Abstract
Although there is considerable evidence implicating posttranslational mechanisms in the development of epithelial cell polarity, little is known about the patterns of gene expression and transcriptional regulation during this process. We characterized the temporal program of gene expression during cell–cell adhesion–initiated polarization of human Caco-2 cells in tissue culture, which develop structural and functional polarity similar to that of enterocytes in vivo. A distinctive switch in gene expression patterns occurred upon formation of cell–cell contacts between neighboring cells. Expression of genes involved in cell proliferation was down-regulated concomitant with induction of genes necessary for functional specialization of polarized epithelial cells. Transcriptional up-regulation of these latter genes correlated with formation of important structural and functional features in enterocyte differentiation and establishment of structural and functional cell polarity; components of the apical microvilli were induced as the brush border formed during polarization; as barrier function was established, expression of tight junction transmembrane proteins peaked; transcripts encoding components of the apical, but not the basal-lateral trafficking machinery were increased during polarization. Coordinated expression of genes encoding components of functional cell structures were often observed indicating temporal control of expression and assembly of multiprotein complexes.
INTRODUCTION
The human intestinal epithelium has a distinctive organization in which a small population of stem cells in the crypt gives rise to postmitotic cells that differentiate as they migrate away from the crypt into the villus (Clatworthy and Subramanian, 2001; Vidrich et al., 2003; Pinto and Clevers, 2005). The major polarized cell type, the enterocyte, forms a tight epithelial monolayer along the crypt-villus axis of the intestine, serving as a permeability barrier between luminal contents and the blood supply and as a conduit for nutrient absorption and enzyme secretion (Clatworthy and Subramanian, 2001).
Enterocyte polarization requires the formation of functionally and structurally distinct plasma membrane domains, termed apical and basal-lateral. The apical surface faces the lumen and consists of a dense array of microvilli, the “brush border,” which increases the absorptive surface area and contains enzymes and transporters to facilitate uptake of nutrients. The basal-lateral surface, which is separated from the apical surface by the tight junction, faces neighboring cells and the extracellular matrix (ECM). Basal-lateral membranes contain intercellular junctional complexes, ECM receptors, and channels and transporters that regulate ion/solute transfer from the intestinal lumen to the interstitium (Yeaman et al., 1999b).
Differentiation and polarization of epithelial cells involves the reorganization of cytoskeletal structures to initiate changes in cell shape and correct orientation of vesicle trafficking machineries to generate and maintain functionally specialized membrane domains (Yeaman et al., 1999a; Rodriguez-Boulan et al., 2005). A major unanswered question is how progenitor cells leaving the crypt are programmed to develop the structural and functional polarity characteristic of enterocytes. However, the inaccessibility to biochemical analysis coupled with the complexity of cell types along the intestinal crypt-villus axis has rendered direct analysis of enterocyte differentiation in situ difficult.
Analysis of mechanisms involved in the development of epithelial cell polarity has focused on cell lines that have retained the ability to polarize in vitro, including Caco-2 cells, which are derived from a human colon adenocarcinoma (Grasset et al., 1985; Wice et al., 1985). When cultured at low density, Caco-2 cells divide every 24 h and generally exhibit a nonpolarized distribution of proteins over the cell membrane, similar to that of other epithelial cells in culture (Yeaman et al., 1999b). However, upon formation of Ca2+-dependent cell–cell contacts, Caco-2 cells gradually form a polarized monolayer of postmitotic cells with structurally and functionally distinct apical and basal-lateral membranes that appear remarkably similar to those of polarized enterocytes in situ (Pinto et al., 1983). Detailed analysis of Caco-2 cells has identified roles for protein sorting and trafficking in the exocytic and endocytic pathways that specify protein distributions in the apical and basal-lateral membrane domains and protein complexes involved in the assembly of specific structures such as the brush border and tight junction that are characteristic of enterocytes (Le Bivic et al., 1989; Le Bivic et al., 1990a; Matter et al., 1990a,b; Soole et al., 1995; Monlauzeur et al., 1998).
Most studies of cell polarization have focused on posttranslational events involved in protein organization and distribution. In contrast, little is known about transcriptional events involved in the development of epithelial polarity in postmitotic cells. For example, do changes that occur in the structural and functional organization of proteins during polarization reflect the reorganization of existing structures in nonpolarized cells, or does reprogramming of gene expression for structures specific to polarized epithelia also play a role? Although it is likely that reprogramming of gene expression programs is important in vivo as cells exit the crypt and start to differentiate, it is less clear whether or to what extent this occurs in cells grown in vitro in which signaling from surrounding stromal cells is absent. However, we found genomic regulation of signaling pathways during Caco-2 polarization (Sääf et al., 2007), raising the possibility that downstream polarity structures may be regulated at this level as well. Here, we investigate the transcriptional modulation of genes encoding prominent structural characteristics of differentiating enterocytes including cell–cell junctions, cell–ECM interactions, brush border assembly, cytoskeletal organization, and membrane trafficking pathways (see Figure 1). The reader is referred to the full data for gene expression profiles of particular interest (http://microarray-pubs.stanford.edu/CACO2). Verification of cell polarization for a given structure or pathway is presented in the context of specific gene expression profiles.
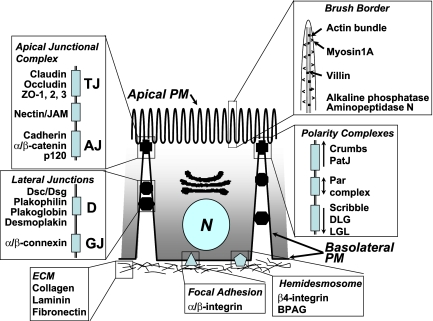
Figure 1.
Structural characteristics of intestinal epithelial cells. Polarized epithelial cells (enterocytes) along the villus are characterized by an apical brush border; lateral adhesion complexes including adherens junctions (AJ), tight junctions (TJ), desmosomes (D), and gap junctions (GJ); polarity complexes at the apex of the lateral membrane; and contacts with the underlying matrix through hemodesmosomes and focal adhesions.
MATERIALS AND METHODS
Microarray Analysis
Caco-2 cells were cultured, RNA was isolated, and microarray experiments were done as reported in the accompanying article in this issue (Sääf et al., 2007). In figures with zero-transformed data, the temporal expression profiles for each gene were translated to set the log2 expression to zero at time 0. Profiles for each IMAGE clone were averaged between experiments and all IMAGE clones representing the same gene were then averaged together (provided the clone had values for at least 7 of the 11 time points) to obtain final values. GeneSpring software (Agilent Technologies, Wilmington, DE) was used for analysis, and clustering was done with Cluster and TreeView programs (Eisen lab; http://rana.lbl.gov/index.htm). MiniTab and R software were used for statistical tests.
Target Validation
Select gene profiles were validated using semiquantitative RT-PCR from RNA isolated as previously described (Sääf et al., 2007). All reactions were done using SuperScript III One-Step RT-PCR Kit with Platinum Taq (Invitrogen, Carlsbad, CA), following the manufacturer's specifications. Between 8 and 16 pg total RNA (depending on target) was added to each reaction. Annealing temperature was 55°C. Primer sequences were as follows: (5′ to 3′) MAPRE1: GGC TGG CCC TGG TGT GGT G (sense), AAT TCC GAT GTT GCT CTG CTG GTC (antisense); MAPRE2: AGC CCC CGC AGC AGG AAG AGT A (sense), AGG GGT GGT GGC AGT GGT GTT G (anti-sense); KRT20: ATG GAT TTC AGT CGC AGA AGC TTC C (sense), CGC AGC TCT TCA ATT TGT CTG TAA T (antisense); PAK1: TGT CAA ATA ACG GCC TAG ACA TTC A (sense), ATG GAT CGG TAA AAT CGG TCC TTC T (antisense); and ARFGAP3: AAG CAG GAC ATC TTG ACC ATC TTC A (sense), GAA CCA CAC AAC TAT CAA GCC ACA G (antisense).
Other Assays
Assays to measure [3H]inulin permeability of the tight junction, the distribution of cell plasma membrane proteins using cell surface biotinylation or immunofluorescence microscopy, and processing of cells for electron microscopy have been published previously (Jou et al., 1998; Yeaman et al., 2001). In situ hybridization was performed as is described in Iacobuzio-Donahue et al. (2002).
RESULTS
Caco-2 cells are often used as a model of enterocyte polarization (Chantret et al., 1988). When grown on permeable filter supports, Caco-2 cells develop structural and functional polarity over a period of ∼3 wk, forming a postmitotic monolayer of cells with fully developed apical and basal-lateral membrane domains, a functional tight junction, and polarized organization of the cytoskeleton similar to that of enterocytes in situ. As described in the preceding study, we characterized the transcriptional program of polarizing Caco-2 cells. Monolayer formation was initiated by plating cells to confluency and mRNA was isolated at 11 time points over the subsequent 26 d time course. Microarray analysis was performed on a total of five replicate data sets, and the results were averaged to obtain final values (Sääf et al., 2007).
Brush Border
A distinctive feature of intestinal epithelial cells is the apical brush border (BB), composed of numerous membrane extensions (microvilli) from the apical plasma membrane (Figure 1). The core of each microvillus comprises a bundle of parallel actin filaments and associated actin binding proteins, including villin which is expressed only in intestinal cells and plays an important role in maintenance of brush border architecture (Mooseker, 1985; Athman et al., 2002).
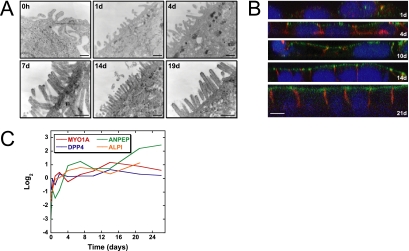
Transmission electron microscopy of Caco-2 cells at intervals during development of polarity demonstrated the gradual formation of the apical brush border (Figure 2A). Initially, the apical surface contained a few short membrane extensions, but later in the time course a dense array of long microvilli characteristic of the brush border formed. We used immunofluorescence confocal microscopy to examine expression of villin (Figure 2B), a protein characteristic of the enterocyte brush border (Athman et al., 2002). Levels of villin staining were low in the first 2 d but increased over time, and villin eventually concentrated at the apical membrane in fully polarized cells, consistent with formation of the brush border.
Figure 2.
Formation of the apical brush border (BB), a distinctive feature of intestinal epithelial cells, is accompanied by increased expression of genes encoding brush border enzymes and structural components during in vitro Caco-2 cell differentiation. (A) Transmission electron microscopy of Caco-2 cells demonstrates the gradual formation of the apical BB during development of polarity. Scale bar, 250 nm. (B) Protein expression and subcellular localization during Caco-2 cell differentiation was examined using immunofluorescence confocal microscopy. Expression of villin, a structural component of the enterocyte brush border, and E-cadherin, a lateral cell–cell adhesion protein, are shown in green and red, respectively; DAPI (blue) stains the cell nucleus. Scale bar, 10 μm. (C) cDNA microarrays were used to analyze transcriptional profiles for genes encoding BB enzymes and structural components during the in vitro assembly of an epithelial layer. Brush border transcripts, including myosin 1A (MYO1A), dipeptidylpeptidase IV (DPP4), aminopeptidase N (ANPEP), and intestinal alkaline phosphatase (ALPI) increased significantly in expression over time during Caco-2 cell polarization. Y-axis indicates fold change of transcript levels relative to a reference pool of human mRNAs on a Log2 scale.
Analysis of the gene expression program in polarizing Caco-2 cells revealed an increase in expression of genes encoding proteins with structural/functional roles in the brush border concomitant with its assembly. The expression pattern of myosin 1A (MYO1A) is illustrated in Figure 2C. Myosin 1A gene expression was low in nonpolarized cells, increased over time, and plateaued as the cells developed full polarity. In contrast, another structural component of microvilli, villin 2 (ezrin), was expressed early in the time course, and levels were maintained as cells transitioned to the postmitotic polarized state (data not shown), indicating that villin 2 gene expression is not specifically timed to the assembly of the brush border. Analysis of gene expression profiles for three brush border enzymes, dipeptidyl-peptidase 4 (DPP4), aminopeptidase N (ANPEP), and intestinal alkaline phosphatase (ALPI; Figure 2C) revealed that their expression profiles are similar to that of myosin 1A. Transcripts encoding another apical protein, mucin-13 (MUC13), also increased over time as Caco-2 cells developed polarity, paralleling the expression pattern of the MUC13 gene in vivo as enterocytes differentiate along the intestinal crypt-villus axis (Supplementary Figure S1).
