Abstract
The insulin-like growth factor (IGF) signaling pathway plays a crucial role in the regulation of cell growth, differentiation, apoptosis, and aging. IGF-binding proteins (IGFBPs) are important members of the IGF axis. IGFBP-5 is up-regulated during cellular senescence in human dermal fibroblasts and endothelial cells, but the function of IGFBP-5 in cellular senescence is unknown. Here we show that IGFBP-5 plays important roles in the regulation of cellular senescence. Knockdown of IGFBP-5 in old human umbilical endothelial cells (HUVECs) with IGFBP-5 micro-RNA lentivirus caused partial reduction of a variety of senescent phenotypes, such as changes in cell morphology, increases in cell proliferation, and decreases in senescence-associated β-galactosidase (SA-β-gal) staining. In addition, treatment with IGFBP-5 protein or up-regulation of IGFBP-5 in young cells accelerates cellular senescence, as confirmed by cell proliferation and SA-β-gal staining. Premature senescence induced by IGFBP-5 up-regulation in young cells was rescued by knockdown of p53, but not by knockdown of p16. Furthermore, atherosclerotic arteries exhibited strong IGFBP-5–positive staining along intimal plaques. These results suggest that IGFBP-5 plays a role in the regulation of cellular senescence via a p53-dependent pathway and in aging-associated vascular diseases.
INTRODUCTION
Senescence is the complex process of deterioration that occurs over the period of development of an organism, resulting in progressive functional decline and eventual death. Cellular senescence is a stress-response phenomenon where cells lose the ability to proliferate (McCormick and Campisi, 1991). Normal somatic cells cultured in vitro have a limited ability to divide and then enter a state of irreversible proliferative arrest, termed replicative senescence (Hayflick and Moorhead, 1961). Irreversible growth arrest is also induced in primary cells by the expression of activated oncogenes such as Ras (Serrano et al., 1997) or Raf (Zhu et al., 1998), by activation of tumor suppressor genes (Tyner et al., 2002; Jacobs and de Lange, 2004). Senescent cells have a characteristic enlarged, flattened morphology (Wagner et al., 2001), express senescence-associated β-galactosidase (SA-β-gal; Dimri et al., 1995), are resistant to mitogen-induced proliferation (Park et al., 2000), and show altered gene expression (Smith and Pereira-Smith, 1996). Accumulating evidence implicates the tumor suppressors, p53, p16, and Rb, as the common major effectors of cellular senescence in normal somatic cells (Beausejour et al., 2003; Campisi, 2005).
In addition, genetic analyses have demonstrated that the insulin/insulin-like growth factor-1 (IGF1) signal transduction pathway is involved in the aging of many organisms, including nematodes, fruit flies, and mammals (Kenyon, 2001; Longo and Finch, 2003). IGF-binding proteins (IGFBPs) are important components of IGF-signaling pathways. The IGFBPs comprise a family of six proteins, IGFBP-1 to -6, that bind to IGFs with high affinity (Firth and Baxter, 2002). Although structurally related, IGFBPs are secreted by many cell types and have differential cell- and tissue-type–dependent expression patterns (Schneider et al., 2002). Binding of IGF to IGFBPs restricts IGF's access to the IGF1 receptor, resulting in inhibition of cell proliferation, differentiation, survival, and other IGF-stimulated signaling events (Firth and Baxter, 2002). Some IGFBPs can interact with biomolecules other than IGFs, including extracellular matrix (ECM) glycosaminoglycans (Arai et al., 1994), ECM proteins (Jones et al., 1993; Gui and Murphy, 2001), and inorganic bone matrix hydroxyapatite (Campbell and Andress, 1997). Because IGFBP interaction with ECM and other proteins decreases the affinity of the IGF-IGFBP interaction, these other interactions are also important for the regulation of both IGF and IGFBP activities. IGFBP proteolysis by specific proteases also reduces the affinity between IGFs and IGFBPs and modulates IGF bioactivity (Nam et al., 1994; Moralez et al., 2003). In addition to IGF-dependent activity of IGFBPs, IGF-independent IGFBP actions were also reported to be important for the regulation of IGFBP activity (Andress and Birnbaum, 1992; Valentinis et al., 1995).
IGFBP-5 is the most conserved of the IGFBPs (Allander et al., 1994). IGFBP-5 is up-regulated during the differentiation of myoblasts (Cobb et al., 2004) and in some tumors, including breast cancers (Pekonen et al., 1992; Sheikh et al., 1992), thyroid cancers (Stolf et al., 2003), and uterine leiomyomas (Giudice et al., 1993), suggesting that IGFBP-5 has an important role in controlling differentiation, cell growth, and apoptosis. The role of IGFBP-5 in cell growth is complicated, and it has been reported that it can either stimulate (Jones et al., 1993; Nam et al., 2000; Cobb et al., 2004) or inhibit cell proliferation (Butt et al., 2003; Salih et al., 2004) in various experimental systems, which is explained by cell- and context-specific effects and also is suggested by IGF-dependent and -independent mechanisms (Beattie et al., 2006). Recently, IGFBP-5 expression was reported to be substantially up-regulated in human dermal fibroblasts (Yoon et al., 2004) and endothelial cells (Hampel et al., 2006) during replicative senescence. IGF1 has been shown to extend the in vitro replicative life span of satellite cells by modulating cell cycle regulatory molecules (Chakravarthy et al., 2000). Repression of insulin/IGF1 signal pathway by deletion of growth hormone receptor (Shimokawa et al., 2002; Coschigano et al., 2003) or IGF1R (Holzenberger et al., 2003) increased life span. Regulation of IGF1 activity by IGFBPs suggests that IGFBPs play an important role in in vivo and in vitro aging; however, the function of IGFBP-5 in cellular senescence remains unidentified.
In the present study, we show that knockdown of IGFBP-5 in old human umbilical vein endothelial cells (HUVECs) partially reversed senescence phenotypes. Overexpression of IGFBP-5 or treatment with exogenous IGFBP-5 induced senescence in young HUVECs. Furthermore, IGFBP-5–induced senescence was associated with engagement of the tumor suppressor p53. Notably, strong IGFBP-5 immunoreactivity was observed in atherosclerotic plaques. Our data suggest that IGFBP-5 plays an important role in cellular senescence through a p53-dependent signaling pathway and in vascular diseases associated with aging.
MATERIALS AND METHODS
MATERIALS
Human umbilical vein endothelial cells (HUVECs) and endothelial cell basal medium-2 (EBM-2) containing several growth factors and supplements (EGM-2) were purchased from Cambrex Bio Science (Walkersville, MD). p16- or p53-null mouse embryo fibroblasts (MEFs) were provided by Dr. H. W. Lee (Yonsei University, Korea). The PCR primer oligonucleotides for IGFBP-5 (forward, agatgaaatgagtggcgtcc; reverse, caagagaaagcagtgcaaacc), IGF1 (forward, cccctgaaaaagttaatgca; reverse, aaggattctcaagggtgg), and IGF2 (forward, ggagaattcgtctgattgtccag; reverse, tttctctccgtgctgttctctc) were obtained from Bioneer (Daejeon, Korea). The pRetroSuper-p53sh and pRetroSuper-p16sh vectors were kindly provided by Dr. R. Agami (Division of Tumor Biology, The Netherlands Cancer Institute, Amsterdam, Netherlands). Small interfering RNAs (siRNAs) against Ataxia Telangiectasia Mutated kinase (ATM) were purchased from Dharmacon (Chicago, IL). The pCMV-Sport6 vector encoding the full-sequence IGFBP-5 gene was purchased from Open Biosystems (Huntsville, AL). The pLenti6/V5 directional TOPO cloning kit was from Invitrogen (Carlsbad, CA). A rabbit polyclonal antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was kindly donated by Dr. K. S. Kwon (KRIBB, Daejeon, Korea). Antibodies against p21, p53, and cyclin A were purchased from Santa Cruz Biotechnology, (Santa Cruz, CA) and antibodies against phospho-p53 and phospho-Rb from Cell Signaling Technology (Danvers, MA). Nonglycosylated recombinant human IGFBP-5 (rhIGFBP-5) and antibody against IGFBP-5 were obtained from R&D Systems (Minneapolis, MN). Twenty vascular specimens from normal and atherosclerosis patients were obtained from the Department of Pathology of Yeungnam University Hospital (Daegu, Korea) from 2000 to 2007: young normal patients (n = 30; average, 24 y old), old normal patients (n = 25; average, 63 y old), and old atherosclerosis patients (n = 31; average, 68 y old).
