have the capacity to phosphorylate the Na+,K+-ATPase within the A-domain and the large cytoplasmic loop of the α-subunit.
Coimmunoprecipitations of the Na+,K+-ATPase α-Subunit and 14-3-3 Proteins
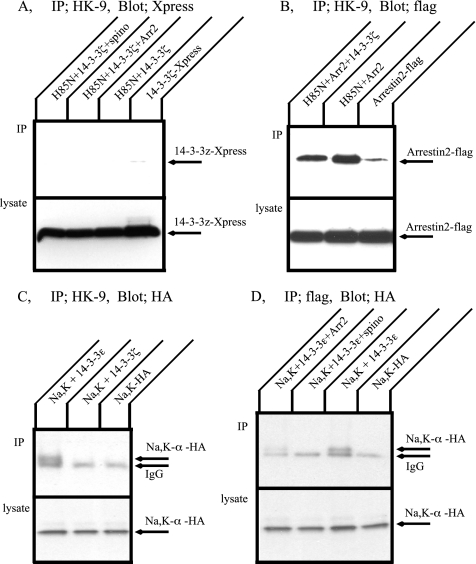
It has been shown that ζ-isoform of 14-3-3 associates with GPCRs and regulates GPCR signaling (Prezeau et al., 1999; Couve et al., 2001). Because arrestin and spinophilin bind to the Na+,K+-ATPase, 14-3-3 ζ may also associate with the Na+,K+-ATPase and induce effects similar to those it exerts on GPCRs. Immunoprecipitation was performed to assess the possibility of an interaction between the Na+,K+- ATPase and Xpress-tagged 14-3-3 ζ. Figure 10A depicts Western blot patterns of immunoprecipitates obtained from transfected COS cell lysates with the HK9 antibody and an anti-Xpress antibody. 14-3-3 ζ was not immunoprecipitated with the H85N α-subunit under any of the conditions tested, including 14-3-3 ζ alone, together with arrestin 2, or with spinophilin. Figure 10B shows the effect of 14-3-3 ζ on the association between arrestin 2 and the Na+,K+-ATPase. Although 14-3-3 ζ did not itself associate with the Na+,K+-ATPase, it appeared to exert a modest inhibitory effect on arrestin binding to this pump.
Figure 10.
Coimmunoprecipitation of the Na,K-ATPase and 14-3-3 proteins expressed in COS cells. (A) COS cells were transfected with Xpress-14-3-3 ζ alone, H85N plus Xpress-14-3-3 ζ, or H85N, Xpress-14-3-3 ζ and flag-arrestin 2 or spinophilin. Immunoprecipitation was performed with HK9 antibody and 14-3-3 ζ was detected by Western blotting with anti-Xpress antibody. 14-3-3 ζ was not immunoprecipitated with the H85N α-subunit under any of the conditions tested. (B) COS cells were transfected with flag-arrestin 2 alone, H85N plus flag-arrestin 2, or H85N, flag-arrestin 2 and 14-3-3 ζ. Immunoprecipitation was performed with HK9 antibody and arrestin 2 was detected by Western blotting with anti-flag antibody. 14-3-3 ζ partially inhibited arrestin 2 binding with the Na+,K+-ATPase, even though 14-3-3 ζ did not itself associate with the Na+,K+-ATPase. (C) COS cells were transfected with HA-Na+,K+-ATPase α-subunit and β-subunit, HA-Na+,K+-ATPase α- and β-subunit plus flag-14-3-3 ε or flag-14-3-3 ζ. Immunoprecipitation was performed with anti-flag antibody, and the Na+,K+-ATPase α-subunit was detected by Western blotting with anti-HA antibody. The Na,K-ATPase α-subunit was coprecipitated with 14-3-3 ε but not with 14-3-3 ζ. (D) Immunoprecipitation between HA-Na+,K+-ATPase α-subunit and 14-3-3 ε in the presence of spinophilin or arrestins. Association between Na+,K+-ATPase α-subunit and 14-3-3 ε was inhibited by expression of spinophilin. Typical results from one of five experiments are shown.