Taking the microscopy and microarray data together, we found that formation of the apical brush border is accompanied by increased expression of genes encoding brush border enzymes and structural components. These data show that brush border assembly is transcriptionally regulated in conjunction with posttranscriptional regulation (Mooseker, 1985) of components such as villin-2.
Cell–Cell Adhesion Complexes
Epithelial cells adhere to each other and to ECM using several different adhesion complexes (Yeaman et al., 1999b; illustrated in Figure 1). The apical junction complex (AJC), desmosomes, and gap junctions are localized in the lateral membrane domain and specify adhesion between neighboring cells. The AJC is localized at the most apical aspect of the lateral plasma membrane and is composed of different protein subcomplexes that function to establish and maintain an intact polarized cell monolayer. These complexes include the membrane proteins Crumbs, JAM, claudin/occludin (tight junctions), nectin and cadherin (adherens junctions), and their associated cytoplasmic adaptor proteins (Nelson, 2003). Some components of the AJC, including β-catenin, have additional roles in regulating gene transcription and mRNA processing (Nelson and Nusse, 2004). Additional junctions present in differentiated enterocytes include desmosomes and gap junctions. We discuss below the analysis of gene expression profiles for these junctional complexes.
Tight Junction.
The tight junction (zonula occludens) is located at the boundary between the apical and basal-lateral membrane domains and regulates paracellular transport (gate function) and diffusion of membrane proteins and lipids between the membrane domains (fence function; Aijaz et al., 2006). The structural composition of tight junction membrane proteins (claudins) appears to influence the ion permeability and selectivity of the paracellular pathway (Laukoetter et al., 2006).
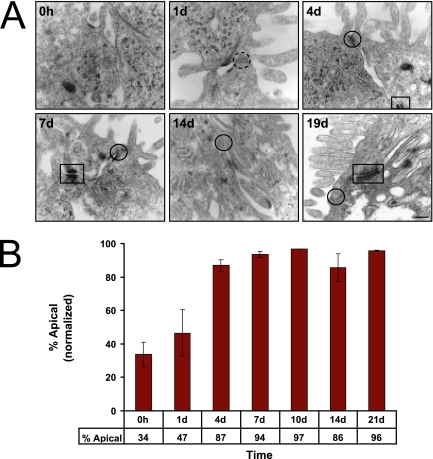
Transmission electron microscopy of Caco-2 cells during the time course revealed the formation at about day 4 of localized electron-dense areas of closely opposing plasma membranes (kissing points) between cells at the apex of the lateral membrane characteristic of forming tight junctions (Figure 3A). To assess functional formation of tight junctions, monolayers were tested for diffusion of a small molecule, [3H]inulin, between the apical and basal-lateral compartments of the filter insert on which the cell monolayer had formed. Initially the monolayer was leaky, allowing rapid equilibration of [3H]inulin between the compartments. However, by day 4 [3H]inulin diffusion across the monolayer was dramatically reduced to a background level, indicative of the formation of functional tight junctions (Figure 3B).
Figure 3.
Formation of tight junctions at the boundary between the apical and basal-lateral membranes. (A) Transmission electron microscopy of Caco-2 cells during the time course revealed the formation of localized electron-dense, closely opposing plasma membranes between cells at the apex of the lateral membrane characteristic of tight junctions by day 4 (solid circle). Dashed circle indicates the location of the future tight junction before its formation. As the time course progressed, desmosomes were observed as electron-dense plaques on the lateral membrane of differentiating Caco-2 cells (box). Scale bar, 100 nm. (B) To functionally assess tight junction formation, Caco-2 monolayers were assayed for permeability of a small molecule, [3H]inulin. At early time points apically applied [3H]inulin rapidly equilibrated between the apical and basal-lateral compartments. By day 4 [3H]inulin diffusion across the monolayer was restricted. Error bars, n = 2.
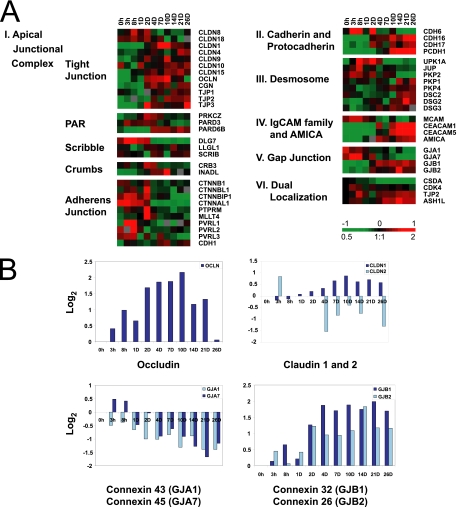
The expression patterns of genes encoding tight junction integral membrane proteins (occludin and claudins) and protein scaffolds (ZO-1, -2, and -3, and cingulin) are shown in Figure 4A, cluster I. The temporal expression patterns of occludin (OCLN) and claudin-1 (CLDN1), found in tight epithelial monolayers, increased initially after cell–cell adhesion and peaked at a time coincident with formation of functional tight junctions (Figure 4B). Claudin-2 (CLDN2) mRNA, which is expressed in leaky epithelia (Furuse et al., 2001), decreased as the cells polarized (Figure 4B). Expression of several other claudins, in particular CLDN 4, 9, 10, and 15, increased gradually over time after induction of cell–cell adhesion (Figure 4, A, cluster I). Transcripts encoding the tight junction adaptor proteins ZO-1 and -2 and particularly ZO-3 (TJP1, -2, -3) and cingulin (CGN) also increased during the time course.
Figure 4.
Temporal expression patterns of genes encoding cell–cell adhesion molecules during in vitro Caco-2 epithelial cell polarization. (A) Genes are displayed and grouped according to adhesion complex or protein family: apical junction complex (cluster I), cadherin/protocadherin families (cluster II), desmosome (cluster III), immunoglobulin superfamily of adhesion molecules (cluster IV), gap junction (cluster V), and dual localization proteins (cluster VI). (B) Zero-transformed expression profiles of genes encoding the tight junction protein occludin, claudin-1 and -2, and connexin family members (α and β subunits of the gap junction). Transcript levels determined by microarray analysis are shown relative to a reference pool of human mRNAs.
Several proteins found at tight junctions also localize to the nucleus, including ZONAB (CSDA), ZO-2, and huASH1 (ASH1L). ZONAB is a Y-box transcription factor that may control proliferation by facilitating the transport of CDK4 into the nucleus (Sherr, 1995; Balda et al., 2003; Sourisseau et al., 2006). ZONAB transcript levels declined concomitantly with decreased levels of CDK4 transcripts as Caco-2 cells polarized (Figure 4A, cluster VI). huASH1 is the human homologue of Drosophila ASH1, which interacts with trithorax, a multiprotein complex that activates gene transcription by altering chromatin structure. In contrast to ZONAB, huASH1 and ZO-2 transcripts increased during Caco-2 cell polarization (Figure 4A, cluster VI).
In summary, we found that expression of tight junction transcripts and the formation of the tight junction barrier appear to be temporally coupled. For example, expression of claudin-1, which decreases junction permeability (Colegio et al., 2003; Van Itallie and Anderson, 2006), mRNA increased gradually and continuously over time after induction of cell–cell adhesion, whereas that of claudin-2, which is expressed in leaky epithelia, decreased during polarization. This switch in claudin expression is consistent with the fact that Caco-2 cells develop over time a “tight” (villus-like) epithelium.
Crumbs, Scribble, and the PAR Complex.
Recent studies have shown that polarity complexes that localize to the AJC play instructional roles in specifying the structural and functional identities of the apical and basal-lateral membrane domains (Nelson, 2003). The PAR-complex (Par3/Par6/aPKC) regulates formation of the apical membrane domain in Drosophila (Bilder et al., 2003; Tanentzapf and Tepass, 2003) and is involved in the development of cell polarity in mammalian epithelial cells (Etienne-Manneville and Hall, 2003; Henrique and Schweisguth, 2003). Our data showed that increases in the levels of Par3 (PARD3) and aPKCζ (PRKCZ) mRNAs preceded increases in Par6b (PARD6B) mRNA during Caco-2 cell differentiation (Figure 4A, cluster I).
The Scribble functional complex (including Lethal giant larva/Scribble/Discs large) appears to antagonize the spread of the apical membrane down the lateral membrane domain (Roh and Margolis, 2003; Bilder, 2004). In differentiating Caco-2 cells, we observed high transcript levels of the Discs Large gene DLG7 in proliferating cells before polarization, followed by a significant decrease in expression level upon cell differentiation. Lethal giant larva (LLGL1) and scribble (SCRIB), however, did not show significant changes in transcriptional levels over time as Caco-2 cells polarized (Figure 4, cluster I).
In turn, the Scribble complex is functionally antagonized by the more apically localized Crumbs complex, which comprises the membrane protein Crumbs and two cytoplasmic proteins, Pals1 and PatJ, and has an important role in maintaining apical membrane identity (Roh and Margolis, 2003). In contrast to the expression profile of the Scribble complex component DLG7, genes encoding Crumbs complex components, Crumbs-3 (CRB3) and Patj (INADL), were initially expressed at low levels and then increased after extensive cell–cell contacts were established. Expression of CRB3 was highest between day 2 and 4, whereas INADL transcript levels peaked later during polarization at day 10 (Figure 4A, cluster I).
Adherens Junction.
Ca2+-dependent cell–cell contacts are formed by the cell adhesion protein E-cadherin, the membrane component of the adherens junctions (AJ; Gumbiner, 2005); the nectin family of Ig superfamily adhesion proteins is also thought to play a role in the initiation of cell–cell adhesion (Irie et al., 2004). E-cadherin binds β-catenin, which in turn binds α-catenin which regulates actin dynamics (Gates and Peifer, 2005; Gumbiner, 2005).
Confocal immunofluorescence microscopy (Figure 2B) shows that E-cadherin was expressed in single proliferating cells and throughout the time course and became restricted to the lateral membrane upon Caco-2 cell polarization. We observed a weak reciprocal trend between the slight increase in E-cadherin (CDH1) transcripts and a decrease in β-catenin and β-catenin-like-1 (CTNNB1, CTNNBL1) transcripts as Caco-2 cells polarized (Figure 4A, cluster I). Levels of transcripts encoding α-catenin-like-1 (CTNNAL1), and β-catenin–interacting protein 1 (CTNNBIP1), and members of the nectin family (PVRL1-3) decreased after establishment of cell–cell contacts, whereas levels of α-catenin transcripts did not change significantly. Afadin (MLLT4) expression peaked at 2 d. The level of expression of PTPμ (PTPRM), a phosphatase that interacts with the AJ complex and positively regulates adhesion (Brady-Kalnay and Tonks, 1995; Ostman et al., 2006), also had a strong peak at day 2 when extensive cell–cell contacts were first formed (Figure 4A, cluster I). Other members of the cadherin and proto-cadherin families showed significant reciprocal (CDH6 vs. CDH16, CDH17, and PCDH1) increases in transcript levels (Figure 4A, cluster II) coincident with the development of cell polarity.
Desmosomes.
Resistance to shear stress across the epithelium is regulated by desmosomes composed of desmosomal cadherins (desmocollins, desmogleins) linked to cytokeratin intermediate filaments through a plaque of cytoplasmic proteins (desmoplakin, plakoglobin, and plakophillin; Getsios et al., 2004). Desmosomes are observed in electron micrographs in Figure 3A as individual electron-dense plaques on lateral membranes between Caco-2 cells.