Cell Culture and Treatment
HUVECs in EGM-2 media were plated at 2 × 105 cells per 100-mm culture plate and cultured at 37°C in a 5% CO2 humidified incubator. When subcultures reached 80–90% confluence, serial passaging was performed by trypsinization, and the number of population doublings (PDs) was monitored for further experiments. For experiments, cells were used in either passage 6 (PD <24) or passage 13 (PD >44). These are referred to as “young” and “old” cells, respectively. PD was calculated using the geometric equation: PD = log2F/log2I, where F is the final population number and I is the initial population number. Primary human dermal fibroblasts (HDFs) were obtained and characterized as previously described (Yoon et al., 2004). For induction of cellular senescence by adriamycin, HUVECs (2 × 105 cells) were seeded in 60-mm dishes and incubated for 24 h in EGM-2 media. Cells were washed three times with DMEM and treated with 500 nM adriamycin for 4 h. After discarding the media containing adriamycin, cells were washed three times with DMEM and incubated in EGM-2 media for the indicated times. Adriamycin-induced cellular senescence was confirmed by SA-β-gal activity staining. For IGFBP-5 treatment, HUVECs (2 × 105 cells) were seeded in 60-mm dishes and incubated for 24 h in EGM-2 media. Cells were treated with 100 ng/ml rhIGFBP-5 for 0, 30, 60, 90, and 120 min or treated with 0, 50, 100, and 200 ng/ml rhIGFBP-5 for 24 h.
Protein Extraction
HUVECs (2 × 105 cells) were seeded in 60-mm dishes and incubated for 24 h in EGM-2 media. Cells were washed with ice-cold phosphate-buffered saline (PBS), lysed in 50 μl of ice-cold RIPA buffer (25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 0.5% SDS, 1 mM Na3VO4, 5 mM NaF, and 1 mM phenylmethylsulfonyl fluoride), and collected by scraping with a rubber policeman. Cell disruption was achieved by vortexing repeatedly for 30-s intervals on ice. Particulate debris was removed by centrifugation at 13,600 × g for 10 min. Protein concentrations in the supernatants were quantified by the bicinchoninic acid (BCA) method (Pierce Biotechnology, Rockford, IL) using bovine serum albumin as a standard.
Western Blot Analysis
Proteins (35 μg) were separated on 12% SDS-polyacrylamide gels and then transferred to nitrocellulose membranes. The membranes were incubated overnight at 4°C with one of the specific antibodies. After washing three times in TTBS, horseradish peroxidase–conjugated goat anti-mouse, goat anti-rabbit, or donkey anti-goat antibodies were applied. The proteins were visualized using enhanced chemiluminescence with a LAS-3000 image system (Fujifilm, Stanford, CT). Some membranes were stripped with an antibody stripping buffer (2% SDS, 100 mM β-mercaptoethanol, and 50 mM Tris-HCl, pH 7.0) at 55°C for 20 min. The membranes were then reprobed with a GAPDH antibody as a control for protein loading. The phosphorylation or acetylation levels of p53 were quantified using the Multi Gauge software, version 3.0 (Fujifilm) by averaging three separate experiments.
RT-PCR
Total RNA was extracted from young and old HUVECs using easy-BLUE total RNA extraction kit (Intron Biotechnology, Sungnam, Korea) according to the manufacturer's protocols and was quantified by measuring absorbance at 260 nm. RNA was reverse-transcribed using 2.5 μM oligo-dT primers, 1 mM dNTPs, and Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega, Madison, WI), and the resulting cDNAs were amplified with Super-Therm DNA polymerase (SR Product, Kent, United Kingdom). GAPDH primers were used to standardize the amount of RNA in each sample. PCR products were resolved on 1.5% agarose gels and visualized by ethidium bromide staining.
Real-Time PCR
Real-time quantitative PCR analysis for IGFBP-5 was performed using a LightCycler 1.5 Instrument (Roche, Mannheim, Germany). PCR was performed in a LightCycler capillary in a 10-μl reaction volume that contained 1× DNA Master SYBR Green I, 2.5 mM MgCl2, 1 μl cDNA, and 0.4 μM primers. The PCR protocol was as follows: initial denaturation for 2 min at 95°C, 45 cycles of 95°C for 10 s, 60°C for 5 s, and 72°C for 12 s. Results were analyzed with LightCycler software, version 3.5.3.
Preparation and Transduction of IGFBP-5 Micro-RNA Lentivirus
Single-stranded DNA oligomers were designed using Invitrogen RNA interference (RNAi) Designer (http://www.invitrogen.com/rnai) as pre-micro-RNA sequences for IGFBP-5 and purchased from Bioneer (Daejeon, Korea). The oligomer sequences are as follows: TGCTGACAATTGGGCAGGTACACAGCGTTTTGGCCACTGACTGACGCTGTGTATGCCCAATTGT and CCTGACAATTGGGCATACACAGCGTCAGTCAGTGGCCAAAACGCTGTG- TACCTGCCCAATTGTC. The two single-stranded oligonucleotides were annealed to generate a double-stranded oligonucleotide and cloned into the pcDNA6.2-GW/EmGFP-miR vector. The resulting construct was transformed into competent Escherichia coli, and the correct expression vector was identified by DNA sequencing. The IGFBP-5 micro-RNA expression vector was transferred to the destination vector, pLenti6/V5-DEST, using a BLOCK-iT Pol II miR RNA expression vector kit (Invitrogen) according to the manufacturer's protocol.
For knockdown of IGFBP-5, old cells (2 × 105) were plated in 60-mm culture plates and incubated for 24 h. Cells were transduced with IGFBP-5 micro-RNA lentivirus or empty lentivirus as a control. After overnight incubation, fresh culture media were exchanged, and the transduced cells were cultured in a CO2 incubator for 3 d. The transduced cells were harvested and analyzed for cell proliferation, SA-β-gal staining and Western blotting.
Preparation and Transduction of IGFBP-5 Lentivirus
For overexpression of IGFBP-5, IGFBP-5 lentivirus was prepared according to manufacturer's suggestions (Invitrogen). IGFBP-5 cDNA was amplified from pCMV-Sport6 IGFBP-5 by PCR and cloned into a pLenti6/V5-d-TOPO vector. Nucleotide sequences of IGFBP-5 were confirmed by dideoxy sequencing. After cells were transduced with IGFBP-5 lentivirus and incubated for 3 d, cells were harvested and analyzed for cell proliferation and SA-β-gal staining. In all experiments, cells were transduced with an empty lentivirus as a control.
Transfection of ATM siRNAs
For knockdown of ATM, transfection of four other siRNAs against ATM (200 nM) in young HUVECs was carried out using FuGENE HD transfection reagent (Roche, Basel, Switzerland) according to the manufacturer's protocol. ATM siRNA-transfected cells were transduced with IGFBP-5 lentivirus or control lentivirus. After incubation of 3 d, p53 and phosphorylated-p53 protein levels were measured by Western blotting, and cell proliferation was analyzed by the MTT [3-(4, 5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide] assay.
MTT Assay
Cell proliferation was measured by the MTT assay. Cells were seeded on 96-well plates at a density of 2 × 103 cells per well. After treatments, cells were incubated with 1 mg/ml MTT solution for 2 h. The medium was aspirated, and the resulting formazan product was solubilized with 100 μl dimethylsulfoxide. Viability was assessed by measuring absorbance at 570 nm with a Bio-Rad microplate reader (Richmond, CA).
SA-β-gal Activity Assay
SA-β-gal activity in cells was measured as described previously (Dimri et al., 1995). After SA-β-gal staining, cells were counterstained with 1% eosin for 5 min and then washed two times with ethanol. The percentage of blue cells per 400 cells observed under a light microscope was calculated.