It has been demonstrated that the N-terminal domain of the Na+,K+-ATPase α-subunit interacts with 14-3-3 ε. This interaction increases the endocytosis of the Na+,K+-ATPase through activation of phosphoinositide 3-kinase (Efendiev et al., 2005). The H85N chimera in which the first 85 residues of the Na+,K+-ATPase α-subunit are replaced by those of the gastric H+,K+-ATPase does not contain the 14-3-3 ε binding site. COS cells were transfected with HA-tagged full-length Na+,K+-ATPase α-subunit alone or together with flag-tagged 14-3-3 proteins. Cell lysates were immunoprecipitated with flag antibody and Na+,K+-ATPase was detected by Western blotting with anti-HA antibody. As expected, when cells expressed the Na+,K+-ATPase and 14-3-3 ε, the Na+,K+-ATPase was precipitated. On the other hand, 14-3-3 ζ did not coprecipitate the Na+,K+-ATPase. This result shows that the pump exhibits isoform specificity in binding 14-3-3 proteins. We also investigated the effect of arrestin 2 and spinophilin on the association between the Na+,K+-ATPase and 14-3-3 ε (Figure 10D). COS cells were transfected with the HA-tagged Na+,K+-ATPase α-subunit and 14-3-3 ε together with spinophilin or arrestin. Cell lysates were subjected to immunoprecipitation with the flag antibody, and then the Na+,K+-ATPase was detected with the anti-HA antibody. 14-3-3 ε binding with the Na+,K+-ATPase was completely blocked in the presence of spinophilin and arrestin. These results suggest that arrestin and spinophilin may regulate trafficking in part by inhibiting 14-3-3 ε association, as well as through their own direct binding to the Na+,K+-ATPase.
DISCUSSION
Arrestins, GRKs and spinophilin collaborate in the regulation of GPCR surface expression and activity. In this article we demonstrate that these same proteins act in very similar ways to modulate the function of the Na+,K+-ATPase, a physiologically important effector of GPCR signaling. The Na+,K+-ATPase is subject to regulation by a large number of GPCR signaling processes. Norepinephrine, for example, acts through the α1A-adrenoceptor to increase the activity of the Na+,K+-ATPase in rat brain by inducing its dephosphorylation (Mallick et al., 2000). Vasopressin treatment of a rat cortical collecting duct cell line stimulates the translocation of the Na+,K+-ATPase to the plasma membrane and decreases the quantity of pump associated with intracellular compartments (Djelidi et al., 2001). PTH inhibits the activity of the Na+,K+-ATPase in opossum kidney cells (Khundmiri and Lederer, 2002). Dopamine also alters the functional properties of the Na+,K+-ATPase. Through activation of a D2-like receptor, dopamine has been found to stimulate the activity of the Na+,K+-ATPase in the proximal tubules of the kidney (Brismar et al., 2002; Narkar et al., 2002). Dopamine has also been shown to inhibit the Na+,K+-ATPase through D1- and D2-like receptors operating through the phospholipase C-PKC cascade and PKA-dependent pathways, respectively (Aperia et al., 1987; Bertorello and Katz, 1993; Baines and Drangova, 1997; Pinto-do et al., 1997; Khundmiri and Lederer, 2002). In general the effects of GPCR signaling on sodium pump activity are thought to be mediated by the phosphorylation/dephosphorylation of the Na+,K+-ATPase α-subunit at its PKC, PKA, or tyrosine phosphorylation sites, which in turn increases/decreases the size of the cell surface population and activity of the Na+,K+-ATPase (Chibalin et al., 1999; Mallick et al., 2000; Djelidi et al., 2001; Narkar et al., 2002). We propose a novel additional mechanism for GPCR-mediated regulation of Na,K-ATPase activity that involves arrestins, GRKs, spinophilin, and 14-3-3 proteins, the same cellular machinery that regulates signaling through the GPCRs themselves.
Four distinct mammalian arrestin proteins have been identified, two of which (visual and cone arrestins) are restricted to the phototransduction pathway. Two somatic forms, arrestin 2 (β-arrestin 1) and arrestin 3 (β-arrestin 2) are ubiquitously expressed and are thought to regulate signaling as well as internalization of many different heptahelical receptors (Sterne-Marr and Benovic, 1995). Arrestins can also enhance the internalization and modulate the signaling of other classes of non-GPCR receptors, including single membrane-spanning receptors for insulin-like growth factor I (Lin et al., 1998), transforming growth factor β (TGFβR-III; Chen et al., 2003), and low-density lipoprotein (LDLR; Wu et al., 2003). Recent studies have shown that the NHE5 isoform of the 12-membrane–spanning Na+/H+ exchanger can also associate with arrestins. Furthermore, interactions with arrestins lead to a decreased cell surface expression of the NHE 5 (Szabo et al., 2005). Interestingly, TGFβR-III, LDLR and NHE 5 do not require any specific activation in order to be susceptible to arrestin effects. In the case of TGFβR-III and LDLR, association with arrestins was found to be ligand independent and was associated with constitutive endocytosis. The arrestin effects on the cell surface expression and activity of NHE 5 also occur without the involvement any ligands or effectors. It remains unclear as to whether specific ligands or stimuli are required to induce the interaction of the Na+,K+-ATPase with arrestins and thus to decrease cell surface expression via enhanced internalization. When we overexpressed arrestins in COS cells, we found that the Na+,K+-ATPase was retained intracellularly in the absence of any added stimuli. On the other hand, expression of exogenous arrestins enhanced the internalization of the Na+,K+-ATPase induced by PKC activation in LLC-PK1 cells. Furthermore, GRK phosphorylation of the Na+,K+-ATPase increased in the presence of ouabain. It is likely that there are cell type–specific influences on the effects induced by arrestins on Na+,K+-ATPase trafficking and function, because the expression of other interacting proteins such as spinophilin, GRKs and 14-3-3 will modulate the influence of arrestins on pump function.