Levels of transcripts encoding desmosomal cadherins (DSG2, DSG3, and DSC2) and two members of the plakophillin protein family (PKP1 and PKP4) increased as Caco-2 cells polarized (Figure 4, cluster III). DSG2 and DSC2 are ubiquitously expressed in tissues with desmosomal junctions (Garrod et al., 2002). Conversely, transcripts encoding the cytoplasmic linker plakoglobin (PKG/JUP) and two other plaque proteins (UPK1A, PKP2) decreased over time, similarly to those encoding other members of this protein family, including β-catenin (see above). These results demonstrate that adhesive components of desmosomes, which have a specialized role in maintaining the structural integrity of the epithelial monolayer, are expressed later in cell polarization when their function may become important.
Other Adhesion Molecules (IgCAM-Super Family).
Transcripts of genes encoding two members of the carcinoembryonic antigen (CEA)-related family of cell adhesion molecules (Fakih and Padmanabhan, 2006), CEACAM1 and CEACAM5 (Figure 4A, cluster IV), increased dramatically after initiation of polarization. CEACAM1 isoforms are localized at lateral membranes of polarized MDCK epithelial cells and may contribute to the organization of desmosomes (Sundberg et al., 2004). JAML/AMICA, a member of the junctional adhesion molecule family, also increased with differentiation and epithelia formation. Conversely, expression of the melanoma cell adhesion molecule MCAM/MUC18 decreased as the cells became postmitotic and started to polarize. This trend, which is consistent with the role of MCAM in metastasis, was reversed here as Caco-2 cells transitioned from single cells to an epithelial sheet (Luca et al., 1993).
Gap Junctions.
Gap junctions (GJ) regulate the exchange of ions and metabolites between cells and are composed of α and β connexins (Cx) that assemble into homotypic or heterotypic channels; combinations of different gap junction proteins form gap junctions with distinct ion selectivity and permeability (Wei et al., 2004; Evans et al., 2006). Genes encoding connexin α and β subunits were expressed in a reciprocal temporal pattern during Caco-2 cell differentiation (Figure 4, A, cluster V, and 4B). GJ-α subunits (GJA1/Cx43 and GJA7/Cx45) were expressed in proliferating Caco-2 cells, but decreased over time during cell polarization. Conversely, expression of GJ-β subunits (GJB1/Cx32 and GJB2/Cx26) increased as cells became postmitotic and developed polarity. These different expression patterns of α- and β-connexin transcripts in polarizing Caco-2 cells are consistent with studies showing that in vitro–translated α/α subunits or β/β subunits interact to form gap junction channels, but α and β subunits do not mix (Saez et al., 2003; Segretain and Falk, 2004) and that Cx26 and Cx32 are coexpressed in the rat intestine (Zhang and Nicholson, 1989).
The significance of the switch between connexin α and β subunits upon formation of an epithelium is unknown. However, members of the α and β family of connexins may have different roles in proliferating epithelial cells and polarized postmitotic cells that form an epithelium. For example, connexins expressed in proliferating cells could have a nonjunctional function, an idea supported by evidence that the carboxy-terminal tail of Cx43 is localized to the nucleus and acts as a growth suppressor in HeLa cells (Dang et al., 2003). The expression of Cx43 in proliferating Caco-2 cells is noteworthy since it is also overexpressed in more proliferative, metastatic cells (Husoy et al., 2005), whereas Cx26, which was more highly expressed in polarized Caco-2 cells, can reverse a malignant phenotype in cultured breast cancer cells (Momiyama et al., 2003), indicating that Cx26 may act as a tumor suppressor.
In summary, these results demonstrate that expression of proteins of epithelial junctional complexes is regulated at the level of transcript abundance. Thus, rather than simply responding to cell–cell contact to reorganize proteins posttranslationally, our results suggest a more programmed transcriptional regulation of expression and assembly of different adhesion complex components upon Caco-2 cell differentiation.
Cell-ECM Adhesion Complexes
Cell–ECM interactions are important for cell development, differentiation, and survival and function in generating and maintaining the apical-basal axis in polarized epithelial cells (O'Brien et al., 2002; Nelson and Bissell, 2005). The ECM of epithelial cells consists principally of laminins, collagens, and proteoglycans, and associated proteins that regulate the organization of these main constituents. Integrins, the major family of receptors that attach cells to the ECM (Figure 1), are comprised of α/β-heterodimers that bind to specific components of the ECM and to cytoplasmic proteins that link to cytoskeleton and signaling complexes (Zamir and Geiger, 2001; Hynes, 2002). These receptors are clustered with many structural and signaling proteins at specific sites at the epithelial basal membrane to form two major adhesion signaling complexes, focal adhesions (FA; Zamir and Geiger, 2001), and hemidesmosomes (HD; Figure 1) that are linked to actin and intermediate filaments, respectively (Hahn and Labouesse, 2001). In addition to their participation in adhesion, these complexes play important roles in cell signaling to regulate cell proliferation, differentiation, and cytoskeleton organization (Zamir and Geiger, 2001; DeMali et al., 2003).
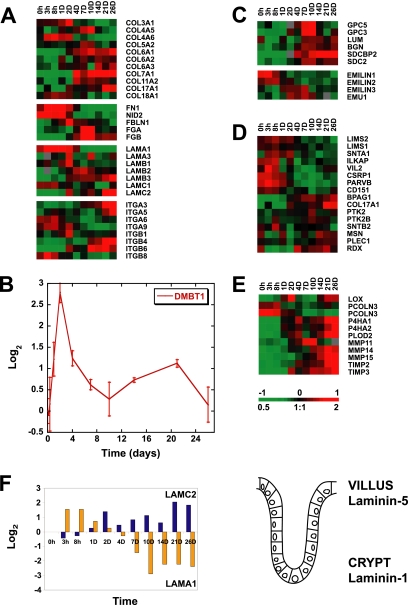
We found that transcripts encoding ECM components were finely regulated during formation of a polarized epithelium in vitro (Figure 5). Collagens, fibronectin, laminins, and integrin receptors, along with their associated proteins at focal adhesions and hemidesmosomes, are all regulated at the level of transcript abundance during polarization. Expression of genes encoding heparin sulfate proteoglycans and matrix synthesis and remodeling enzymes are also modulated. Individual molecules with summaries of their functions in epithelial cells are listed in Supplementary Table S1.
Figure 5.
Temporal expression patterns identified for genes encoding cell-ECM adhesion molecules during in vitro development of cell polarity, including family members of collagen, laminin and integrin receptors (A), hensin (DMBT1) (B), heparan sulfate proteoglycans (C), and hemidesmosome and focal adhesion complex components (D). Enzymes with roles in the modification or remodeling of ECM are also under fine transcriptional regulation during Caco-2 cell polarization (E). (F) Left, the reciprocal expression pattern observed for transcripts encoding components of laminin-1 and -5 (LAMA1 and LAMC2, respectively) during in vitro establishment of an epithelial layer, which mimic in vivo expression trends of laminin chains, previously identified along the human intestinal crypt villus axis (shown on the right; Leivo et al., 1996; Orian-Rousseau et al., 1996). Transcript levels determined by microarray analysis are shown relative to a reference pool of human mRNAs. A summary of the roles of individual cell–ECM components is listed in Supplementary Table S1.
The ECM component hensin has been shown to induce formation of columnar epithelia (van Adelsberg et al., 1994), and mouse embryos lacking hensin protein die between embryonic day (E) 4.5 and E5.5, concomitant with formation of the first columnar epithelium, the trophectoderm (Takito and Al-Awqati, 2004). In humans, deletion of hensin has been observed in a number of cancers (Mollenhauer et al., 1997; Mori et al., 1999; Takito et al., 1999). Expression of hensin (DMBT1) had a particularly interesting expression profile, showing a strong peak at day 2 of Caco-2 polarization (Figure 5B) consistent with a role in initiating formation of a polarized columnar epithelial monolayer.
Laminins are important in intestinal morphogenesis and differentiation (Simon-Assmann et al., 1998). Laminin gene expression during Caco-2 polarization paralleled their expression patterns along the intestinal crypt-villus axis in vivo (Figure 5, A and F). The three chains comprising laminin-1 (LAMA1, LAMB1, and LAMC1) are all most highly expressed at early time points, and LAMA1 transcript levels, in particular, peak early in the time course. This is consistent with the role of laminin-1 in enterocyte differentiation, and particularly the α-1 chain (LAMA1), which is required for the efficient secretion of the other two chains and deposition of ECM (De Arcangelis et al., 1996; Turck et al., 2005). The three chains of laminin-5 (LAMA3, LAMB3, and LAMC2) were all up-regulated as cell polarization proceeded. Interestingly, laminin-5 expression increases along the crypt-villus axis in vivo and is the ligand for the hemidesmosome cell–ECM adhesion complex.
The apparent intrinsic gene-expression program regulating interactions with the ECM may function to effect the large-scale cytoskeletal changes that occur during polarization. In addition, the temporally staged secretion of specific ECM components may enable polarizing Caco-2 cells in culture to mimic changes in the basal membrane structure and surrounding ECM composition presented to enterocytes migrating up the crypt-villus axis in vivo. These changes in basal membrane transcript expression during polarization may be important in the development of cell polarity (O'Brien et al., 2002; Nelson and Bissell, 2005). Modulation of other components of the ECM was observed during Caco-2 polarization; the reader is referred to the full data set for these expression patterns.
Actin Cytoskeleton
The cytoskeleton is organized to regulate and maintain epithelial polarity. Different actin cytoskeleton organizations are associated with each plasma membrane domain (Figure 1). On the basal-lateral surface, actin associates with the basal membrane at focal adhesions involved in attachment to the extracellular matrix (ECM; Zamir and Geiger, 2001) and with the lateral membrane cell–cell contacts (Kobielak and Fuchs, 2004). On the apical surface, actin bundles comprise the core structure of the brush border microvilli (Mooseker, 1985; Athman et al., 2002). Like many cell types, single Caco-2 cells exhibit a dynamic cell surface in which lamellipodia supported by branched actin networks extend over the substratum. On initiation of polarization through cell–cell contact formation, actin reorganizes to form a cortical bundle around the cell periphery. This transition from a highly dynamic branched actin organization to a more stable bundled structure may be reflected in the transcription of genes encoding actin and actin-associated proteins.
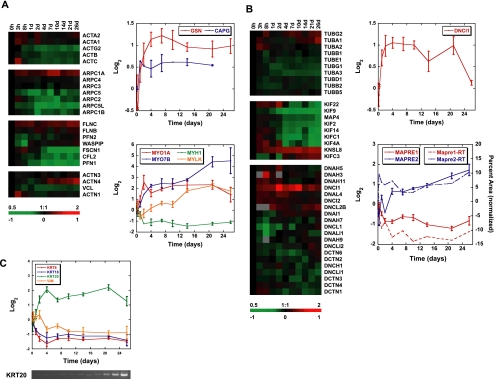
Hierarchical clustering of the time courses of expression of actin and its associated proteins identified several potentially significant patterns of regulation that could affect the organization of the actin cytoskeleton during cell polarization (Figure 6A; functions summarized in Supplementary Table S2). Transcripts encoding the ubiquitous G-actin–sequestering protein profilin-1 (PFN1; Carlsson et al., 1977) decreased during the time course. Components of the Arp2/3 actin-nucleating complex (Welch, 1999), with the exception of ARPC1A, were most highly expressed early in the time course when the cells had not polarized. Profilin and the Arp2/3 complex are important in polymerization of branched actin networks and in cell migration (Pollard and Borisy, 2003); decreases in the levels of their mRNAs could signal a change in actin dynamics as cells become more sedentary and develop polarity. Expression of the Wiskott-Aldrich syndrome protein (WASP), which facilitates Arp2/3-dependent actin nucleation, was not temporally regulated at the transcriptional level, but its interacting protein WASPIP, or WIP (Aspenstrom, 2005), was sharply down-regulated initially and then rapidly recovered to a level similar to that before the commencement of polarization.
Figure 6.