Flow Cytometric Analyses for Apoptosis and Cell Cycle
Induction of apoptosis was examined by Annexin V-fluorescein isothiocyanate (FITC) staining (BD Biosciences, San Jose, CA) according to the manufacturer's suggestion. Cells were seeded at 2 × 105 in 60-mm dishes and incubated overnight. Cells were treated with rhIGFBP-5 for 2 d and then stained with Annexin V-FITC in the dark. The FITC fluorescence intensity of 10,000 cells was measured using a Becton-Dickinson FACS Caliber flow cytometer (San Jose, CA).
Cell cycle profiles were analyzed by propidium iodide (PI) staining. A minimum of 10,000 cells in each sample was detected according to intracellular PI fluorescence intensity by flow cytometry, and cell cycle was analyzed by Cell Quest software (Becton-Dickinson).
Immunohistochemical Staining
Formalin-fixed, paraffin-embedded tissues were immunohistochemically stained. These tissues were: young normal human artery (n = 30), old normal human artery (n = 25), and old human aortic artery with atheromatous plaque (n = 31). Tissue blocks were sectioned at 4 μm, attached to silane-coated slides, deparaffinized in xylene, and rehydrated in graded alcohol. Tissue samples were immunohistochemically stained with an IGFBP-5 antibody (R&D Systems) and the reagents supplied with the kit (ChemMate, DAKO Envision, Glostrup, Denmark) according to the manufacturer's instructions. Mayer's hematoxylin was used for counterstaining. As a negative control, the primary antibody was replaced with nonimmune serum.
RESULTS
Differential Expression of IGFBP-5 in Cellular Senescence
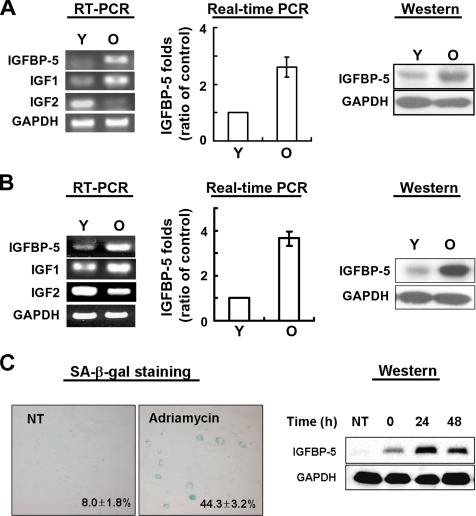
To investigate whether IGFBP-5 is associated with cellular senescence of normal cells, we examined the expression levels of IGFBP-5 in young and old cells by semiquantitative RT-PCR, real-time PCR, and Western blot analysis. The expression level of IGFBP-5 mRNA in old HUVECs was threefold higher than that in young HUVECs. The IGFBP-5 protein level was also increased 2.5-fold in old HUVECs compared with young HUVECs (Figure 1A). Both mRNA and protein levels of IGFBP-5 were up-regulated four-fold or more in old HDFs (Figure 1B). Furthermore, because cytotoxic agents such as adriamycin are known to induce cellular senescence, we also measured the expression levels of IGFBP-5 in HUVECs treated with adriamycin. As expected, IGFBP-5 levels were increased by adriamycin treatment (Figure 1C).
Figure 1.
Expression levels of IGFBP-5 mRNA and protein in cellular senescence IGFBP-5 mRNA expression levels were measured by semiquantitative RT-PCR and real-time PCR analysis, and IGFBP-5 protein levels were analyzed by Western blotting with antibodies against IGFBP-5 and GAPDH (loading control) in HUVECs (A) and HDFs (B) with age. IGFBP-5 expression levels in HUVECs treated with adriamycin (C). Cells were treated with 500 nM adriamycin for 4 h and exchanged to fresh media. The cells were incubated for 0, 24, and 48 h, and IGFBP-5 was detected by Western blotting with antibodies against IGFBP-5 and GAPDH (loading control). Values are means ± SD of three independent experiments. The figure shows representative data from three independent experiments. Y, young cells; O, old cells.
Partial Reversal of Cellular Senescence in old HUVECs by IGFBP-5 Knockdown
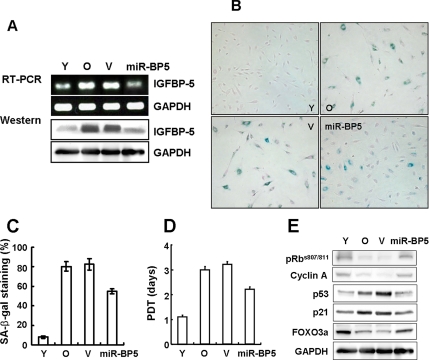
Old cells are resistant to mitogen-induced proliferation, express SA-β-gal, and have a characteristically enlarged and flattened morphology. In our study, old HUVECs (passage 13, PD >44) displayed senescence phenotypes that distinguished them from early passage cells. To investigate the role of IGFBP-5 in cellular senescence, the levels of IGFBP-5 mRNA and protein in old cells were down-regulated by gene silencing using IGFBP-5 micro-RNA lentivirus (Figure 2A). Transduction with IGFBP-5 micro-RNA lentivirus caused ∼65% decrease in IGFBP-5 levels. Repression of IGFBP-5 levels in old cells caused morphological changes similar to young cells and a decrease in SA-β-gal activity (Figure 2, B and C). Population doubling time (PDT) was also decreased in IGFBP-5 micro-RNA cells compared with vector-transduced cells (Figure 2D). In addition, the expression levels of p53 and p21 proteins were reduced and phosphorylated-Rb at serine 807 and serine 811 and cyclin A protein levels were increased in IGFBP-5 micro-RNA cells (Figure 2E). Because we reported that FOXO3a levels were decreased in old HDFs, and down-regulation of FOXO3a accelerated cellular senescence in HDFs (Kyoung Kim et al., 2005), FOXO3a level was also measured. As expected, FOXO3a protein level was up-regulated in IGFBP-5 micro-RNA cells (Figure 2E). These results suggest that knockdown of IGFBP-5 in old cells partially reverses senescence phenotypes.
Figure 2.
Partial reversal of cellular senescence by down-regulation of IGFBP-5 in old HUVECs. Old cells were transduced with IGFBP-5 micro-RNA lentivirus and incubated for 3 d. IGFBP-5 knockdown was confirmed by RT-PCR and Western blot analysis (A). Effects of IGFBP-5 knockdown on cellular senescence in IGFBP-5 micro-RNA–transduced old cells were examined by cell morphology and SA-β-gal staining (×100; B), percentages of SA-β-gal–positive cells (C), measurement of population doubling time (PDT; D), and Western blotting using pRb, cyclin A, p53, p21, and FOXO3a antibodies (E). The figure shows representative data from three independent experiments. Values are means ± SD of three independent experiments. Y, young cells; O, old cells; V, empty virus-transduced cells; miR-BP5, IGFBP-5 micro-RNA–transduced old cells.
Acceleration of Cellular Senescence in Young HUVECs by Exogenous IGFBP-5 Treatment
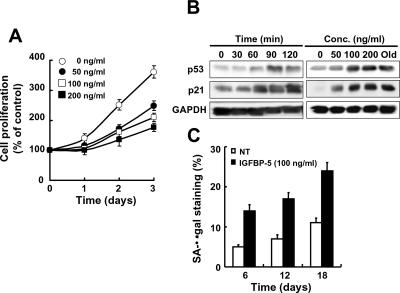
Because senescence phenotypes in old cells were reversed in IGFBP-5 micro-RNA cells, we tested whether exogenous rhIGFBP-5 protein affected cellular senescence in young HUVECs. Treatment of young cells with rhIGFBP-5 decreased cell proliferation both time- and dose-dependently (Figure 3A). The expression levels of p53 and p21 protein were also increased in rhIGFBP-5–treated cells (Figure 3B). Furthermore, prolonged treatment with rhIGFBP-5 increased SA-β-gal staining (Figure 3C) and caused a characteristically enlarged and flattened morphology (data not shown).
Figure 3.