Spinophilin participates in a variety of processes in addition to those involving GPCR signaling. Spinophilin was originally identified and cloned based on its activity as a protein phosphatase 1 binding protein (Allen et al., 1997). It serves as a targeting subunit for protein phosphatase 1 (PP-1), linking this enzyme to a variety of substrate phosphoproteins. PP-1 is known as one of the phosphatases that regulate the activity of the Na+,K+-ATPase (Li et al., 1995). Shortly after the original cloning of spinophilin, another group cloned Neurabin II based on its ability to act as a F-actin–binding protein (Satoh et al., 1998). Neurabin II and spinophilin are one in the same protein. It has also been demonstrated that TGN38, which constitutively cycles between the trans-Golgi network (TGN) and the plasma membrane, interacts directly with spinophilin, and it has been suggested that spinophilin provides a link between TGN-containing membranes and the actin cytoskeleton (Stephens and Banting, 1999). These observations suggest that spinophilin may play key roles in several facets of the Na+,K+-ATPase's complex life cycle, including connecting it to PP1 to regulate its activity, linking it to F-actin and thus to the actin cytoskeleton, and inhibiting its associations with arrestins, GRKs and 14-3-3.
Arrestin 2 and/or arrestin 3 associate with most classes of GPCRs, which exhibit agonist-evoked conformational changes and are susceptible to phosphorylation by GRKs. Spinophilin also interacts with at least two subfamilies of GPCRs; the α2-adrenergic receptor subtypes (Richman et al., 2001; Wang and Limbird, 2002; Brady et al., 2003) and the D2 dopamine receptor (Smith et al., 1999). Arrestins and spinophilin appear to act competitively in regulating these receptors. Arrestins accelerate signaling desensitization and receptor internalization, whereas spinophilin stabilizes the retention of receptors at the cell surface by preventing GRK phosphorylation and arrestin binding both in vitro and in vivo (Brady et al., 2003; Wang et al., 2004a). Our observations suggest that arrestins and spinophilin may operate in a highly analaogous manner to modulate the function of the Na+,K+-ATPase. Arrestins were able to associate with the Na+,K+-ATPase, and this association increased the pump's internalization. Spinophilin blocked arrestin binding and inhibited GRK association, as well as arrestin-induced internalization of the Na+,K+-ATPase. We were not able to show that GRK phosphorylation of the Na+,K+-ATPase directly leads to arrestin binding to the sodium pump. Because spinophilin was able to compete the binding between both arrestin and GRK and the Na+,K+-ATPase, it is possible that spinophilin may inhibit GRK phosphorylation of the Na+,K+-ATPase and the sodium pump's association with arrestin in a manner similar to that observed with GPCRs. Alternatively, the inhibitory effects of spinophilin on interactions between the Na+,K+-ATPase and arrestin or GRK may be due to overlapping interaction sites, as suggested by the GST pulldown study. This is the first evidence that arrestins and spinophilin can act competitively to regulate a nonreceptor membrane protein such as the sodium pump. Because the activities of many transport proteins and ion pumps are regulated by GPCR signaling, it is interesting to speculate that arrestins and spinophilin may influence other transport systems in a similar manner.