Genomic regulation of cytoskeletal components during polarization. (A) Transcript profiles of actin and actin-associated proteins. Hierarchical clusters of actin isotype, Arp2/3 subunit, and actin-associated protein mRNA. Expression levels of each gene over time relative to the 0 h time point (zero-transformed) are displayed in log2 scale. Red or green color indicates expression levels above or below expression at 0 h over the time course, respectively. Top graph shows actin-capping protein transcript expression over time. Gene profiles are zero-transformed and displayed in log2 scale. Bottom graph displays selected myosin gene expression over the time course. (B) Gene expression of microtubule subunits and microtubule-associated proteins. Hierarchical clusters of tubulin, kinesin, and dynein/dynactin transcripts. Top graph shows cytoplasmic dynein IC 1 (DNCI1) mRNA expression over time. Bottom graph displays expression of the MAPRE gene family during cell polarization. Solid lines represent the average expression profile of each gene from microarray experiments displayed in log2 scale. Dashed lines show mRNA profiles from RT-PCR verification as percent band area normalized to total. (C) Expression of intermediate filament components during Caco-2 polarization. Graph shows the transcriptional profiles of selected keratins and vimentin over time. Bottom panel shows verification of KRT20 array profile by RT-PCR. Transcript levels determined by microarray analysis are shown relative to a reference pool of human mRNAs. Error bars, SE.
Microtubules
As epithelial cells polarize, the organization of the microtubule cytoskeleton changes from a radial array nucleated by the centrosome to a complex organization of acentrosomal parallel bundles that extend along the vertical axis of the cell and mesh-like networks on the apical and basal surfaces (Figure 1; Bre et al., 1987; Bacallao et al., 1989; Gilbert et al., 1991). These organizations of microtubules provide structural rigidity as cells increase in height during polarization and are required for vesicle transport and organelle positioning (Busson-Mabillot et al., 1982). Thus, changes in expression of microtubule-interacting proteins and tubulin isotypes may be important during cell reorganization.
We observed altered expression of centrosomal tubulins, microtubule-associated proteins, and motors as cells became postmitotic (Figure 6B; functions summarized in Supplementary Table S2). These included MAP4, which increases the rescue frequency of microtubules (Permana et al., 2005) and reduces vesicle motility and organelle movement (Bulinski et al., 1997), and was down-regulated over the time course. The expression of KIFC3, which has been implicated in Golgi positioning and apical transport (Noda et al., 2001; Xu et al., 2002) was increased. Transcripts encoding microtubule end-binding (EB) proteins EB1 (MAPRE1, the best characterized member) and EB2 (MAPRE2, largely uncharacterized), which localize to the plus ends of microtubules (Tirnauer and Bierer, 2000), differed in their temporal expression programs (Figure 6B). MAPRE1, which plays a well-established role in mitosis in regulating spindle dynamics, was strongly down-regulated coincident with the cells becoming postmitotic. In contrast, transcripts of MAPRE2 increased as cell polarization proceeded. mRNA levels of the adenomatous polyposis coli (APC) protein, a known binding partner of MAPRE1 (Su et al., 1995) and tumor suppressor mutated in familial colon cancer, showed only modest changes over the time course (Sääf et al., 2007).
Intermediate Filaments
Intermediate filaments connect to hemidesmosomes at the basal cell surface and to desmosomes at cell–cell contacts forming a structural continuum to withstand shear stress across the epithelium (Figure 1; Hahn and Labouesse, 2001; Getsios et al., 2004). Although a considerable amount of information is available concerning cytoskeletal organization in polarized epithelial cells, less is known about the regulatory mechanisms involved.
Generally, different intermediate filament proteins are expressed in mesenchymal cells (vimentin) and epithelial cells (keratins; Franke et al., 1978). During polarization of Caco-2 cells, levels of vimentin transcript (VIM) decreased (Figure 6C). Intermediate filaments composed of cytokeratins bind to desmosomes and provide epithelial cell layers with tensile strength (Getsios et al., 2004). Typically, keratin polymers comprise heterodimers composed of one acidic (type I) and one basic (type II) keratin. The expression of keratin isoforms along the intestinal crypt-villus axis has been previously characterized; KRT18 (type I) localizes to the crypt, KRT20 (also type I) to fully differentiated cells along the villus, and the corresponding type II keratin, KRT8, localizes along the entire axis (Calnek and Quaroni, 1993). We find that this in vivo pattern of cytokerain expression is paralleled in polarizing Caco-2 cells (Figure 6C); Krt20 mRNA levels increased, and correspondingly Krt18 transcripts decreased during polarization. Interestingly, Krt8 transcripts were down-regulated as Caco-2 cells polarized, perhaps indicating changes in protein stability.
Rho GTPases
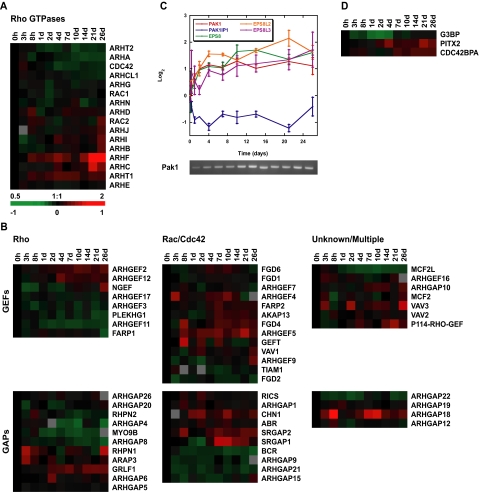
Small GTPases play diverse roles in regulating the cytoskeleton and protein trafficking, and hence, morphological changes in cells (Jaffe and Hall, 2005). Small GTPases including members of the Rho superfamily (Rho, Rac, and Cdc42) cycle through active (GTP-bound) and inactive (GDP-bound) states. The time spent in each state, and thus their activity, is regulated by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs), which promote transition into and out of the active GTP-bound state, respectively. Transcripts of two of the best-characterized members of the Rho GTPase family, RhoA (ARHA) and Cdc42 (CDC42) implicated in stress fiber and filopodial formation, respectively (Fujita and Braga, 2005), were slightly down-regulated during Caco-2 polarization (Figure 7A). Transcripts encoding RAC1 (involved in lamellipodia activity Fujita and Braga, 2005) were relatively unchanged. Transcripts of some of the more recently identified Rho GTPases, in particular RHOF, were up-regulated as Caco-2 cells polarized.
Figure 7.
Expression profiles of Rho GTPases and GTPase-interacting proteins. (A) Hierarchical clustering of Rho GTPase transcripts: Rho (ARH), Rac, and Cdc42 over time. (B) Clustering of GEFs and GAPs based on specificity for Rho or Rac/Cdc42 or those of unknown or promiscuous Rho GTPase interaction. (C) Graph of PAK1, PAK1IP1, and EPS8 family mRNA expression during polarization. DNA gel below verifies PAK1 expression profile by RT-PCR. (D) Transcript profiles of MRCK (CDC42BPA), PITX2, and G3BP over the time course. Transcript levels determined by microarray analysis are shown relative to a reference pool of human mRNAs. Error bars, SE.
In addition to direct regulation of expression of these GTPases, the function of the GEFs and GAPs that modulate their activity can be affected by other proteins or through transcriptional regulation, allowing for finer spatial and temporal control of GTPase activation (Rossman et al., 2005). When expression patterns of GEFs and GAPs were clustered based on their GTPase specificity, the expression of those that act on Rho (based largely on studies using the RhoA family member) were generally down-regulated, whereas a greater fraction of the Rac1- and Cdc42-specific GEFs and GAPs had increased expression during cell polarization (Figure 7B). This difference between the regulation of Rho and Rac1-Cdc42 GEFs and GAPs suggests a greater role for the latter GTPases in polarized Caco-2 cells, consistent with recent data implicating Cdc42 in apical membrane organization (Martin-Belmonte et al., 2007). Known functions of individual GEFs and GAPs whose transcripts were regulated during polarization are summarized in Supplementary Table S3.
In addition to GEFs and GAPs, effectors of small GTPases were also regulated during Caco-2 cell polarization (Figure 7, C and D). Pak1 expression increased and transcripts of the Pak1 inhibitor Pak1IP1 (Xia et al., 2001) decreased over the time course (Figure 7C). Pak1 is activated by Cdc42 and Rac1, leading to actin cytoskeleton reorganization and lamellipodia formation or membrane ruffling (Vadlamudi et al., 2005). Pak1 also modifies microtubule dynamics (Jaffer and Chernoff, 2002). As Caco-2 cells polarized, expression of EPS8 and two closely related genes, EPS8L2 and EPS8L3, increased (Figure 7C). Eps8 homologues in other organisms transduce signals from Ras, Rac, and growth factor receptors to regulate actin remodeling, to cap actin barbed ends, and to regulate apical morphology (Croce et al., 2004; Disanza et al., 2004; Offenhauser et al., 2004).
Although transcripts encoding Rho GTPases were only slightly down-regulated during Caco-2 cell polarization, we found that regulation of Rho GTPase signaling pathways occurred indirectly through regulation of their modulators (GAPs and GEFs) and effectors. The pathways primarily affected by this level of regulation are Rac1- and Cdc42-dependent, whereas components of Rho signaling were generally down-regulated, suggesting that Rac1 and Cdc42 play a more dominant role in the polarized Caco-2 cell.
Protein Trafficking Machinery
The generation and maintenance of functionally and structurally distinct plasma membrane domains involves sorting signal-specific trafficking of proteins within the exocytic and endocytic pathways (Rodriguez-Boulan et al., 2005). Localized protein delivery to distinct membrane domains enables selective uptake and directional transport of nutrients, secretion of enzymes, diffusible morphogens and ECM proteins into correct compartments, and receptor localization to maintain intercellular communication (Le Bivic et al., 1990b; Matter et al., 1990c). The extent to which components of membrane trafficking pathways between different compartments are specifically programmed by patterns of new gene expression in polarized epithelial cells or adapted from nonpolarized cells (Yoshimori et al., 1996) is poorly understood.
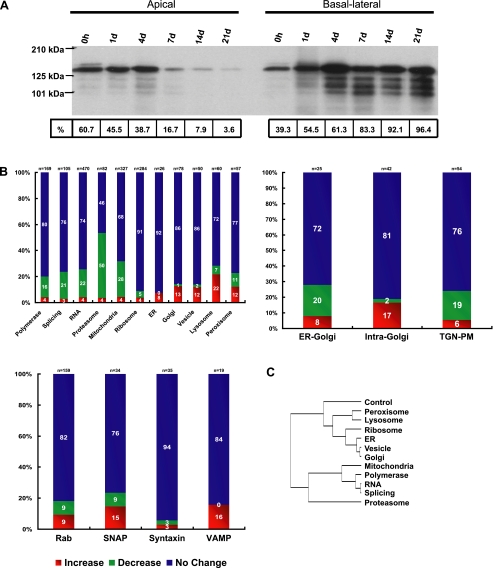
We verified the induction of differential membrane trafficking during polarization by selectively biotinylating either apical or basal-lateral cell surface proteins and visualizing the distribution of biotinylated basal-lateral marker protein E-cadherin (Figure 8A). To examine changes in transcripts encoding components of protein trafficking pathways during Caco-2 cell polarization, we began by assembling supervised clusters for different steps of the protein sorting and membrane trafficking pathways between the endoplasmic reticulum and plasma membrane. We compared their overall expression patterns with those of mRNAs encoding components of several other basic cellular processes (polymerases, splicing machinery, other RNA processing factors, proteasomal components, and mitochondrial proteins); we did not expect the latter to be linked to the establishment and maintenance of differential plasma membrane domains, and therefore they provided a base level for comparison to get a broad sense of any differential regulation of trafficking pathways during polarization. We classified the transcripts that increased, decreased, or did not significantly change (using a twofold threshold) during cell polarization for each functional gene group and then compared these distributions between groups (Figure 8B).
Figure 8.