Acceleration of cellular senescence by exogenous rhIGFBP-5 in young HUVECs. Cells were treated with rhIGFBP-5 for the times and at the doses indicated. Cell proliferation was then measured by the MTT assay (A). Induction of p53 and p21 was observed by Western blotting of cells treated with 100 ng/ml rhIGFBP-5 for the indicated times or with increasing concentrations of rhIGFBP-5 for 24 h (B). SA-β-gal activity was measured after prolonged treatment with 100 ng/ml rhIGFBP-5 for 6, 12, and 18 d (C). Values are means ± SD of three independent experiments. Representative data from three independent experiments are shown.
Induction of Cell Cycle Arrest by Exogenous IGFBP-5 in HUVECs
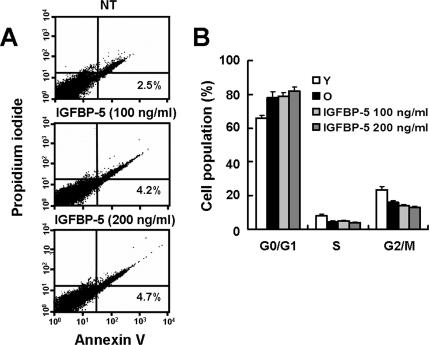
IGFBP-5 can enhance apoptosis by modulating IGF-mediated survival (Marshman et al., 2003) or through an IGF-independent DNA-damaging pathway (Tonner et al., 2000; Butt et al., 2003; Butt et al., 2005). To examine the possibility that decreased cell proliferation is the result of apoptotic cell death induced by IGFBP-5, we treated young HUVECs with 100 or 200 ng/ml rhIGFBP-5 for 2 d and measured apoptosis by flow cytometric analysis with Annexin V staining (R&D Systems). IGFBP-5–treated cells display no apoptotic cell death (Figure 4A). To confirm that rhIGFBP-5–treated cells entered senescence-related cell cycle arrest, we analyzed DNA content by flow cytometry with PI staining. Most cells stimulated with rhIGFBP-5 for 2 d were arrested in G1 phase (Figure 4B), which is one of the typical phenotypes in cellular senescence (Wagner et al., 2001).
Figure 4.
Effects of rhIGFBP-5 on apoptosis and cell cycle profiles in HUVECs. Cells were treated with rhIGFBP-5 (0, 100, and 200 ng/ml) for 2 d and stained with Annexin V-FITC and propidium iodide (PI). Fluorescence intensity of Annexin V-FITC and PI was analyzed by flow cytometry (A). Cell cycle profiles were analyzed by PI staining and flow cytometry of cells treated with rhIGFBP-5 (100 or 200 ng/ml) for 2 d (B). Values are means ± SD of three independent experiments. Representative data from three independent experiments are shown.
Acceleration of Cellular Senescence in Young HUVECs by IGFBP-5 Up-Regulation
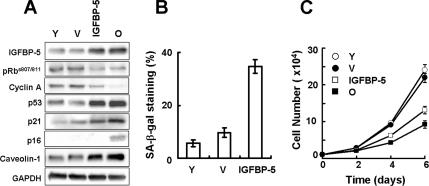
Because down-regulation of IGFBP-5 reversed cellular senescence in old cells and treatment with exogenous IGFBP-5 induced cellular senescence in young cells, we also tested whether IGFBP-5 overexpression impacts cellular senescence in young HUVECs. Young cells were transduced with IGFBP-5 lentivirus, and senescence markers in IGFBP-5 overexpressing cells were examined. The expression level of IGFBP-5 was increased 2.6-fold in young cells transduced with IGFBP-5 lentivirus. Up-regulation of IGFBP-5 in young cells caused decreases in phosphorylated Rb at serine 807 and serine 811 and in cyclin A protein levels and increases in p53, p21, p16, and in caveolin-1 (Cho et al., 2004; Cho and Park, 2005) protein levels, which were known to be increased during cellular senescence (Figure 5A). Also, overexpression of IGFBP-5 in young cells caused an increase in SA-β-gal staining and a decrease in cell proliferation compared with control virus-transduced cells (Figure 5, B and C). Taken together, these results suggest that IGFBP-5 plays an important role in replicative senescence of HUVECs.
Figure 5.
Acceleration of cellular senescence by IGFBP-5 up-regulation in young HUVECs. Young cells were transduced with IGFBP-5 lentivirus or control lentivirus and incubated for 3 d. The levels of IGFBP-5, pRb, cyclin A, p53, p21, p16, and caveolin-1 proteins were detected by Western blot analysis (A). The percentages of SA-β-gal–positive cells were analyzed (B), and cell proliferation was measured by cell counting (C). Values are means ± SD of three independent experiments. Representative data from three independent experiments are shown. Y, young cells; O, old cells; IGFBP-5, IGFBP-5-lentivirus transduced cells; V, empty virus-transduced cells.
Induction of Cellular Senescence by IGFBP-5 through a p53-dependent Pathway
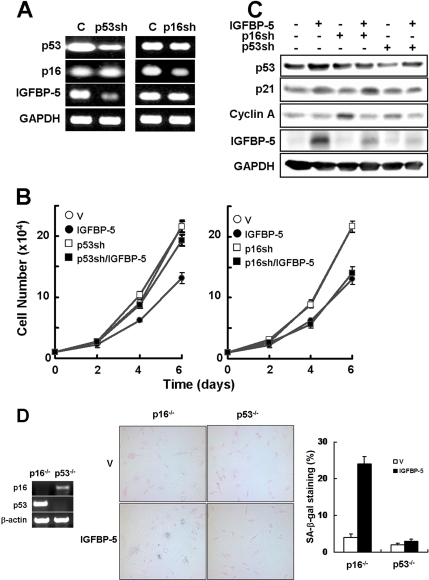
The p53 and the p16/Rb tumor suppressor pathways play a critical role in the senescence response (Ferbeyre et al., 2002; Ben-Porath and Weinberg, 2005). It is reasonable to expect that IGFBP-5 activates specific signaling pathways to engage p53 via p21 protein and/or p16/Rb proteins. To determine which pathway is involved in cellular senescence by IGFBP-5, we generated p16 or p53 knockdowns in young cells using shRNA retrovirus and measured the effects of IGFBP-5 on cellular senescence. Knockdown of p53, but not p16, in young cells decreased the level of IGFBP-5 expression (Figure 6A). The p16 knockdown (p16sh) cells showed a decrease in cell proliferation in response to IGFBP-5 overexpression similar to that in control cells. In contrast, overexpression of IGFBP-5 had no effect on cell proliferation in the p53 knockdown (p53sh) cells (Figure 6B). The p21 protein level increased in the p16sh/IGFBP-5 cells compared with the other cells, particularly the p53sh/IGFBP-5 cells (Figure 6C). To further confirm which pathway is involved in IGFBP-5–mediated cellular senescence, the effects of IGFBP-5 up-regulation on cellular senescence were measured in p53−/− MEFs and p16−/− MEFs. We found that IGFBP-5 overexpression induced an increase in SA-β-gal activity in p16−/− MEFs but not p53−/− MEFs (Figure 6D). Therefore, these results suggest that cellular senescence induced by IGFBP-5 is mediated through the p53-dependent pathway.
Figure 6.
Induction of cellular senescence by IGFBP-5 through a p53-dependent pathway. (A) Young HUVECs were transduced with p53- or p16-shRNA retrovirus and incubated for 3 d and knockdown of p53 and p16 was then confirmed by RT-PCR analysis. (B) The p53- or p16-shRNA cells were transduced with IGFBP-5 lentivirus or control lentivirus and incubated for 3 d. Cell proliferation was measured by cell counting at 2-d intervals. (C) The expression levels of p53, p21 cyclin A, and IGFBP-5 were analyzed by Western blotting. (D) Effects of IGFBP-5 up-regulation on cellular senescence were measured in p16−/− or p53−/− MEFs. Knockout of p16 or p53 in MEFs was confirmed by RT-PCR. p16−/− or p53−/− MEFs were transduced with IGFBP-5 lentivirus or control lentivirus and incubated for 6 d. Cells were stained for SA-β-gal activity (blue) and counterstained with eosin (red). The percentages of SA-β-gal–positive cells were analyzed (×100). Values are means ± SD of three independent experiments. Representative data from three independent experiments are shown. C, control cells; p53sh, p53-shRNA cells; p16sh, p16-shRNA cells; V, empty virus-transduced cells.