14-3-3 proteins bind and modulate the function of phosphorylated target proteins. Because the 14-3-3 ζ isoform cooperates with arrestins and spinophilin to orchestrate the regulation of the α2-adrenergic receptor (Prezeau et al., 1999; Wang and Limbird, 2002), we expected that the Na+,K+-ATPase might also associate with 14-3-3 ζ. We were surprised to find, therefore, that 14-3-3 ζ was not coimmunoprecipitated with the H85N α-subunit construct. During the preparation of this article, Efendiev et al. (2005) reported that the 14-3-3 ε isoform interacted with the Na+,K+-ATPase at the N-terminus of the α-subunit. Because in the H85N α-subunit construct the first 85 residues of the Na+,K+-ATPase are replaced with the complementary sequence from the gastric H,K-ATPase, we wondered whether this modification might have altered the capacity of the H85N construct to interact with 14-3-3 proteins. To test this possibility, we expressed a HA-tagged full-length Na+,K+-ATPase α-subunit and assessed its ability to coimmunoprecipitate the 14-3-3 ζ or ε isoforms. In contrast to the behavior found with the α2-adrenergic receptor, 14-3-3 ε but not 14-3-3 ζ coimmunoprecipitated with the Na+,K+-ATPase. Similar inhibitory effects of arrestin and spinophilin on 14-3-3 association were detected, even though these proteins appear to interact with different domains of the Na+,K+-ATPase α-subunit sequence. Different 14-3-3 isoform proteins may introduce specificity into the relationships between receptors and ion pumps. Recent studies show that the plasma membrane Ca2+-ATPase isoform 4, which, like the Na+,K+-ATPase, belongs to the P-type ATPase family, also associates with the 14-3-3 ε isoform and this association leads to inactivation of the Ca2+-ATPase (Rimessi et al., 2005). This interaction is isoform specific with respect to both the Ca2+-ATPases and the 14-3-3s, because the Ca2+-ATPase isoform 2 does not interact with the 14-3-3 ε isoform, and the Ca2+-ATPase isoform 4 does not coprecipitate with the 14-3-3 ζ and θ isoforms. These findings are consistent with the premise that there is likely to be specificity in the interactions between signaling proteins and their effectors.
Arrestins participate in complexes with many interesting proteins that may contribute to the regulation of the Na+,K+-ATPase or be involved in signaling through the Na+,K+-ATPase. These include clathrin, clathrin adaptor protein AP-2, the nonreceptor tyrosine kinase Src, and the extracellular signal-regulated kinase 2 (ERK2), which is a member of the mitogen-activated protein kinase (MAPK) family (Claing et al., 2002; Shenoy and Lefkowitz, 2003; Lefkowitz and Shenoy, 2005). It has been reported that the endocytosis of the Na+,K+-ATPase in response to GPCR-mediated signaling depends on clathrin and the AP-2 adaptor protein (Chibalin et al., 1997). Because we find that arrestins can bind directly to the Na+,K+-ATPase, it is possible that arrestins may act as a linker that connects clathrin and clathrin adaptor proteins to the pump.
Recently it has become clear that, in addition to serving in its capacity as an ion pump, the Na+,K+-ATPase is engaged in the assembly of multiple protein complexes that transmit signals to different intracellular compartments (Xie and Cai, 2003). It has been demonstrated that the binding of the specific inhibitor ouabain to the Na+,K+-ATPase causes rapid activation of Src family kinases in many different cell types, including cardiac myocytes, smooth muscle, and kidney epithelial cells (Xie and Cai, 2003). It has also been demonstrated that ouabain is a potent promoter of ERK1/2 activation in rat renal epithelial cells (Dmitrieva and Doris, 2003). It is interesting that several independent investigators have shown that mammalian tissues contain digitalis-like compounds, and they suggest that the Na+,K+-ATPase is the principal molecular receptor for these putative endogenous signaling substances (Fishman, 1979; Haupert and Sancho, 1979; Lichtstein and Samuelov, 1980). Digitalis-like compounds have been shown to be present in many mammalian tissues, including the brain, heart, and adrenal glands (Lichtstein et al., 1992). Here, we showed that arrestins are colocalized with the Na+,K+-ATPase in the kidney and in the choroid plexus of the brain, which produces cerebrospinal fluid. We also found that arrestin coimmunoprecipitated with the Na+,K+-ATPase from homogenates of tissue. GRK phosphorylation of the Na+,K+-ATPase α-subunit was stimulated in the presence of ouabain, and we propose that binding of ouabain and other digitalis-like compounds to the Na+,K+-ATPase may lead to Src and ERK activation through GRK phosphorylation and arrestin binding.
In summary, we have identified three new families of binding partners that interact with the Na+,K+-ATPase: arrestins, GRKs, and spinophilin. We have also found that these proteins regulate the trafficking of the Na+,K+-ATPase. Future studies will be required to elucidate further the possible connections between signaling pathways involving GPCRs or digitalis-like compounds and interaction of the Na+,K+-ATPase with arrestins, GRKs, spinophilin, and 14-3-3 proteins. We anticipate that these novel interactions will be involved in regulating the contributions of the Na+,K+-ATPase to a variety of physiologically important processes.
ACKNOWLEDGMENTS
We thank Dr. Kazue Hashimoto-Torii (Yale University) and SueAnn Mentone for technical assistance. We also thank members of the Caplan lab group for technical assistance, comments, and helpful discussions. This work was supported by National Institutes of Health Grants DK072614 and DK17433 (M.J.C.), and Grant MH40899 and MH074866 (A.C.N.).