Expression of protein trafficking pathways in polarizing Caco-2 cells. (A) Immunoblots of selectively biotinylated membranes (either apical or basal-lateral) that had been immunoprecipitated with an antibody against E-cadherin and were then probed with labeled streptavidin. E-cadherin was expressed in single proliferating cells and throughout the time course and accumulated almost exclusively in the basal-lateral membrane upon Caco-2 polarization. (B) Graphs showing the number of genes that increase, decrease, or do not significantly change (using a twofold threshold) for each of the selected cell structures, functions, or protein families. Results are displayed as percentages of the total number of genes in each category to allow comparison between groups. (C) Statistical analysis of the distributions in B using pairwise chi-squared tests clustered based on similarity. Included is a control gene set of 155 randomly chosen genes. Notice that the distributions of protein trafficking pathways cluster away from those of proteasome and polymerase/splicing/RNA/mitochondria.
The expression patterns of each functional cluster indicate that a larger fraction of transcripts encoding proteins involved in protein sorting and vesicle transport increased compared with transcripts of proteins with roles in other cellular functions. Correspondingly, a smaller fraction of the trafficking proteins decreased. To confirm the significance of this observation, we used pair-wise chi-square analysis to determine the relative similarity between distributions and then clustered the gene lists based on similarity, including a control gene set consisting of 155 random genes (Figure 8C). We found that the distributions of genes involved in protein trafficking and recycling clustered together. Those involved in DNA replication, transcription, and mRNA processing clustered away from trafficking components, and with mitochondrial and proteasomal components, likely reflective of substantial roles in the cell cycle.
When the data were clustered by intercompartment transport (ER-Golgi, intra-Golgi, or trans-Golgi network [TGN]-plasma membrane [PM]), additional differences emerged (Figure 8B). ER-Golgi and TGN-PM profiles were similar to the reference clusters, whereas intra-Golgi transport was the only pathway to exhibit an up-regulation of more genes (∼17%) and down-regulation of few (∼2%). Clustering can be further restricted to core components and proteins with well-established roles in each stage of trafficking (Figure 9A). Within the ER-Golgi (COPII) pathway, the regulation was subtle and unexpected. Vesicle exit from the ER requires recruitment of the Sar1 GTPase, cargo selection by the Sec23/24 complex, and finally Sec13/31-mediated budding (Barlowe, 2002). With the exception of Sec23A, components of the Sec23/24 and Sec13/31 complexes showed inverse expression patterns. Additionally, although mRNA of the homolog of the Sar1 GEF Sec12 (PREB) was moderately down-regulated that of the represented Sar1 homolog, Sara2 increased as the cells polarized.
Figure 9.
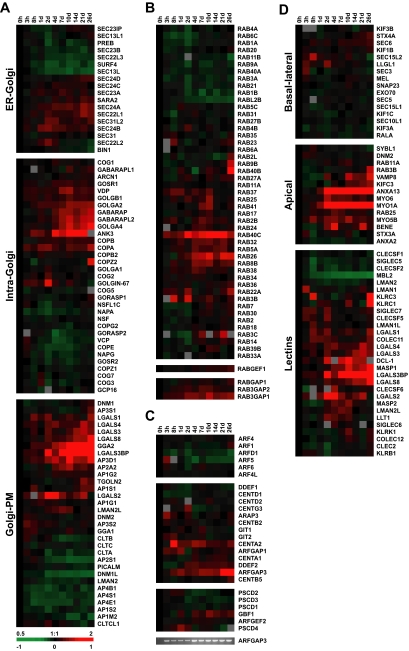
Expression profiles of individual trafficking components. (A) Hierarchical gene expression clusters of the ER-Golgi, intra-Golgi, and Golgi-PM steps of protein trafficking. (B) Rab, RabGEF, and RabGAP gene expression clusters. (C) Arf and ArfGEF and GAP mRNA profiles during polarization. DNA gel shows verification of ARFGAP3 profile by RT-PCR. (D) Clustering of transcripts encoding trafficking pathway components based on basal-lateral or apical (lectins are separated out from the other apical targeting factors for clarity) targeting functions. Transcript levels determined by microarray analysis are shown relative to a reference pool of human mRNAs.
Examination of the intra-Golgi transport cluster revealed that many integral components were transcriptionally up-regulated as cell polarization proceeded (Figure 9A). Among these were several golgins, including GolgA2 (GM-130), GolgA4 (p230), and GolgB1 (Giantin); GolgA2 activates the ste-20 kinases Ysk1 and Mst4 (Preisinger et al., 2004); GolgA4 localizes to non-clathrin–coated vesicles budding from the TGN, interacts with the microtubule and actin cross-linker Macf1, and has been implicated in the transport of GPI-anchored proteins (Kakinuma et al., 2004); and GolgB1 is a docking protein that interacts directly with VDP (p115), a Golgi tether (Barr and Short, 2003), whose expression also increases as Caco-2 cells polarize, as does that of the Golgi ankyrin (ANK3). The GRASP family (GORASP1 or GRASP65 and GORASP2 or GRASP55; Seemann et al., 2000), however, were down-regulated during polarization and the two Golgi SNAPs, GOSR1 (Gos-28) and GOSR2 (Membrin), had opposite expression profiles, with GOSR1 increasing and GOSR2 decreasing during cell polarization.
The expression program of the TGN-PM pathway was dominated by increased expression of genes encoding lectins (Figure 9A). These proteins may function in apical trafficking by binding and clustering glycosylated substrates (Gut et al., 1998; Hauri et al., 2000). Galectin-3 and -4 (LGALS3, 4) have been implicated in apical transport (Delacour et al., 2005, 2006). However, transcripts encoding the lectin VIP-36 (LMAN2), which also has a putative role in apical trafficking (Hara-Kuge et al., 2002), decreased over the time course. A related gene, LMAN2L, had increased levels of transcripts as the apical membrane domain was established during cell polarization. Clathrin has also been implicated in protein sorting and trafficking in polarized epithelial cells (Folsch, 2005), but both clathrin light chains (CLTA and CLTB) and heavy chain (CLTC) were down-regulated during Caco-2 cell polarization.
Isolation of specific protein families involved in regulating protein-trafficking pathways (Chen and Scheller, 2001; Zerial and McBride, 2001) gave more variable results, as expected for a smaller sample size with less statistical power (Figure 8B). Rabs and syntaxins comprised approximately equal percentages of genes up- and down-regulated (∼9% and ∼3%, respectively), whereas SNAP and VAMP clusters had more genes that increased over time than decreased. When Rab GTPases were examined in detail both clusters of mRNAs up- and down-regulated during polarization were observed (Figure 9B; functions summarized in Supplementary Table S4). As in the case of the Rho family, several of the transcriptionally modulated genes (Rab2B, 31, 37, 40A, 40C, and 41) were among the less well studied. Of the relatively few Rab GEFs and GAPs represented, all had increased transcripts as the cells became polarized (Figure 9B). Arf GTPases (D'Souza-Schorey and Chavrier, 2006) all showed moderate decreases or no change in expression as polarization proceeded (Figure 9C), but transcripts of several Arf-GEFs and Arf-GAPs were also up-regulated over the time course. ARFGAP3, implicated in COPI cargo loading and vesicle formation (Lee et al., 2005), increased in expression during polarization (Figure 9C).
Finally, genes were clustered based on their roles in either apical or basal-lateral trafficking. The results from this analysis were dramatic. Expression of genes with roles in basal-lateral trafficking was virtually unchanged throughout the time course, whereas the expression of apical targeting genes was strongly up-regulated as cells polarized (Figure 9D). This suggests that the basal-lateral trafficking pathway may be used by nonpolarized cells for constitutive secretion, whereas the ability to fully sort and retain apically localized proteins is acquired only after the initiation of polarization.
DISCUSSION
The development of epithelial cell polarity requires the acquisition and assembly of functional characteristics and structural features of fully differentiated epithelia. Early studies of polarization focused on posttranslational pathways involving protein trafficking and cytoskeleton rearrangement in the development of structurally and functionally distinct apical and basal-lateral plasma membrane domains (Le Bivic et al., 1990a; Matter et al., 1990a) and on the role of extracellular cues (cell–cell and cell-matrix adhesion) in initiating and orienting cellular reorganization (Yeaman et al., 1999b) in epithelial cell lines. However the transcriptional regulation of these processes has not been studied at the genomic level. We used microarray analysis to examine the transcriptional changes that accompany polarization in Caco-2 cells and relate them to key steps in the development of cell polarity. Our results have uncovered a complex pattern of regulation of genes that correlates with the formation of structural and functional characteristics of polarized epithelial cells. Genes involved in regulating functional differentiation were up-regulated at 4 d, as those necessary for proliferation were down-regulated (Sääf et al., 2007). This time point in vitro may therefore correspond to the in vivo transition from mitotic crypt cells to postmitotic, differentiating enterocytes in the villus.
Cell Adhesion Complexes
Development of a continuous monolayer of cells requires cell–cell adhesion, and we found that there was differential regulation of transcripts encoding components of several cell adhesion complexes. For example, expression levels of nectins (adherens junction), which function early in cell contact formation (Irie et al., 2004), were high before the formation of cell–cell adhesion, whereas expression levels of E-cadherin were constant. Interestingly, several genes encoding components of desmosomes (desmosomal cadherins and desmoplakin and plakophilin-4), the adhesion complex that allows epithelial sheets to withstand shear stress (Getsios et al., 2004), and tight junctions (claudins1, -4, and -15), which prevent mixing of membrane domains and regulate paracellular permeability (Van Itallie and Anderson, 2006), were induced during differentiation. Another junctional complex, the gap junction, showed a switch in expression patterns from α subunit transcripts (Cx43 and Cx45) in proliferating cells to β subunits (Cx26 and Cx32) in polarized cells. These distinct patterns of gene expression within each functional multiprotein complex (tight junction, desmosome, gap junction) suggests that there are important temporal controls for patterns of component gene expression that accompany induction of the assembly of each functional group during polarization of Caco-2 cells. Again, this analysis provides important new data in an experimentally tractable system and the impetus to study coregulation of these cohorts of genes.
Genes encoding ECM components, integrin receptors and downstream signaling proteins were also regulated during Caco-2 cell polarization. In general, the patterns of expression reflected the switch from proliferating, motile cells to postmitotic, sedentary cells. The regulation of interactions with ECM may affect large-scale cytoskeletal changes that occur during polarization. In addition, secretion of specific ECM components may allow polarizing Caco-2 cells in culture to mimic the changing ECM the enterocyte normally encounters during its migration up the crypt-villus axis and in certain cases such as hensin directly impact cell polarization.
Cytoskeleton
Cell–cell contact leads to restructuring of the cytoskeleton during the transition from migratory, single cells to a stable, nonmigratory epithelium. This change is reflected in the temporal regulation of the actin cytoskeleton and factors that modify its stability and structure. Transcripts encoding β-actin and proteins that affect polymerization and turnover of the actin network decreased as cells become postmitotic and sedentary. The observed down-regulation of the Arp2/3 complex, profilin, fascin and vinculin, and up-regulation of α-actinin4 may be related to decreased lamellipodia activity as cells form stable contacts and become nonmotile (Ehrlich et al., 2002). For example, fascin is up-regulated in colorectal cancer, and its expression correlates with increased cell motility and invasiveness (Kureishy et al., 2002), whereas expression of the tumor suppressor α-actinin4 correlates with decreased cell motility (Nikolopoulos et al., 2000).
Although expression of several genes that affect overall actin dynamics decreased, actin is required to form the apical brush border, a specialized structure of enterocytes. We found that myosin 1A and 7B transcripts accumulated as the brush border assembled; myosin 1A links actin bundles to the plasma membrane along the shaft of microvilli, whereas myosin 7B accumulates at the tips (Cheney and Mooseker, 1992; Chen et al., 2001). Induction of myosin 1A and 7B expression coincided with up-regulation of other components of the brush border, including brush border enzymes. MYLK, whose transcript increased during polarization, is known to be highly expressed on the intestinal villus end where it has been shown to be required for rapid changes in the paracellular permeability of tight junctions through actomyosin ring contraction in response to increased extracellular sodium and glucose (Clayburgh et al., 2004).