Posttranslational Modification of p53 by IGFBP-5 through DNA Damage Signaling
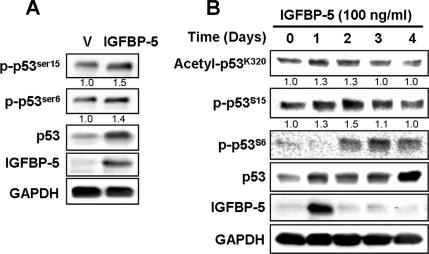
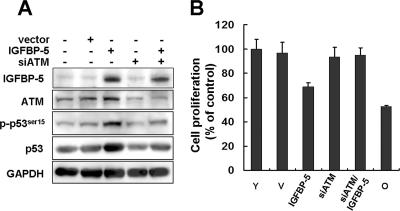
To further investigate the role of p53 in IGFBP-5–mediated cellular senescence, we measured the posttranslational modifications of p53 by IGFBP-5. The phosphorylation of p53 at serine 15 is a critical senescence-inducible modification (Webley et al., 2000) that is known to be regulated by ATM kinase (Moiseeva et al., 2006). The phosphorylation of p53 at serine 6 is induced by taxol and nocodazole and the acetylation at lysine 320 by β-interferon (Moiseeva et al., 2006). We therefore measured the phosphorylation at serine 15 and serine 6 and the acetylation at lysine 320 in response to IGFBP-5. Overexpression of IGFBP-5 induced phosphorylation of serine 6 and serine 15 (Figure 7A). Treatment with rhIGFBP-5 also induced phosphorylation at serine 6 and serine 15 as well as acetylation at lysine 320 (Figure 7B). To further confirm that the p53 activation by IGFBP-5 might be mediated through DNA damage signaling, ATM level was down-regulated using siRNAs against ATM and the effects of IGFBP-5 overexpression were measured. Increases in p53 and phospho-p53 levels induced by IGFBP-5 up-regulation were repressed by knockdown of ATM (Figure 8A). Furthermore, inhibition of cell proliferation induced by IGFBP-5 up-regulation was rescued by knockdown of ATM (Figure 8B). These results suggested that IGFBP-5–induced cellular senescence is mediated by posttranslational modification of p53 such as phosphorylation and acetylation through DNA damage signaling.
Figure 7.
Posttranslational modifications of p53 by both endogenous and exogenous IGFBP-5 in HUVECs. Cells were transduced with IGFBP-5 lentivirus or control lentivirus and incubated for 3 d. The expression levels of IGFBP-5 and p53 and the phosphorylation of p53 at serine 6 and serine 15 were analyzed by Western blotting (A). Cells were treated with rhIGFBP-5 (100 ng/ml) for the indicated times, and the phosphorylation of p53 at serine 6 and serine 15 and the acetylation at lysine 320 were analyzed by Western blotting (B). The relative intensity of phosphorylated or acetylated p53 band, compared with that of the respective GAPDH signal, was determined by using the Multi Gauge software, version 3.0 (Fujifilm), and normalized to 1.0 for control cells without IGFBP-5 by averaging three separate experiments. Representative data from three independent experiments are shown.
Figure 8.
Involvement of DNA damage signaling pathway in IGFBP-5–induced senescence. HUVECs were transfected with siRNA against ATM (200 nM) and then transduced with IGFBP-5 lentivirus or control lentivirus. After 3-d incubation, the expression levels of IGFBP-5, ATM, p53 and the phosphorylation of p53 at serine 15 were analyzed by Western blotting (A). Cell proliferation was measured by MTT assay for 4 d (B). Representative data from three independent experiments are shown. Values are means ± SD of three independent experiments.
Strong IGFBP-5 Immunoreactivity in Human Atherosclerotic Plaques
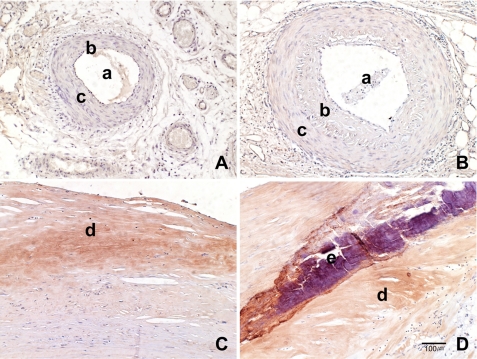
To elucidate whether IGFBP-5 is involved in in vivo vascular aging and aging-associated vascular diseases, paraffin-embedded vessel sections from normal and atherosclerosis patients were stained with an IGFBP-5 antibody. No immunopositive reaction to IGFBP-5 was observed in young arteries (0/30). Weak IGFBP-5 immunoreactivity was found in 6 of 25 (24%) old normal arteries. Strong IGFBP-5–positive areas were seen in the fibrotic stromal matrix of the intimal plaque in 28 of 31 (90%) atherosclerotic vessels, and this immunopositive reaction was especially prominent around calcifications (Figure 9). These results suggest that IGFBP-5 plays an important role in vascular aging, as well as in vascular disease associated with aging.
Figure 9.
Immunohistochemical staining in normal and atherosclerotic arteries with an IGFBP-5 antibody. Although no immunoreactivity to IGFBP-5 was observed in young control group (A) and weak immunoexpression was found in the vascular wall of the old control group (B), IGFBP-5 immunopositive reaction was strong in the fibrotic stromal matrix of the intimal plaque (C); this immunopositive reaction is especially prominent around calcifications (D; ×100). a, arterial lumen; b, intima; c, muscle layer; d, atherosclerotic plaque; e, calcification area.
DISCUSSION
In the present study, we provide the first evidence of the involvement of IGFBP-5 in cellular senescence of human primary endothelial cells through the p53 signaling pathway. We demonstrated that IGFBP-5 plays an important role in cellular senescence of endothelial cells through three different findings: 1) knockdown of IGFBP-5 in old cells partially reversed senescence phenotypes (Figure 2); 2) treatment of young cells with rhIGFBP-5 induced growth arrest and cellular senescence (Figure 3); and 3) overexpression of IGFBP-5 in young cells accelerated cellular senescence (Figure 5). Although a variety of evidence suggests that insulin/IGF signaling pathways are important in aging and longevity of many organisms (Kenyon, 2001; Longo and Finch, 2003), and IGFBPs are important components of these signaling pathways, the role of IGFBPs in senescence was not well understood. Because IGF1 and IGF2 are essential for growth and development and bioavailable IGFs are tightly regulated by six related IGFBPs, regulation of IGFs by IGFBPs was known to affect cell proliferation, differentiation, and survival. Especially, IGFBP-5 transgenic mice display significantly increased neonatal mortality, reduced female fertility, whole-body growth inhibition, and retarded muscle development, suggesting that IGFBP-5 compromises survival, growth, muscle development, and fertility in mice (Salih et al., 2004). IGFBP-5 inhibits the growth of human breast cancer cells by G2/M cell cycle arrest and apoptosis (Butt et al., 2003). IGFBP-5 is also known to play an important role in bone physiology (Devlin et al., 2002; Durant et al., 2004). Several reports have suggested a role for IGFBPs in in vivo aging and in vitro cellular senescence. The expression levels of IGFBP-3 are increased in senescent human fibroblasts (Goldstein et al., 1993; Yoon et al., 2004) and endothelial cells (Grillari et al., 2000). IGFBP-3 accumulation in conditioned medium of senescent fibroblasts contributes to growth arrest of human fibroblasts, and the failure to endocytose IGFBP-3 and the absence of nuclear IGFBP-3 may contribute to the apoptosis resistance of senescent human fibroblasts (Hampel et al., 2005). The loss of Sirt1 protein, an NAD-dependent deacetylase, in mice results in increased expression of IGFBP-1, and a number of the anatomical characteristics of Sirt1-null mice closely resemble those of transgenic mice overexpressing IGFBP-1, suggesting that Sirt1 is part of a regulatory loop that limits IGFBP-1 production, thereby modulating IGF signaling (Lemieux et al., 2005). In addition, indirect evidence suggests that IGFBP-5 plays a role in cellular senescence. IGFBP-5 induces skin fibrosis with increased collagen deposition, denser dermal connective tissue, and increased collagen bundle thickness in IGFBP-5-adenovirus–injected mice (Yasuoka et al., 2006). The senescence-accelerated-prone (SAM P10) mice exhibit remarkable increases in the density of collagen fibers in the dermis compared with the senescence-resistant (SAM R1) mice, which is similar to aged human skin (Cerimele et al., 1990). Collagen synthesis was also altered in senescent fibroblasts and in fibroblasts from Werner's syndrome patients (Basler et al., 1979). These data all suggest that IGFBP-5 is involved in in vitro and in vivo skin aging by alteration of collagen synthesis.