Abbreviations used:
- PKA
protein kinase A
- PKC
protein kinase C
- HA
hemagglutinin
- GPCR
G-protein–coupled receptor
- GRK
G-protein–coupled receptor kinase.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-08-0711) on September 5, 2007.
REFERENCES
- Allen P. B., Ouimet C. C., Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc. Natl. Acad. Sci. USA. 1997;94:9956–9961. doi: 10.1073/pnas.94.18.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aperia A., Bertorello A., Seri I. Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am. J. Physiol. 1987;252:F39–F45. doi: 10.1152/ajprenal.1987.252.1.F39. [DOI] [PubMed] [Google Scholar]
- Attramadal H., Arriza J. L., Aoki C., Dawson T. M., Codina J., Kwatra M. M., Snyder S. H., Caron M. G., Lefkowitz R. J. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J. Biol. Chem. 1992;267:17882–17890. [PubMed] [Google Scholar]
- Baines A. D., Drangova R. Regulation of sodium transport by endogenous dopamine production in proximal tubular and OK cells. Clin. Exp. Hypertens. 1997;19:87–91. doi: 10.3109/10641969709080806. [DOI] [PubMed] [Google Scholar]
- Bertorello A. M., Katz A. I. Short-term regulation of renal Na-K-ATPase activity: physiological relevance and cellular mechanisms. Am. J. Physiol. 1993;265:F743–F755. doi: 10.1152/ajprenal.1993.265.6.F743. [DOI] [PubMed] [Google Scholar]
- Biemesderfer D., Dekan G., Aronson P. S., Farquhar M. G. Assembly of distinctive coated pit and microvillar microdomains in the renal brush border. Am. J. Physiol. 1992;262:F55–F67. doi: 10.1152/ajprenal.1992.262.1.F55. [DOI] [PubMed] [Google Scholar]
- Brady A. E., Wang Q., Colbran R. J., Allen P. B., Greengard P., Limbird L. E. Spinophilin stabilizes cell surface expression of alpha 2B-adrenergic receptors. J. Biol. Chem. 2003;278:32405–32412. doi: 10.1074/jbc.M304195200. [DOI] [PubMed] [Google Scholar]
- Brismar H., Agren M., Holtback U. beta-Adrenoceptor agonist sensitizes the dopamine-1 receptor in renal tubular cells. Acta Physiol. Scand. 2002;175:333–340. doi: 10.1046/j.1365-201X.2002.00996.x. [DOI] [PubMed] [Google Scholar]
- Chen W., Kirkbride K. C., How T., Nelson C. D., Mo J., Frederick J. P., Wang X. F., Lefkowitz R. J., Blobe G. C. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003;301:1394–1397. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- Chibalin A. V., Katz A. I., Berggren P. O., Bertorello A. M. Receptor-mediated inhibition of renal Na(+)-K(+)-ATPase is associated with endocytosis of its alpha- and beta-subunits. Am. J. Physiol. 1997;273:C1458–C1465. doi: 10.1152/ajpcell.1997.273.5.C1458. [DOI] [PubMed] [Google Scholar]
- Chibalin A. V., Ogimoto G., Pedemonte C. H., Pressley T. A., Katz A. I., Feraille E., Berggren P. O., Bertorello A. M. Dopamine-induced endocytosis of Na+,K+-ATPase is initiated by phosphorylation of Ser-18 in the rat alpha subunit and is responsible for the decreased activity in epithelial cells. J. Biol. Chem. 1999;274:1920–1927. doi: 10.1074/jbc.274.4.1920. [DOI] [PubMed] [Google Scholar]
- Claing A., Laporte S. A., Caron M. G., Lefkowitz R. J. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog. Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- Couve A., Kittler J. T., Uren J. M., Calver A. R., Pangalos M. N., Walsh F. S., Moss S. J. Association of GABA(B) receptors and members of the 14-3-3 family of signaling proteins. Mol. Cell Neurosci. 2001;17:317–328. doi: 10.1006/mcne.2000.0938. [DOI] [PubMed] [Google Scholar]
- Djelidi S., Beggah A., Courtois-Coutry N., Fay M., Cluzeaud F., Viengchareun S., Bonvalet J. P., Farman N., Blot-Chabaud M. Basolateral translocation by vasopressin of the aldosterone-induced pool of latent Na-K-ATPases is accompanied by alpha1 subunit dephosphorylation: study in a new aldosterone-sensitive rat cortical collecting duct cell line. J. Am. Soc. Nephrol. 2001;12:1805–1818. doi: 10.1681/ASN.V1291805. [DOI] [PubMed] [Google Scholar]