The shift from proliferating to postmitotic cells as Caco-2 cells polarized is also reflected in the down-regulation of genes encoding microtubule components (TUBG1, TUBE1) and regulators (EB1, mitotic kinesins, Kif2, and Kif9) involved in cell cycle–dependent centrosome and mitotic functions. In addition, during this time Caco-2 cell height increases from ∼5 to 12–15 μm (Figure 2), which necessitates additional cytoskeletal support for the taller cells, as well as polarized vesicle delivery to selectively expand the apical and lateral membranes. As cells become postmitotic, the length of microtubules increases, concordant with decreased expression of microtubule depolymerizing proteins such as Kif2. Coordinated reorganization of actin and microtubule cytoskeletons during mitosis is also no longer necessary and may be reflected in the down-regulation of Kif9 (Piddini et al., 2001; Homma et al., 2003). The mRNAs encoding most dynein and dynactin subunits decreased as polarization proceeded, perhaps because polarized Caco-2 cells are no longer dividing. However, increased transcripts of a minority of minus-end motor subunits was observed, perhaps related to their roles in organelle positioning or apical exocytosis (Karki and Holzbaur, 1999).
The change in intermediate filament composition during Caco-2 cell polarization may also reflect decreased migratory capacity and the requirement for increased resistance to shear stress through formation of keratin-desmosome interactions in fully polarized cells. Cells expressing vimentin have increased motility relative to those with cytokeratin intermediate filaments alone (Hendrix et al., 1996). Interestingly, we also found that several genes encoding components of desmosomes (desmosomal cadherins and desmoplakin and plakophilin-4), which establish strong epithelial adhesion that resists shear stress, were induced during differentiation (see above). We observed a striking correlation between in vivo expression profiles of keratins during enterocyte differentiation and polarization of Caco-2 cells in culture, which may prove useful in addressing the functional significance and regulation of cytokeratin modulation during enterocyte differentiation.
Protein Trafficking Pathways
There is strong evidence that modifications of constitutive protein-sorting pathways facilitate protein trafficking upon epithelial cell polarization (Kreitzer et al., 2003; Rodriguez-Boulan et al., 2005). By examining protein-trafficking pathways in the context of other metabolic pathways, we were able to establish that significant transcriptional regulation of these pathways occurred during Caco-2 cell polarization. Of the stages of exocytosis examined, the most affected was intra-Golgi transport, which plays a central role in sorting protein cargos to different organelles and plasma membrane domains (Ponnambalam and Baldwin, 2003). Although it is difficult to draw conclusions from the overall expression profile of individual protein families, several proteins implicated in regulated exocytosis (Rab27a and Rab37) and vesicle trafficking (GolgA2, GolgA4, Vamp4, and Kifc3) were up-regulated during Caco-2 polarization, suggesting that they may play a special role in the establishment of polarized membrane domains.
Genes encoding many core functions in intra-Golgi transport were strongly up-regulated during polarization, suggesting that key changes in trafficking necessary for polarized vesicle delivery may occur at this level. In addition, examination of the TGN-PM stage of exocytosis revealed a coordinate up-regulation of lectins implicated in cargo specification in the apical pathway, whereas clathrins, as post-Golgi vesicle coat proteins, were down-regulated. Components of the apical trafficking machinery, but not the basal-lateral trafficking machinery, are up-regulated during polarization. This is consistent with the idea that the basal-lateral sorting machinery is used in nonpolarized cells as the de facto pathway for exocytosis (Yoshimori et al., 1996). Our results suggest that the apical transport machinery is refined by transcriptional regulation during cell polarization and is superimposed on the pre-existing basal-lateral trafficking machinery.
The absence of widespread changes in expression of regulated trafficking components such as the exocyst (Sec 6/8 complex), SNARE proteins, Rabs, and vesicle coat and adapter proteins underscores the idea that these pathways are largely intact in nonpolarized cells before cell–cell adhesion and development of polarity. These pathways may be reoriented around new signals from extracellular cues as cells form cell–cell contacts and alter the organization of the ECM, and a few select changes in gene expression that are used to establish the high degree of selectivity and retention observed in a polarized cell monolayer. These changes are most apparent in the up-regulation of intra-Golgi transport molecules and components of the apical trafficking pathway. This study provides a starting point for investigating how these events are coordinated.
Although the mechanisms underlying these dramatic changes in gene expression profiles are unknown, it is noteworthy that Caco-2 cells have retained an intrinsic ability to develop into a functional epithelium in a process that includes large-scale reprogramming of gene expression in the absence of specific signals from stromal cells or extracellular factors normally present in the intestine (e.g., Wnts, BMPs; Clevers and Batlle, 2006). The dominant feature of the gene expression program was a transition at ∼4 d after the initiation of cell adhesion. Within the limits of temporal resolution for the time course, this suggests that the genomic differentiation program in these cells is a highly synchronous one. Had sequential events been required, modulation of gene expression would be expected in waves along the time course as distinct complexes were formed in a predetermined order. Thus, the expression programs for a diverse set of cell structures and functions, from multiple cell–cell junction complexes to formation of a brush border to secretion of ECM as well as others, are induced together with exquisite temporal fidelity. Together, our results establish the existence of a complex expression program under fine temporal control required for polarization of intestinal epithelial cells in vitro, which has striking similarities to patterns of gene expression in enterocytes in vivo and which is independent of extracellular signals present in the intestinal niche. The ability of Caco-2 cells to execute this program in culture suggests either that this cell line was originally derived from a tumor consisting of stem cells or proliferating progenitors in the intestinal crypt with an inherent ability to differentiate into functional enterocytes or that when the cells were isolated from an adenocarcinoma along the villus, they retained an imprinted “memory” of their differentiated state that could be reactivated by cell–cell contact formation. Although both possibilities are intriguing, further studies will be required to distinguish between them.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Brown and Nelson laboratories, Robert Tibshirani, and Ronald S. Rock for helpful discussions. We also thank members of the Stanford Functional Genomics Facility (SFGF) and Stanford Microarray Database (SMD) for their advice and Nafisa Ghori for her help with electron microscopy. We gratefully acknowledge financial assistance from the Swedish Research Council, “Vetenskapsrådet” (A.S). This work was supported by National Cancer Institute Grant CA77097 (P.O.B.) and by the Howard Hughes Medical Institute. P.O.B. is an Investigator of the Howard Hughes Medical Institute. Work from the Nelson laboratory is supported by the National Institutes of Health Grant GM35527 and a HHMI predoctoral Fellowship (J.M.H).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-04-0308) on August 15, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Aijaz S., Balda M. S., Matter K. Tight junctions: molecular architecture and function. Int. Rev. Cytol. 2006;248:261–298. doi: 10.1016/S0074-7696(06)48005-0. [DOI] [PubMed] [Google Scholar]
- Aspenstrom P. The verprolin family of proteins: regulators of cell morphogenesis and endocytosis. FEBS Lett. 2005;579:5253–5259. doi: 10.1016/j.febslet.2005.08.053. [DOI] [PubMed] [Google Scholar]
- Athman R., Louvard D., Robine S. The epithelial cell cytoskeleton and intracellular trafficking. III. How is villin involved in the actin cytoskeleton dynamics in intestinal cells? Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G496–G502. doi: 10.1152/ajpgi.00207.2002. [DOI] [PubMed] [Google Scholar]
- Bacallao R., Antony C., Dotti C., Karsenti E., Stelzer E. H., Simons K. The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium. J. Cell Biol. 1989;109:2817–2832. doi: 10.1083/jcb.109.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda M. S., Garrett M. D., Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J. Cell Biol. 2003;160:423–432. doi: 10.1083/jcb.200210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C. COPII-dependent transport from the endoplasmic reticulum. Curr. Opin. Cell Biol. 2002;14:417–422. doi: 10.1016/s0955-0674(02)00348-4. [DOI] [PubMed] [Google Scholar]
- Barr F. A., Short B. Golgins in the structure and dynamics of the Golgi apparatus. Curr. Opin. Cell Biol. 2003;15:405–413. doi: 10.1016/s0955-0674(03)00054-1. [DOI] [PubMed] [Google Scholar]
- Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- Bilder D., Schober M., Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat. Cell Biol. 2003;5:53–58. doi: 10.1038/ncb897. [DOI] [PubMed] [Google Scholar]
- Brady-Kalnay S. M., Tonks N. K. Protein tyrosine phosphatases as adhesion receptors. Curr. Opin. Cell Biol. 1995;7:650–657. doi: 10.1016/0955-0674(95)80106-5. [DOI] [PubMed] [Google Scholar]
- Bre M. H., Kreis T. E., Karsenti E. Control of microtubule nucleation and stability in Madin-Darby canine kidney cells: the occurrence of noncentrosomal, stable detyrosinated microtubules. J. Cell Biol. 1987;105:1283–1296. doi: 10.1083/jcb.105.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski J. C., McGraw T. E., Gruber D., Nguyen H. L., Sheetz M. P. Overexpression of MAP4 inhibits organelle motility and trafficking in vivo. J. Cell Sci. 1997;110(Pt 24):3055–3064. doi: 10.1242/jcs.110.24.3055. [DOI] [PubMed] [Google Scholar]
- Busson-Mabillot S., Chambaut-Guerin A. M., Ovtracht L., Muller P., Rossignol B. Microtubules and protein secretion in rat lacrimal glands: localization of short-term effects of colchicine on the secretory process. J. Cell Biol. 1982;95:105–117. doi: 10.1083/jcb.95.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calnek D., Quaroni A. Differential localization by in situ hybridization of distinct keratin mRNA species during intestinal epithelial cell development and differentiation. Differentiation. 1993;53:95–104. doi: 10.1111/j.1432-0436.1993.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Nystrom L. E., Sundkvist I., Markey F., Lindberg U. Actin polymerizability is influenced by profilin, a low molecular weight protein in non-muscle cells. J. Mol. Biol. 1977;115:465–483. doi: 10.1016/0022-2836(77)90166-8. [DOI] [PubMed] [Google Scholar]
- Chantret I., Barbat A., Dussaulx E., Brattain M. G., Zweibaum A. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res. 1988;48:1936–1942. [PubMed] [Google Scholar]
- Chen Y. A., Scheller R. H. SNARE-mediated membrane fusion. Nat. Rev. Mol. Cell Biol. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- Chen Z. Y., Hasson T., Zhang D. S., Schwender B. J., Derfler B. H., Mooseker M. S., Corey D. P. Myosin-VIIb, a novel unconventional myosin, is a constituent of microvilli in transporting epithelia. Genomics. 2001;72:285–296. doi: 10.1006/geno.2000.6456. [DOI] [PubMed] [Google Scholar]