One important question is what signal mediates cellular senescence induced by IGFBP-5 in HUVECs. Accumulating evidence suggests that p53 and p16/Rb tumor suppressor pathways are key regulators of the senescence response (Ferbeyre et al., 2002; Ben-Porath and Weinberg, 2005). We found that p53 is required for IGFBP-5–induced senescence: 1) unlike knockdown of p53, knockdown of p16 did not influence the inhibition of proliferation in young cells induced by IGFBP-5 up-regulation (Figure 6B); 2) SA-β-gal staining by IGFBP-5 up-regulation was increased in p16−/− MEFs but not in p53−/− MEFs (Figure 6D); and 3) IGFBP-5 induced posttranslational modification of p53, including phosphorylation at serine 6 and serine 15 and acetylation at lysine 320 (Figure 7). Recently, cellular senescence induced by several genes or molecules, such as ING2 (Pedeux et al., 2005), β-interferon (Moiseeva et al., 2006), or 5-lipoxygenase (Catalano et al., 2005), was shown to be regulated by the p53-dependent pathway. Taken together, the posttranslational modifications of p53 by IGFBP-5 suggest that IGFBP-5 induces cellular senescence through p53 activation in HUVECs.
Recently, plasminogen activator inhibitor-1 (PAI-1) was reported to be a critical downstream target of p53 in the induction of replicative senescence in MEFs (Kortlever et al., 2006). IGFBP-5 binds to PAI-1, which partially protects IGFBP-5 from proteolysis (Nam et al., 1997), and PAI-1 and IGFBP-3 are transcriptional targets of p53 (Buckbinder et al., 1995; Zhao et al., 2000). Furthermore, we found that IGFBP-5 protein levels were decreased by knockdown of p53, but not of p16, in young cells, suggesting that IGFBP-5 is a p53-responsive gene. Therefore, senescence induced by IGFBP-5 might also be associated with PAI-1 induction by p53, and further studies are needed.
IGFBP-5 expression is regulated by various signaling molecules in vitro, including IGF1, IGF2, insulin, and dexamethasone (Schneider et al., 2002). IGF1 is the most important regulator of in vitro IGFBP-5 expression in a variety of cell types from different species. IGFBP-5 induction by IGF1 can occur by direct stimulation of IGFBP-5 gene transcription (Duan et al., 1999) or by posttranslational protection from proteolysis via binding to secreted IGFBP-5 protein (Camacho-Hubner et al., 1992). We found that IGF1 mRNA levels were higher in old cells than in young cells, whereas IGF2 levels were lower in old cells (Figure 1A). Because insulin/IGF-signaling pathways are known to be involved in organismal aging and longevity, further studies are needed to investigate the association of IGFBP-5 in cellular senescence with increased IGF1 levels in cells with age.
Our results regarding the role of IGFBP-5 in cellular senescence suggest that the IGF/IGFBP system could be related to cardiovascular risk factors and atherosclerosis because cellular senescence might contribute to in vivo organismal aging and aging-associated diseases. In the present study, we showed that IGFBP-5 immunoreactivity was increased in old human arteries compared with young human arteries and was also strong in atherosclerotic plaques (Figure 9), suggesting that IGFBP-5 plays a role in in vivo vascular aging as well as in aging-associated vascular diseases. There are some controversies surrounding the association of the IGF/IGFBP system with cardiovascular diseases. Colao et al., (2005) reported that circulating IGF1 and IGFBP-3 levels were negatively correlated with common cardiovascular risk factors in healthy subjects, independent of age. However, Kawachi et al. (2005) showed that circulating IGF1 and IGFBP-3 were associated with early carotid atherosclerosis. IGFBP-3 was reported to be associated with the presence and extent of coronary arteriosclerosis (Schuler-Luttmann et al., 2000) and with the development of carotid atherosclerosis in hypertensive patients (Watanabe et al., 2003). To our knowledge, there are no reports of IGFBP-5 in association with aging-associated vascular diseases. However, our results suggest that cellular senescence induced by IGFBP-5 in endothelial cells might also contribute to vascular aging and the development of aging-associated cardiovascular diseases.
ACKNOWLEDGMENTS
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST; R13-2005-005-01003-0 (2006)) and by a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (02-PJ10-PG6-AG01-0003).
Abbreviations used:
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HUVEC
human umbilical vein endothelial cell
- HDF
human dermal fibroblast
- IGF
insulin-like growth factor
- IGFBP-5
IGF-binding protein-5
- PD
population doublings
- SA-β-gal
senescence-associated β-galactosidase.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-03-0280) on September 5, 2007.
REFERENCES
- Allander S. V., Larsson C., Ehrenborg E., Suwanichkul A., Weber G., Morris S. L., Bajalica S., Kiefer M. C., Luthman H., Powell D. R. Characterization of the chromosomal gene and promoter for human insulin-like growth factor binding protein-5. J. Biol. Chem. 1994;269:10891–10898. [PubMed] [Google Scholar]
- Andress D. L., Birnbaum R. S. Human osteoblast-derived insulin-like growth factor (IGF) binding protein-5 stimulates osteoblast mitogenesis and potentiates IGF action. J. Biol. Chem. 1992;267:22467–22472. [PubMed] [Google Scholar]
- Arai T., Arai A., Busby W. H., Jr, Clemmons D. R. Glycosaminoglycans inhibit degradation of insulin-like growth factor-binding protein-5. Endocrinology. 1994;135:2358–2363. doi: 10.1210/endo.135.6.7527332. [DOI] [PubMed] [Google Scholar]
- Basler J. W., David J. D., Agris P. F. Deteriorating collagen synthesis and cell ultrastructure accompanying senescence of human normal and Werner's syndrome fibroblast cell strains. Exp. Cell Res. 1979;118:73–84. doi: 10.1016/0014-4827(79)90585-8. [DOI] [PubMed] [Google Scholar]
- Beattie J., Allan G. J., Lochrie J. D., Flint D. J. Insulin-like growth factor-binding protein-5 (IGFBP-5): a critical member of the IGF axis. Biochem. J. 2006;395:1–19. doi: 10.1042/BJ20060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beausejour C. M., Krtolica A., Galimi F., Narita M., Lowe S. W., Yaswen P., Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porath I., Weinberg R. A. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Buckbinder L., Talbott R., Velasco-Miguel S., Takenaka I., Faha B., Seizinger B. R., Kley N. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature. 1995;377:646–649. doi: 10.1038/377646a0. [DOI] [PubMed] [Google Scholar]
- Butt A. J., Dickson K. A., Jambazov S., Baxter R. C. Enhancement of tumor necrosis factor-alpha-induced growth inhibition by insulin-like growth factor-binding protein-5 (IGFBP-5), but not IGFBP-3 in human breast cancer cells. Endocrinology. 2005;146:3113–3122. doi: 10.1210/en.2004-1408. [DOI] [PubMed] [Google Scholar]
- Butt A. J., Dickson K. A., McDougall F., Baxter R. C. Insulin-like growth factor-binding protein-5 inhibits the growth of human breast cancer cells in vitro and in vivo. J. Biol. Chem. 2003;278:29676–29685. doi: 10.1074/jbc.M301965200. [DOI] [PubMed] [Google Scholar]
- Camacho-Hubner C., Busby W. H., Jr, McCusker R. H., Wright G., Clemmons D. R. Identification of the forms of insulin-like growth factor-binding proteins produced by human fibroblasts and the mechanisms that regulate their secretion. J. Biol. Chem. 1992;267:11949–11956. [PubMed] [Google Scholar]