- Dmitrieva R. I., Doris P. A. Ouabain is a potent promoter of growth and activator of ERK1/2 in ouabain-resistant rat renal epithelial cells. J. Biol. Chem. 2003;278:28160–28166. doi: 10.1074/jbc.M303768200. [DOI] [PubMed] [Google Scholar]
- Dunbar L. A., Aronson P., Caplan M. J. A transmembrane segment determines the steady-state localization of an ion-transporting adenosine triphosphatase. J. Cell Biol. 2000;148:769–778. doi: 10.1083/jcb.148.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efendiev R., Chen Z., Krmar R. T., Uhles S., Katz A. I., Pedemonte C. H., Bertorello A. M. The 14-3-3 protein translates the NA+,K+-ATPase α1-subunit phosphorylation signal into binding and activation of phosphoinositide 3-kinase during endocytosis. J. Biol. Chem. 2005;280:16272–16277. doi: 10.1074/jbc.M500486200. [DOI] [PubMed] [Google Scholar]
- Fishman M. C. Endogenous digitalis-like activity in mammalian brain. Proc. Natl. Acad. Sci. USA. 1979;76:4661–4663. doi: 10.1073/pnas.76.9.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes P., Soares-da-Silva P. Dopamine acutely decreases type 3 Na(+)/H(+) exchanger activity in renal OK cells through the activation of protein kinases A and C signalling cascades. Eur. J. Pharmacol. 2004;488:51–59. doi: 10.1016/j.ejphar.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Gottardi C. J., Caplan M. J. Molecular requirements for the cell-surface expression of multisubunit ion-transporting ATPases. Identification of protein domains that participate in Na,K-ATPase and H,K-ATPase subunit assembly. J. Biol. Chem. 1993;268:14342–14347. [PubMed] [Google Scholar]
- Haupert G. T., Jr, Sancho J. M. Sodium transport inhibitor from bovine hypothalamus. Proc. Natl. Acad. Sci. USA. 1979;76:4658–4660. doi: 10.1073/pnas.76.9.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Lemas M. V., Takeyasu K. Na(+)-, ouabain-, Ca(2+)-, and thapsigargin-sensitive ATPase activity expressed in chimeras between the calcium and the sodium pump alpha subunits. Proc. Natl. Acad. Sci. USA. 1994;91:6103–6107. doi: 10.1073/pnas.91.13.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khundmiri S. J., Lederer E. PTH and DA regulate Na-K ATPase through divergent pathways. Am. J. Physiol. Renal Physiol. 2002;282:F512–F522. doi: 10.1152/ajprenal.00111.2000. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Shenoy S. K. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Li D., Aperia A., Celsi G., da Cruz e Silva E. F., Greengard P., Meister B. Protein phosphatase-1 in the kidney: evidence for a role in the regulation of medullary Na(+)-K(+)-ATPase. Am. J. Physiol. 1995;269:F673–F680. doi: 10.1152/ajprenal.1995.269.5.F673. [DOI] [PubMed] [Google Scholar]
- Lichtstein D., Samuelov S. Endogenous 'ouabain like' activity in rat brain. Biochem. Biophys. Res. Commun. 1980;96:1518–1523. doi: 10.1016/0006-291x(80)91346-7. [DOI] [PubMed] [Google Scholar]
- Lichtstein D., Samuelov S., Gati I., Wechter W. J. Digitalis-like compounds in animal tissues. J. Basic Clin. Physiol. Pharmacol. 1992;3:269–292. doi: 10.1515/jbcpp.1992.3.4.269. [DOI] [PubMed] [Google Scholar]
- Lin F. T., Daaka Y., Lefkowitz R. J. beta-arrestins regulate mitogenic signaling and clathrin-mediated endocytosis of the insulin-like growth factor I receptor. J. Biol. Chem. 1998;273:31640–31643. doi: 10.1074/jbc.273.48.31640. [DOI] [PubMed] [Google Scholar]
- Liu F., Gesek F. A. alpha(1)-Adrenergic receptors activate NHE1 and NHE3 through distinct signaling pathways in epithelial cells. Am. J. Physiol. Renal Physiol. 2001;280:F415–F425. doi: 10.1152/ajprenal.2001.280.3.F415. [DOI] [PubMed] [Google Scholar]
- Liu F., Nesbitt T., Drezner M. K., Friedman P. A., Gesek F. A. Proximal nephron Na+/H+ exchange is regulated by alpha 1A- and alpha 1B-adrenergic receptor subtypes. Mol. Pharmacol. 1997;52:1010–1018. doi: 10.1124/mol.52.6.1010. [DOI] [PubMed] [Google Scholar]