- Cheney R. E., Mooseker M. S. Unconventional myosins. Curr. Opin. Cell Biol. 1992;4:27–35. doi: 10.1016/0955-0674(92)90055-h. [DOI] [PubMed] [Google Scholar]
- Clatworthy J. P., Subramanian V. Stem cells and the regulation of proliferation, differentiation and patterning in the intestinal epithelium: emerging insights from gene expression patterns, transgenic and gene ablation studies. Mech. Dev. 2001;101:3–9. doi: 10.1016/s0925-4773(00)00557-8. [DOI] [PubMed] [Google Scholar]
- Clayburgh D. R., Rosen S., Witkowski E. D., Wang F., Blair S., Dudek S., Garcia J. G., Alverdy J. C., Turner J. R. A differentiation-dependent splice variant of myosin light chain kinase, MLCK1, regulates epithelial tight junction permeability. J. Biol. Chem. 2004;279:55506–55513. doi: 10.1074/jbc.M408822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Batlle E. EphB/EphrinB receptors and Wnt signaling in colorectal cancer. Cancer Res. 2006;66:2–5. doi: 10.1158/0008-5472.CAN-05-3849. [DOI] [PubMed] [Google Scholar]
- Colegio O. R., Van Itallie C., Rahner C., Anderson J. M. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am. J. Physiol. Cell Physiol. 2003;284:C1346–C1354. doi: 10.1152/ajpcell.00547.2002. [DOI] [PubMed] [Google Scholar]
- Croce A., Cassata G., Disanza A., Gagliani M. C., Tacchetti C., Malabarba M. G., Carlier M. F., Scita G., Baumeister R., Di Fiore P. P. A novel actin barbed-end-capping activity in EPS-8 regulates apical morphogenesis in intestinal cells of Caenorhabditis elegans. Nat. Cell Biol. 2004;6:1173–1179. doi: 10.1038/ncb1198. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- Dang X., Doble B. W., Kardami E. The carboxy-tail of connexin-43 localizes to the nucleus and inhibits cell growth. Mol. Cell Biochem. 2003;242:35–38. [PubMed] [Google Scholar]
- De Arcangelis A., Neuville P., Boukamel R., Lefebvre O., Kedinger M., Simon-Assmann P. Inhibition of laminin alpha 1-chain expression leads to alteration of basement membrane assembly and cell differentiation. J. Cell Biol. 1996;133:417–430. doi: 10.1083/jcb.133.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacour D., Cramm-Behrens C. I., Drobecq H., Le Bivic A., Naim H. Y., Jacob R. Requirement for galectin-3 in apical protein sorting. Curr. Biol. 2006;16:408–414. doi: 10.1016/j.cub.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Delacour D., et al. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J. Cell Biol. 2005;169:491–501. doi: 10.1083/jcb.200407073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMali K. A., Wennerberg K., Burridge K. Integrin signaling to the actin cytoskeleton. Curr. Opin. Cell Biol. 2003;15:572–582. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- Disanza A., Carlier M. F., Stradal T. E., Didry D., Frittoli E., Confalonieri S., Croce A., Wehland J., Di Fiore P. P., Scita G. Eps8 controls actin-based motility by capping the barbed ends of actin filaments. Nat. Cell Biol. 2004;6:1180–1188. doi: 10.1038/ncb1199. [DOI] [PubMed] [Google Scholar]
- Ehrlich J. S., Hansen M. D., Nelson W. J. Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev. Cell. 2002;3:259–270. doi: 10.1016/s1534-5807(02)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. Cell polarity: Par6, aPKC and cytoskeletal crosstalk. Curr. Opin. Cell Biol. 2003;15:67–72. doi: 10.1016/s0955-0674(02)00005-4. [DOI] [PubMed] [Google Scholar]
- Evans W. H., De Vuyst E., Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem. J. 2006;397:1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakih M. G., Padmanabhan A. CEA monitoring in colorectal cancer. What you should know. Oncology (Williston Park) 2006;20:579–587. discussion 588, 594, 596 passim. [PubMed] [Google Scholar]
- Folsch H. The building blocks for basolateral vesicles in polarized epithelial cells. Trends Cell Biol. 2005;15:222–228. doi: 10.1016/j.tcb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc. Natl. Acad. Sci. USA. 1978;75:5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Braga V. Epithelial cell shape and Rho small GTPases. Novartis Found Symp. 2005;269:144–155. discussion 155–148, 223–230. [PubMed] [Google Scholar]
- Furuse M., Furuse K., Sasaki H., Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J. Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod D. R., Merritt A. J., Nie Z. Desmosomal cadherins. Curr. Opin. Cell Biol. 2002;14:537–545. doi: 10.1016/s0955-0674(02)00366-6. [DOI] [PubMed] [Google Scholar]
- Gates J., Peifer M. Can 1000 reviews be wrong? Actin, alpha-Catenin, and adherens junctions. Cell. 2005;123:769–772. doi: 10.1016/j.cell.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Getsios S., Huen A. C., Green K. J. Working out the strength and flexibility of desmosomes. Nat. Rev. Mol. Cell Biol. 2004;5:271–281. doi: 10.1038/nrm1356. [DOI] [PubMed] [Google Scholar]
- Gilbert T., Le Bivic A., Quaroni A., Rodriguez-Boulan E. Microtubular organization and its involvement in the biogenetic pathways of plasma membrane proteins in Caco-2 intestinal epithelial cells. J. Cell Biol. 1991;113:275–288. doi: 10.1083/jcb.113.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasset E., Bernabeu J., Pinto M. Epithelial properties of human colonic carcinoma cell line Caco-2: effect of secretagogues. Am. J. Physiol. 1985;248:C410–C418. doi: 10.1152/ajpcell.1985.248.5.C410. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Gut A., Kappeler F., Hyka N., Balda M. S., Hauri H. P., Matter K. Carbohydrate-mediated Golgi to cell surface transport and apical targeting of membrane proteins. EMBO J. 1998;17:1919–1929. doi: 10.1093/emboj/17.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B. S., Labouesse M. Tissue integrity: hemidesmosomes and resistance to stress. Curr. Biol. 2001;11:R858–R861. doi: 10.1016/s0960-9822(01)00516-4. [DOI] [PubMed] [Google Scholar]
- Hara-Kuge S., Ohkura T., Ideo H., Shimada O., Atsumi S., Yamashita K. Involvement of VIP36 in intracellular transport and secretion of glycoproteins in polarized Madin-Darby canine kidney (MDCK) cells. J. Biol. Chem. 2002;277:16332–16339. doi: 10.1074/jbc.M112188200. [DOI] [PubMed] [Google Scholar]
- Hauri H., Appenzeller C., Kuhn F., Nufer O. Lectins and traffic in the secretory pathway. FEBS Lett. 2000;476:32–37. doi: 10.1016/s0014-5793(00)01665-3. [DOI] [PubMed] [Google Scholar]
- Hendrix M. J., Seftor E. A., Chu Y. W., Trevor K. T., Seftor R. E. Role of intermediate filaments in migration, invasion and metastasis. Cancer Metastasis Rev. 1996;15:507–525. doi: 10.1007/BF00054016. [DOI] [PubMed] [Google Scholar]
- Henrique D., Schweisguth F. Cell polarity: the ups and downs of the Par6/aPKC complex. Curr. Opin. Genet. Dev. 2003;13:341–350. doi: 10.1016/s0959-437x(03)00077-7. [DOI] [PubMed] [Google Scholar]
- Homma N., Takei Y., Tanaka Y., Nakata T., Terada S., Kikkawa M., Noda Y., Hirokawa N. Kinesin superfamily protein 2A (KIF2A) functions in suppression of collateral branch extension. Cell. 2003;114:229–239. doi: 10.1016/s0092-8674(03)00522-1. [DOI] [PubMed] [Google Scholar]
- Husoy T., Knutsen H. K., Cruciani V., Olstorn H. B., Mikalsen S. O., Loberg E. M., Alexander J. Connexin43 is overexpressed in Apc(Min/+)-mice adenomas and colocalises with COX-2 in myofibroblasts. Int. J. Cancer. 2005;116:351–358. doi: 10.1002/ijc.21025. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Iacobuzio-Donahue C. A., Argani P., Hempen P. M., Jones J., Kern S. E. The desmoplastic response to infiltrating breast carcinoma: gene expression at the site of primary invasion and implications for comparisons between tumor types. Cancer Res. 2002;62:5351–5357. [PubMed] [Google Scholar]
- Irie K., Shimizu K., Sakisaka T., Ikeda W., Takai Y. Roles and modes of action of nectins in cell-cell adhesion. Semin. Cell Dev. Biol. 2004;15:643–656. doi: 10.1016/j.semcdb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Jaffe A. B., Hall A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jaffer Z. M., Chernoff J. p21-activated kinases: three more join the Pak. Int. J. Biochem. Cell Biol. 2002;34:713–717. doi: 10.1016/s1357-2725(01)00158-3. [DOI] [PubMed] [Google Scholar]
- Jou T. S., Schneeberger E. E., Nelson W. J. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J. Cell Biol. 1998;142:101–115. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma T., Ichikawa H., Tsukada Y., Nakamura T., Toh B. H. Interaction between p230 and MACF1 is associated with transport of a glycosyl phosphatidyl inositol-anchored protein from the Golgi to the cell periphery. Exp. Cell Res. 2004;298:388–398. doi: 10.1016/j.yexcr.2004.04.047. [DOI] [PubMed] [Google Scholar]
- Karki S., Holzbaur E. L. Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr. Opin. Cell Biol. 1999;11:45–53. doi: 10.1016/s0955-0674(99)80006-4. [DOI] [PubMed] [Google Scholar]
- Kobielak A., Fuchs E. Alpha-catenin: at the junction of intercellular adhesion and actin dynamics. Nat. Rev. Mol. Cell Biol. 2004;5:614–625. doi: 10.1038/nrm1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer G., Schmoranzer J., Low S. H., Li X., Gan Y., Weimbs T., Simon S. M., Rodriguez-Boulan E. Three-dimensional analysis of post-Golgi carrier exocytosis in epithelial cells. Nat. Cell Biol. 2003;5:126–136. doi: 10.1038/ncb917. [DOI] [PubMed] [Google Scholar]
- Kureishy N., Sapountzi V., Prag S., Anilkumar N., Adams J. C. Fascins, and their roles in cell structure and function. Bioessays. 2002;24:350–361. doi: 10.1002/bies.10070. [DOI] [PubMed] [Google Scholar]
- Laukoetter M. G., Bruewer M., Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr. Opin. Gastroenterol. 2006;22:85–89. doi: 10.1097/01.mog.0000203864.48255.4f. [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Quaroni A., Nichols B., Rodriguez-Boulan E. Biogenetic pathways of plasma membrane proteins in Caco-2, a human intestinal epithelial cell line. J. Cell Biol. 1990a;111:1351–1361. doi: 10.1083/jcb.111.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A., Real F. X., Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc. Natl. Acad. Sci. USA. 1989;86:9313–9317. doi: 10.1073/pnas.86.23.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A., Sambuy Y., Mostov K., Rodriguez-Boulan E. Vectorial targeting of an endogenous apical membrane sialoglycoprotein and uvomorulin in MDCK cells. J. Cell Biol. 1990b;110:1533–1539. doi: 10.1083/jcb.110.5.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Yang J. S., Hong W., Premont R. T., Hsu V. W. ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation. J. Cell Biol. 2005;168:281–290. doi: 10.1083/jcb.200404008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivo I., Tani T., Laitinen L., Bruns R., Kivilaakso E., Lehto V. P., Burgeson R. E., Virtanen I. Anchoring complex components laminin-5 and type VII collagen in intestine: association with migrating and differentiating enterocytes. J. Histochem. Cytochem. 1996;44:1267–1277. doi: 10.1177/44.11.8918902. [DOI] [PubMed] [Google Scholar]
- Luca M., Hunt B., Bucana C. D., Johnson J. P., Fidler I. J., Bar-Eli M. Direct correlation between MUC18 expression and metastatic potential of human melanoma cells. Melanoma Res. 1993;3:35–41. doi: 10.1097/00008390-199304000-00006. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F., Gassama A., Datta A., Yu W., Rescher U., Gerke V., Mostov K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K., Brauchbar M., Bucher K., Hauri H. P. Sorting of endogenous plasma membrane proteins occurs from two sites in cultured human intestinal epithelial cells (Caco-2) Cell. 1990a;60:429–437. doi: 10.1016/0092-8674(90)90594-5. [DOI] [PubMed] [Google Scholar]
- Matter K., Bucher K., Hauri H. P. Microtubule perturbation retards both the direct and the indirect apical pathway but does not affect sorting of plasma membrane proteins in intestinal epithelial cells (Caco-2) EMBO J. 1990b;9:3163–3170. doi: 10.1002/j.1460-2075.1990.tb07514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K., Stieger B., Klumperman J., Ginsel L., Hauri H. P. Endocytosis, recycling, and lysosomal delivery of brush border hydrolases in cultured human intestinal epithelial cells (Caco-2) J. Biol. Chem. 1990c;265:3503–3512. [PubMed] [Google Scholar]