- Campbell P. G., Andress D. L. Insulin-like growth factor (IGF)-binding protein-5-(201–218) region regulates hydroxyapatite and IGF-I binding. Am. J. Physiol. 1997;273:E1005–E1013. doi: 10.1152/ajpendo.1997.273.5.E1005. [DOI] [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Catalano A., Rodilossi S., Caprari P., Coppola V., Procopio A. 5-Lipoxygenase regulates senescence-like growth arrest by promoting ROS-dependent p53 activation. EMBO J. 2005;24:170–179. doi: 10.1038/sj.emboj.7600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerimele D., Celleno L., Serri F. Physiological changes in ageing skin. Br. J. Dermatol. 1990;122(Suppl. 35):13–20. doi: 10.1111/j.1365-2133.1990.tb16120.x. [DOI] [PubMed] [Google Scholar]
- Chakravarthy M. V., Abraha T. W., Schwartz R. J., Fiorotto M. L., Booth F. W. Insulin-like growth factor-I extends in vitro replicative life span of skeletal muscle satellite cells by enhancing G1/S cell cycle progression via the activation of phosphatidylinositol 3′-kinase/Akt signaling pathway. J. Biol. Chem. 2000;275:35942–35952. doi: 10.1074/jbc.M005832200. [DOI] [PubMed] [Google Scholar]
- Cho K. A., Park S. C. Caveolin-1 as a prime modulator of aging: a new modality for phenotypic restoration? Mech. Ageing Dev. 2005;126:105–110. doi: 10.1016/j.mad.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Cho K. A., Ryu S. J., Oh Y. S., Park J. H., Lee J. W., Kim H. P., Kim K. T., Jang I. S., Park S. C. Morphological adjustment of senescent cells by modulating caveolin-1 status. J. Biol. Chem. 2004;279:42270–42278. doi: 10.1074/jbc.M402352200. [DOI] [PubMed] [Google Scholar]
- Cobb L. J., Salih D. A., Gonzalez I., Tripathi G., Carter E. J., Lovett F., Holding C., Pell J. M. Partitioning of IGFBP-5 actions in myogenesis: IGF-independent anti-apoptotic function. J. Cell Sci. 2004;117:1737–1746. doi: 10.1242/jcs.01028. [DOI] [PubMed] [Google Scholar]
- Colao A., Spiezia S., Di Somma C., Pivonello R., Marzullo P., Rota F., Musella T., Auriemma R. S., De Martino M. C., Lombardi G. Circulating insulin-like growth factor-I levels are correlated with the atherosclerotic profile in healthy subjects independently of age. J. Endocrinol. Invest. 2005;28:440–448. doi: 10.1007/BF03347225. [DOI] [PubMed] [Google Scholar]
- Coschigano K. T., Holland A. N., Riders M. E., List E. O., Flyvbjerg A., Kopchick J. J. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- Devlin R. D., Du Z., Buccilli V., Jorgetti V., Canalis E. Transgenic mice overexpressing insulin-like growth factor binding protein-5 display transiently decreased osteoblastic function and osteopenia. Endocrinology. 2002;143:3955–3962. doi: 10.1210/en.2002-220129. [DOI] [PubMed] [Google Scholar]
- Dimri G. P., et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C., Liimatta M. B., Bottum O. L. Insulin-like growth factor (IGF)-I regulates IGF-binding protein-5 gene expression through the phosphatidylinositol 3-kinase, protein kinase B/Akt, and p70 S6 kinase signaling pathway. J. Biol. Chem. 1999;274:37147–37153. doi: 10.1074/jbc.274.52.37147. [DOI] [PubMed] [Google Scholar]
- Durant D., Pereira R., Stadmeyer L., Canalis E. Transgenic mice expressing selected insulin-like growth factor-binding protein-5 fragments do not exhibit enhanced bone formation. Growth Horm. IGF Res. 2004;14:319–327. doi: 10.1016/j.ghir.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Ferbeyre G., de Stanchina E., Lin A. W., Querido E., McCurrach M. E., Hannon G. J., Lowe S. W. Oncogenic ras and p53 cooperate to induce cellular senescence. Mol. Cell. Biol. 2002;22:3497–3508. doi: 10.1128/MCB.22.10.3497-3508.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth S. M., Baxter R. C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- Giudice L. C., Irwin J. C., Dsupin B. A., Pannier E. M., Jin I. H., Vu T. H., Hoffman A. R. Insulin-like growth factor (IGF), IGF binding protein (IGFBP), and IGF receptor gene expression and IGFBP synthesis in human uterine leiomyomata. Hum. Reprod. 1993;8:1796–1806. doi: 10.1093/oxfordjournals.humrep.a137937. [DOI] [PubMed] [Google Scholar]
- Goldstein S., Moerman E. J., Baxter R. C. Accumulation of insulin-like growth factor binding protein-3 in conditioned medium of human fibroblasts increases with chronologic age of donor and senescence in vitro. J. Cell. Physiol. 1993;156:294–302. doi: 10.1002/jcp.1041560211. [DOI] [PubMed] [Google Scholar]
- Grillari J., Hohenwarter O., Grabherr R. M., Katinger H. Subtractive hybridization of mRNA from early passage and senescent endothelial cells. Exp. Gerontol. 2000;35:187–197. doi: 10.1016/s0531-5565(00)00080-2. [DOI] [PubMed] [Google Scholar]
- Gui Y., Murphy L. J. Insulin-like growth factor (IGF)-binding protein-3 (IGFBP-3) binds to fibronectin (FN): demonstration of IGF-I/IGFBP-3/fn ternary complexes in human plasma. J. Clin. Endocrinol. Metab. 2001;86:2104–2110. doi: 10.1210/jcem.86.5.7472. [DOI] [PubMed] [Google Scholar]
- Hampel B., et al. Increased expression of extracellular proteins as a hallmark of human endothelial cell in vitro senescence. Exp. Gerontol. 2006;41:474–481. doi: 10.1016/j.exger.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Hampel B., Wagner M., Teis D., Zwerschke W., Huber L. A., Jansen-Durr P. Apoptosis resistance of senescent human fibroblasts is correlated with the absence of nuclear IGFBP-3. Aging Cell. 2005;4:325–330. doi: 10.1111/j.1474-9726.2005.00180.x. [DOI] [PubMed] [Google Scholar]
- Hayflick L., Moorhead P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Holzenberger M., Dupont J., Ducos B., Leneuve P., Geloen A., Even P. C., Cervera P., Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Jacobs J. J., de Lange T. Significant role for p16INK4a in p53-independent telomere-directed senescence. Curr. Biol. 2004;14:2302–2308. doi: 10.1016/j.cub.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Jones J. I., Gockerman A., Busby W. H., Jr, Camacho-Hubner C., Clemmons D. R. Extracellular matrix contains insulin-like growth factor binding protein-5, potentiation of the effects of IGF-I. J. Cell Biol. 1993;121:679–687. doi: 10.1083/jcb.121.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi S., Takeda N., Sasaki A., Kokubo Y., Takami K., Sarui H., Hayashi M., Yamakita N., Yasuda K. Circulating insulin-like growth factor-1 and insulin-like growth factor binding protein-3 are associated with early carotid atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005;25:617–621. doi: 10.1161/01.ATV.0000154486.03017.35. [DOI] [PubMed] [Google Scholar]
- Kenyon C. A conserved regulatory system for aging. Cell. 2001;105:165–168. doi: 10.1016/s0092-8674(01)00306-3. [DOI] [PubMed] [Google Scholar]
- Kortlever R. M., Higgins P. J., Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat. Cell Biol. 2006;8:877–884. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyoung Kim H., Kyoung Kim Y., Song I. H., Baek S. H., Lee S. R., Hye Kim J., Kim J. R. Down-regulation of a forkhead transcription factor, FOXO3a, accelerates cellular senescence in human dermal fibroblasts. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:4–9. doi: 10.1093/gerona/60.1.4. [DOI] [PubMed] [Google Scholar]