- Mallick B. N., Adya H. V., Faisal M. Norepinephrine-stimulated increase in Na+,K+-ATPase activity in the rat brain is mediated through alpha1A-adrenoceptor possibly by dephosphorylation of the enzyme. J. Neurochem. 2000;74:1574–1578. doi: 10.1046/j.1471-4159.2000.0741574.x. [DOI] [PubMed] [Google Scholar]
- Masuzawa T., Ohta T., Kawamura M., Nakahara N., Sato F. Immunohistochemical localization of Na+, K+-ATPase in the choroid plexus. Brain Res. 1984;302:357–362. doi: 10.1016/0006-8993(84)90250-6. [DOI] [PubMed] [Google Scholar]
- Narkar V. A., Hussain T., Lokhandwala M. F. Activation of D2-like receptors causes recruitment of tyrosine-phosphorylated NKA alpha 1-subunits in kidney. Am. J. Physiol. Renal Physiol. 2002;283:F1290–F1295. doi: 10.1152/ajprenal.00039.2002. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M., Noguchi S., Takeda K., Morohashi M., Kawamura M. Site-directed mutagenesis of Asp-376, the catalytic phosphorylation site, and Lys-507, the putative ATP-binding site, of the alpha-subunit of Torpedo californica Na+/K(+)-ATPase. Biochim. Biophys. Acta. 1990;1021:157–160. doi: 10.1016/0005-2736(90)90028-m. [DOI] [PubMed] [Google Scholar]
- Pagel P., Zatti A., Kimura T., Duffield A., Chauvet V., Rajendran V., Caplan M. J. Ion pump-interacting proteins: promising new partners. Ann. NY Acad. Sci. 2003;986:360–368. doi: 10.1111/j.1749-6632.2003.tb07215.x. [DOI] [PubMed] [Google Scholar]
- Parruti G., Peracchia F., Sallese M., Ambrosini G., Masini M., Rotilio D., De Blasi A. Molecular analysis of human beta-arrestin-1, cloning, tissue distribution, and regulation of expression. Identification of two isoforms generated by alternative splicing. J. Biol. Chem. 1993;268:9753–9761. [PubMed] [Google Scholar]
- Pedrosa R., Gomes P., Soares-da-Silva P. Distinct signalling cascades downstream to Gsalpha coupled dopamine D1-like NHE3 inhibition in rat and opossum renal epithelial cells. Cell Physiol. Biochem. 2004;14:91–100. doi: 10.1159/000076930. [DOI] [PubMed] [Google Scholar]
- Pietrini G., Matteoli M., Banker G., Caplan M. J. Isoforms of the Na,K-ATPase are present in both axons and dendrites of hippocampal neurons in culture. Proc. Natl. Acad. Sci. USA. 1992;89:8414–8418. doi: 10.1073/pnas.89.18.8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-do O. P., Chibalin A. V., Katz A. I., Soares-da-Silva P., Bertorello A. M. Short-term vs. sustained inhibition of proximal tubule Na,K-ATPase activity by dopamine: cellular mechanisms. Clin. Exp. Hypertens. 1997;19:73–86. doi: 10.3109/10641969709080805. [DOI] [PubMed] [Google Scholar]
- Prezeau L., Richman J. G., Edwards S. W., Limbird L. E. The zeta isoform of 14-3-3 proteins interacts with the third intracellular loop of different alpha2-adrenergic receptor subtypes. J. Biol. Chem. 1999;274:13462–13469. doi: 10.1074/jbc.274.19.13462. [DOI] [PubMed] [Google Scholar]
- Richman J. G., Brady A. E., Wang Q., Hensel J. L., Colbran R. J., Limbird L. E. Agonist-regulated Interaction between alpha2-adrenergic receptors and spinophilin. J. Biol. Chem. 2001;276:15003–15008. doi: 10.1074/jbc.M011679200. [DOI] [PubMed] [Google Scholar]
- Rimessi A., Coletto L., Pinton P., Rizzuto R., Brini M., Carafoli E. Inhibitory interaction of the 14-3-3ϵ protein with isoform 4 of the plasma membrane Ca2+-ATPase pump. J. Biol. Chem. 2005;280:37195–37203. doi: 10.1074/jbc.M504921200. [DOI] [PubMed] [Google Scholar]
- Satoh A., et al. Neurabin-II/spinophilin. An actin filament-binding protein with one pdz domain localized at cadherin-based cell-cell adhesion sites. J. Biol. Chem. 1998;273:3470–3475. doi: 10.1074/jbc.273.6.3470. [DOI] [PubMed] [Google Scholar]