- Mollenhauer J., Wiemann S., Scheurlen W., Korn B., Hayashi Y., Wilgenbus K. K., von Deimling A., Poustka A. DMBT1, a new member of the SRCR superfamily, on chromosome 10q25.3–26.1 is deleted in malignant brain tumours. Nat. Genet. 1997;17:32–39. doi: 10.1038/ng0997-32. [DOI] [PubMed] [Google Scholar]
- Momiyama M., Omori Y., Ishizaki Y., Nishikawa Y., Tokairin T., Ogawa J., Enomoto K. Connexin26-mediated gap junctional communication reverses the malignant phenotype of MCF-7 breast cancer cells. Cancer Sci. 2003;94:501–507. doi: 10.1111/j.1349-7006.2003.tb01473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monlauzeur L., Breuza L., Le Bivic A. Putative O-glycosylation sites and a membrane anchor are necessary for apical delivery of the human neurotrophin receptor in Caco-2 cells. J. Biol. Chem. 1998;273:30263–30270. doi: 10.1074/jbc.273.46.30263. [DOI] [PubMed] [Google Scholar]
- Mooseker M. S. Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu. Rev. Cell Biol. 1985;1:209–241. doi: 10.1146/annurev.cb.01.110185.001233. [DOI] [PubMed] [Google Scholar]
- Mori M., Shiraishi T., Tanaka S., Yamagata M., Mafune K., Tanaka Y., Ueo H., Barnard G. F., Sugimachi K. Lack of DMBT1 expression in oesophageal, gastric and colon cancers. Br. J. Cancer. 1999;79:211–213. doi: 10.1038/sj.bjc.6690035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. M., Bissell M. J. Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin. Cancer Biol. 2005;15:342–352. doi: 10.1016/j.semcancer.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos S. N., Spengler B. A., Kisselbach K., Evans A. E., Biedler J. L., Ross R. A. The human non-muscle alpha-actinin protein encoded by the ACTN4 gene suppresses tumorigenicity of human neuroblastoma cells. Oncogene. 2000;19:380–386. doi: 10.1038/sj.onc.1203310. [DOI] [PubMed] [Google Scholar]
- Noda Y., Okada Y., Saito N., Setou M., Xu Y., Zhang Z., Hirokawa N. KIFC3, a microtubule minus end-directed motor for the apical transport of annexin XIIIb-associated Triton-insoluble membranes. J. Cell Biol. 2001;155:77–88. doi: 10.1083/jcb.200108042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien L. E., Zegers M. M., Mostov K. E. Opinion: Building epithelial architecture: insights from three-dimensional culture models. Nat. Rev. Mol. Cell Biol. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- Offenhauser N., Borgonovo A., Disanza A., Romano P., Ponzanelli I., Iannolo G., Di Fiore P. P., Scita G. The eps8 family of proteins links growth factor stimulation to actin reorganization generating functional redundancy in the Ras/Rac pathway. Mol. Biol. Cell. 2004;15:91–98. doi: 10.1091/mbc.E03-06-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orian-Rousseau V., Aberdam D., Fontao L., Chevalier L., Meneguzzi G., Kedinger M., Simon-Assmann P. Developmental expression of laminin-5 and HD1 in the intestine: epithelial to mesenchymal shift for the laminin gamma-2 chain subunit deposition. Dev. Dyn. 1996;206:12–23. doi: 10.1002/(SICI)1097-0177(199605)206:1<12::AID-AJA2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Ostman A., Hellberg C., Bohmer F. D. Protein-tyrosine phosphatases and cancer. Nat. Rev. Cancer. 2006;6:307–320. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- Permana S., Hisanaga S., Nagatomo Y., Iida J., Hotani H., Itoh T. J. Truncation of the projection domain of MAP4 (microtubule-associated protein 4) leads to attenuation of microtubule dynamic instability. Cell Struct. Funct. 2005;29:147–157. doi: 10.1247/csf.29.147. [DOI] [PubMed] [Google Scholar]
- Piddini E., Schmid J. A., de Martin R., Dotti C. G. The Ras-like GTPase Gem is involved in cell shape remodelling and interacts with the novel kinesin-like protein KIF9. EMBO J. 2001;20:4076–4087. doi: 10.1093/emboj/20.15.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D., Clevers H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp. Cell Res. 2005;306:357–363. doi: 10.1016/j.yexcr.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Pinto M., Robine-Leon S., Appay M. D., Kedinger M., Triadou N. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2. Biol. Cell. 1983;47:323–330. [Google Scholar]
- Pollard T. D., Borisy G. G. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Ponnambalam S., Baldwin S. A. Constitutive protein secretion from the trans-Golgi network to the plasma membrane. Mol. Membr. Biol. 2003;20:129–139. doi: 10.1080/0968768031000084172. [DOI] [PubMed] [Google Scholar]
- Preisinger C., Short B., De Corte V., Bruyneel E., Haas A., Kopajtich R., Gettemans J., Barr F. A. YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14–3-3zeta. J. Cell Biol. 2004;164:1009–1020. doi: 10.1083/jcb.200310061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Kreitzer G., Musch A. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- Roh M. H., Margolis B. Composition and function of PDZ protein complexes during cell polarization. Am. J. Physiol. Renal. Physiol. 2003;285:F377–F387. doi: 10.1152/ajprenal.00086.2003. [DOI] [PubMed] [Google Scholar]
- Rossman K. L., Der C. J., Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Sääf A. M., Halbleib J. M., Chen X., Yuen S. T., Leung S. Y., Nelson W. J., Brown P. O. Parallels between global transcriptional programs of polarizing Caco-2 intestinal epithelial cells in vitro and gene expression programs in normal and colon cancer. Mol. Biol. Cell. 2007;18:4245–4260. doi: 10.1091/mbc.E07-04-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez J. C., Berthoud V. M., Branes M. C., Martinez A. D., Beyer E. C. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- Seemann J., Jokitalo E., Pypaert M., Warren G. Matrix proteins can generate the higher order architecture of the Golgi apparatus. Nature. 2000;407:1022–1026. doi: 10.1038/35039538. [DOI] [PubMed] [Google Scholar]
- Segretain D., Falk M. M. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim. Biophys. Acta. 2004;1662:3–21. doi: 10.1016/j.bbamem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. D-type cyclins. Trends Biochem. Sci. 1995;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- Simon-Assmann P., Lefebvre O., Bellissent-Waydelich A., Olsen J., Orian-Rousseau V., De Arcangelis A. The laminins: role in intestinal morphogenesis and differentiation. Ann. NY Acad. Sci. 1998;859:46–64. doi: 10.1111/j.1749-6632.1998.tb11110.x. [DOI] [PubMed] [Google Scholar]
- Soole K. L., Jepson M. A., Hazlewood G. P., Gilbert H. J., Hirst B. H. Epithelial sorting of a glycosylphosphatidylinositol-anchored bacterial protein expressed in polarized renal MDCK and intestinal Caco-2 cells. J. Cell Sci. 1995;108(Pt 1):369–377. doi: 10.1242/jcs.108.1.369. [DOI] [PubMed] [Google Scholar]
- Sourisseau T., Georgiadis A., Tsapara A., Ali R. R., Pestell R., Matter K., Balda M. S. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol. Cell. Biol. 2006;26:2387–2398. doi: 10.1128/MCB.26.6.2387-2398.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L. K., Burrell M., Hill D. E., Gyuris J., Brent R., Wiltshire R., Trent J., Vogelstein B., Kinzler K. W. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–2977. [PubMed] [Google Scholar]
- Sundberg U., Beauchemin N., Obrink B. The cytoplasmic domain of CEACAM1-L controls its lateral localization and the organization of desmosomes in polarized epithelial cells. J. Cell Sci. 2004;117:1091–1104. doi: 10.1242/jcs.00944. [DOI] [PubMed] [Google Scholar]
- Takito J., Al-Awqati Q. Conversion of ES cells to columnar epithelia by hensin and to squamous epithelia by laminin. J. Cell Biol. 2004;166:1093–1102. doi: 10.1083/jcb.200405159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takito J., Yan L., Ma J., Hikita C., Vijayakumar S., Warburton D., Al-Awqati Q. Hensin, the polarity reversal protein, is encoded by DMBT1, a gene frequently deleted in malignant gliomas. Am. J. Physiol. 1999;277:F277–F289. doi: 10.1152/ajprenal.1999.277.2.F277. [DOI] [PubMed] [Google Scholar]
- Tanentzapf G., Tepass U. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat. Cell Biol. 2003;5:46–52. doi: 10.1038/ncb896. [DOI] [PubMed] [Google Scholar]
- Tirnauer J. S., Bierer B. E. EB1 proteins regulate microtubule dynamics, cell polarity, and chromosome stability. J. Cell Biol. 2000;149:761–766. doi: 10.1083/jcb.149.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turck N., Gross I., Gendry P., Stutzmann J., Freund J. N., Kedinger M., Simon-Assmann P., Launay J. F. Laminin isoforms: biological roles and effects on the intracellular distribution of nuclear proteins in intestinal epithelial cells. Exp. Cell Res. 2005;303:494–503. doi: 10.1016/j.yexcr.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Vadlamudi R. K., Barnes C. J., Rayala S., Li F., Balasenthil S., Marcus S., Goodson H. V., Sahin A. A., Kumar R. p21-activated kinase 1 regulates microtubule dynamics by phosphorylating tubulin cofactor B. Mol. Cell. Biol. 2005;25:3726–3736. doi: 10.1128/MCB.25.9.3726-3736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Adelsberg J., Edwards J. C., Takito J., Kiss B., al-Awqati Q. An induced extracellular matrix protein reverses the polarity of band 3 in intercalated epithelial cells. Cell. 1994;76:1053–1061. doi: 10.1016/0092-8674(94)90382-4. [DOI] [PubMed] [Google Scholar]
- Van Itallie C. M., Anderson J. M. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- Vidrich A., Buzan J. M., Cohn S. M. Intestinal stem cells and mucosal gut development. Curr. Opin. Gastroenterol. 2003;19:583–590. doi: 10.1097/00001574-200311000-00012. [DOI] [PubMed] [Google Scholar]
- Wei C. J., Xu X., Lo C. W. Connexins and cell signaling in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:811–838. doi: 10.1146/annurev.cellbio.19.111301.144309. [DOI] [PubMed] [Google Scholar]
- Welch M. D. The world according to Arp: regulation of actin nucleation by the Arp2/3 complex. Trends Cell Biol. 1999;9:423–427. doi: 10.1016/s0962-8924(99)01651-7. [DOI] [PubMed] [Google Scholar]
- Wice B. M., Trugnan G., Pinto M., Rousset M., Chevalier G., Dussaulx E., Lacroix B., Zweibaum A. The intracellular accumulation of UDP-N-acetylhexosamines is concomitant with the inability of human colon cancer cells to differentiate. J. Biol. Chem. 1985;260:139–146. [PubMed] [Google Scholar]
- Xia C., Ma W., Stafford L. J., Marcus S., Xiong W. C., Liu M. Regulation of the p21-activated kinase (PAK) by a human Gbeta -like WD-repeat protein, hPIP1. Proc. Natl. Acad. Sci. USA. 2001;98:6174–6179. doi: 10.1073/pnas.101137298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Takeda S., Nakata T., Noda Y., Tanaka Y., Hirokawa N. Role of KIFC3 motor protein in Golgi positioning and integration. J. Cell Biol. 2002;158:293–303. doi: 10.1083/jcb.200202058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C., Grindstaff K. K., Hansen M. D., Nelson W. J. Cell polarity: Versatile scaffolds keep things in place. Curr. Biol. 1999a;9:R515–R517. doi: 10.1016/s0960-9822(99)80324-8. [DOI] [PubMed] [Google Scholar]
- Yeaman C., Grindstaff K. K., Nelson W. J. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol. Rev. 1999b;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- Yeaman C., Grindstaff K. K., Wright J. R., Nelson W. J. Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells. J. Cell Biol. 2001;155:593–604. doi: 10.1083/jcb.200107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori T., Keller P., Roth M. G., Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J. Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir E., Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J. Cell Sci. 2001;114:3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- Zerial M., McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhang J. T., Nicholson B. J. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J. Cell Biol. 1989;109:3391–3401. doi: 10.1083/jcb.109.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.