- Lemieux M. E., Yang X., Jardine K., He X., Jacobsen K. X., Staines W. A., Harper M. E., McBurney M. W. The Sirt1 deacetylase modulates the insulin-like growth factor signaling pathway in mammals. Mech. Ageing Dev. 2005;126:1097–1105. doi: 10.1016/j.mad.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Longo V. D., Finch C. E. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- Marshman E., Green K. A., Flint D. J., White A., Streuli C. H., Westwood M. Insulin-like growth factor binding protein 5 and apoptosis in mammary epithelial cells. J. Cell Sci. 2003;116:675–682. doi: 10.1242/jcs.00263. [DOI] [PubMed] [Google Scholar]
- McCormick A., Campisi J. Cellular aging and senescence. Curr. Opin. Cell Biol. 1991;3:230–234. doi: 10.1016/0955-0674(91)90144-n. [DOI] [PubMed] [Google Scholar]
- Moiseeva O., Mallette F. A., Mukhopadhyay U. K., Moores A., Ferbeyre G. DNA damage signaling and p53-dependent senescence after prolonged beta-interferon stimulation. Mol. Biol. Cell. 2006;17:1583–1592. doi: 10.1091/mbc.E05-09-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moralez A., Busby W. H., Jr, Clemmons D. Control of insulin-like growth factor binding protein-5 protease synthesis and secretion by human fibroblasts and porcine aortic smooth muscle cells. Endocrinology. 2003;144:2489–2495. doi: 10.1210/en.2002-220896. [DOI] [PubMed] [Google Scholar]
- Nam T. J., Busby W., Jr, Clemmons D. R. Insulin-like growth factor binding protein-5 binds to plasminogen activator inhibitor-I. Endocrinology. 1997;138:2972–2978. doi: 10.1210/endo.138.7.5230. [DOI] [PubMed] [Google Scholar]
- Nam T. J., Busby W. H., Jr, Clemmons D. R. Human fibroblasts secrete a serine protease that cleaves insulin-like growth factor-binding protein-5. Endocrinology. 1994;135:1385–1391. doi: 10.1210/endo.135.4.7523096. [DOI] [PubMed] [Google Scholar]
- Nam T. J., Busby W. H., Jr, Rees C., Clemmons D. R. Thrombospondin and osteopontin bind to insulin-like growth factor (IGF)-binding protein-5 leading to an alteration in IGF-I-stimulated cell growth. Endocrinology. 2000;141:1100–1106. doi: 10.1210/endo.141.3.7386. [DOI] [PubMed] [Google Scholar]
- Park J. I., Jeong J. S., Han J. Y., Kim D. I., Gao Y. H., Park S. C., Rodgers G. P., Kim I. H. Hydroxyurea induces a senescence-like change of K562 human erythroleukemia cell. J. Cancer Res. Clin. Oncol. 2000;126:455–460. [PubMed] [Google Scholar]
- Pedeux R., et al. ING2 regulates the onset of replicative senescence by induction of p300-dependent p53 acetylation. Mol. Cell. Biol. 2005;25:6639–6648. doi: 10.1128/MCB.25.15.6639-6648.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekonen F., Nyman T., Ilvesmaki V., Partanen S. Insulin-like growth factor binding proteins in human breast cancer tissue. Cancer Res. 1992;52:5204–5207. [PubMed] [Google Scholar]
- Salih D. A., Tripathi G., Holding C., Szestak T. A., Gonzalez M. I., Carter E. J., Cobb L. J., Eisemann J. E., Pell J. M. Insulin-like growth factor-binding protein 5 (Igfbp5) compromises survival, growth, muscle development, and fertility in mice. Proc. Natl. Acad. Sci. USA. 2004;101:4314–4319. doi: 10.1073/pnas.0400230101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. R., Wolf E., Hoeflich A., Lahm H. IGF-binding protein- 5, flexible player in the IGF system and effector on its own. J. Endocrinol. 2002;172:423–440. doi: 10.1677/joe.0.1720423. [DOI] [PubMed] [Google Scholar]
- Schuler-Luttmann S., Monnig G., Enbergs A., Schulte H., Breithardt G., Assmann G., Kerber S., von Eckardstein A. Insulin-like growth factor-binding protein-3 is associated with the presence and extent of coronary arteriosclerosis. Arterioscler. Thromb. Vasc. Biol. 2000;20:E10–E15. [PubMed] [Google Scholar]
- Serrano M., Lin A. W., McCurrach M. E., Beach D., Lowe S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Sheikh M. S., Shao Z. M., Clemmons D. R., LeRoith D., Roberts C. T., Jr, Fontana J. A. Identification of the insulin-like growth factor binding proteins 5 and 6 (IGFBP-5 and 6) in human breast cancer cells. Biochem. Biophys. Res. Commun. 1992;183:1003–1010. doi: 10.1016/s0006-291x(05)80290-6. [DOI] [PubMed] [Google Scholar]
- Shimokawa I., Higami Y., Utsuyama M., Tuchiya T., Komatsu T., Chiba T., Yamaza H. Life span extension by reduction in growth hormone-insulin-like growth factor-1 axis in a transgenic rat model. Am. J. Pathol. 2002;160:2259–2265. doi: 10.1016/S0002-9440(10)61173-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. R., Pereira-Smith O. M. Replicative senescence: implications for in vivo aging and tumor suppression. Science. 1996;273:63–67. doi: 10.1126/science.273.5271.63. [DOI] [PubMed] [Google Scholar]
- Stolf B. S., et al. Differential expression of IGFBP-5 and two human ESTs in thyroid glands with goiter, adenoma and papillary or follicular carcinomas. Cancer Lett. 2003;191:193–202. doi: 10.1016/s0304-3835(02)00679-1. [DOI] [PubMed] [Google Scholar]
- Tonner E., Allan G., Shkreta L., Webster J., Whitelaw C. B., Flint D. J. Insulin-like growth factor binding protein-5 (IGFBP-5) potentially regulates programmed cell death and plasminogen activation in the mammary gland. Adv. Exp. Med. Biol. 2000;480:45–53. doi: 10.1007/0-306-46832-8_5. [DOI] [PubMed] [Google Scholar]
- Tyner S. D., et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- Valentinis B., Bhala A., DeAngelis T., Baserga R., Cohen P. The human insulin-like growth factor (IGF) binding protein-3 inhibits the growth of fibroblasts with a targeted disruption of the IGF-I receptor gene. Mol. Endocrinol. 1995;9:361–367. doi: 10.1210/mend.9.3.7539889. [DOI] [PubMed] [Google Scholar]
- Wagner M., Hampel B., Bernhard D., Hala M., Zwerschke W., Jansen-Durr P. Replicative senescence of human endothelial cells in vitro involves G1 arrest, polyploidization and senescence-associated apoptosis. Exp. Gerontol. 2001;36:1327–1347. doi: 10.1016/s0531-5565(01)00105-x. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Itokawa M., Nakagawa Y., Iguchi T., Katagiri T. Increased levels of insulin-like growth factor binding protein-3 in hypertensive patients with carotid atherosclerosis. Am. J. Hypertens. 2003;16:754–760. doi: 10.1016/s0895-7061(03)00985-3. [DOI] [PubMed] [Google Scholar]
- Webley K., Bond J. A., Jones C. J., Blaydes J. P., Craig A., Hupp T., Wynford-Thomas D. Posttranslational modifications of p53 in replicative senescence overlapping but distinct from those induced by DNA damage. Mol. Cell. Biol. 2000;20:2803–2808. doi: 10.1128/mcb.20.8.2803-2808.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuoka H., Jukic D. M., Zhou Z., Choi A. M., Feghali-Bostwick C. A. Insulin-like growth factor binding protein 5 induces skin fibrosis: A novel murine model for dermal fibrosis. Arthritis Rheum. 2006;54:3001–3010. doi: 10.1002/art.22084. [DOI] [PubMed] [Google Scholar]
- Yoon I. K., Kim H. K., Kim Y. K., Song I. H., Kim W., Kim S., Baek S. H., Kim J. H., Kim J. R. Exploration of replicative senescence-associated genes in human dermal fibroblasts by cDNA microarray technology. Exp. Gerontol. 2004;39:1369–1378. doi: 10.1016/j.exger.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Zhao R., Gish K., Murphy M., Yin Y., Notterman D., Hoffman W. H., Tom E., Mack D. H., Levine A. J. Analysis of p53-regulated gene expression patterns using oligonucleotide arrays. Genes Dev. 2000;14:981–993. [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Woods D., McMahon M., Bishop J. M. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]