- Scheiner-Bobis G., Farley R. A. Subunit requirements for expression of functional sodium pumps in yeast cells. Biochim. Biophys. Acta. 1994;1193:226–234. doi: 10.1016/0005-2736(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Shenoy S. K., Lefkowitz R. J. Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem. J. 2003;375:503–515. doi: 10.1042/BJ20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel G. J., Holm C., Schreiber J. H., Desmond T., Ernst S. A. Purification of mouse brain (Na++ K+)-ATPase catalytic unit, characterization of antiserum, and immunocytochemical localization in cerebellum, choroid plexus, and kidney. J. Histochem. Cytochem. 1984;32:1309–1318. doi: 10.1177/32.12.6094658. [DOI] [PubMed] [Google Scholar]
- Skou J. C., Esmann M. The Na,K-ATPase. J. Bioenerget. Biomembr. 1992;24:249–261. doi: 10.1007/BF00768846. [DOI] [PubMed] [Google Scholar]
- Smith F. D., Oxford G. S., Milgram S. L. Association of the D2 dopamine receptor third cytoplasmic loop with spinophilin, a protein phosphatase-1-interacting protein. J. Biol. Chem. 1999;274:19894–19900. doi: 10.1074/jbc.274.28.19894. [DOI] [PubMed] [Google Scholar]
- Stephens D. J., Banting G. Direct interaction of the trans-Golgi network membrane protein, TGN38, with the F-actin binding protein, neurabin. J. Biol. Chem. 1999;274:30080–30086. doi: 10.1074/jbc.274.42.30080. [DOI] [PubMed] [Google Scholar]
- Sterne-Marr R., Benovic J. L. Regulation of G protein-coupled receptors by receptor kinases and arrestins. Vitam. Horm. 1995;51:193–234. doi: 10.1016/s0083-6729(08)61039-0. [DOI] [PubMed] [Google Scholar]
- Szabo E. Z., Numata M., Lukashova V., Iannuzzi P., Orlowski J. beta-Arrestins bind and decrease cell-surface abundance of the Na+/H+ exchanger NHE5 isoform. Proc. Natl. Acad. Sci. USA. 2005;102:2790–2795. doi: 10.1073/pnas.0407444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C. M., Brady A. E., Nickols H. H., Wang Q., Limbird L. E. Membrane trafficking of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2004;44:559–609. doi: 10.1146/annurev.pharmtox.44.101802.121558. [DOI] [PubMed] [Google Scholar]
- Tazawa H., Takahashi S., Zilliacus J. Interaction of the parathyroid hormone receptor with the 14-3-3 protein. Biochim. Biophys. Acta. 2003;1620:32–38. doi: 10.1016/s0304-4165(02)00503-2. [DOI] [PubMed] [Google Scholar]
- Therien A. G., Blostein R. Mechanisms of sodium pump regulation. Am. J. Physiol. Cell Physiol. 2000;279:C541–C566. doi: 10.1152/ajpcell.2000.279.3.C541. [DOI] [PubMed] [Google Scholar]
- Toyoshima C., Mizutani T. Crystal structure of the calcium pump with a bound ATP analogue. Nature. 2004;430:529–535. doi: 10.1038/nature02680. [DOI] [PubMed] [Google Scholar]
- Wang Q., Limbird L. E. Regulated interactions of the alpha 2A adrenergic receptor with spinophilin, 14-3-3zeta, and arrestin 3. J. Biol. Chem. 2002;277:50589–50596. doi: 10.1074/jbc.M208503200. [DOI] [PubMed] [Google Scholar]
- Wang Q., Zhao J., Brady A. E., Feng J., Allen P. B., Lefkowitz R. J., Greengard P., Limbird L. E. Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science. 2004a;304:1940–1944. doi: 10.1126/science.1098274. [DOI] [PubMed] [Google Scholar]
- Wang W., Li C., Kwon T. H., Miller R. T., Knepper M. A., Frokiaer J., Nielsen S. Reduced expression of renal Na+ transporters in rats with PTH-induced hypercalcemia. Am. J. Physiol. Renal Physiol. 2004b;286:F534–F545. doi: 10.1152/ajprenal.00044.2003. [DOI] [PubMed] [Google Scholar]
- Wu J. H., Peppel K., Nelson C. D., Lin F. T., Kohout T. A., Miller W. E., Exum S. T., Freedman N. J. The adaptor protein beta-arrestin2 enhances endocytosis of the low density lipoprotein receptor. J. Biol. Chem. 2003;278:44238–44245. doi: 10.1074/jbc.M309450200. [DOI] [PubMed] [Google Scholar]
- Xie Z., Cai T. Na+-K+-ATPase-mediated signal transduction: from protein interaction to cellular function. Mol. Interv. 2003;3:157–168. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]