Abstract
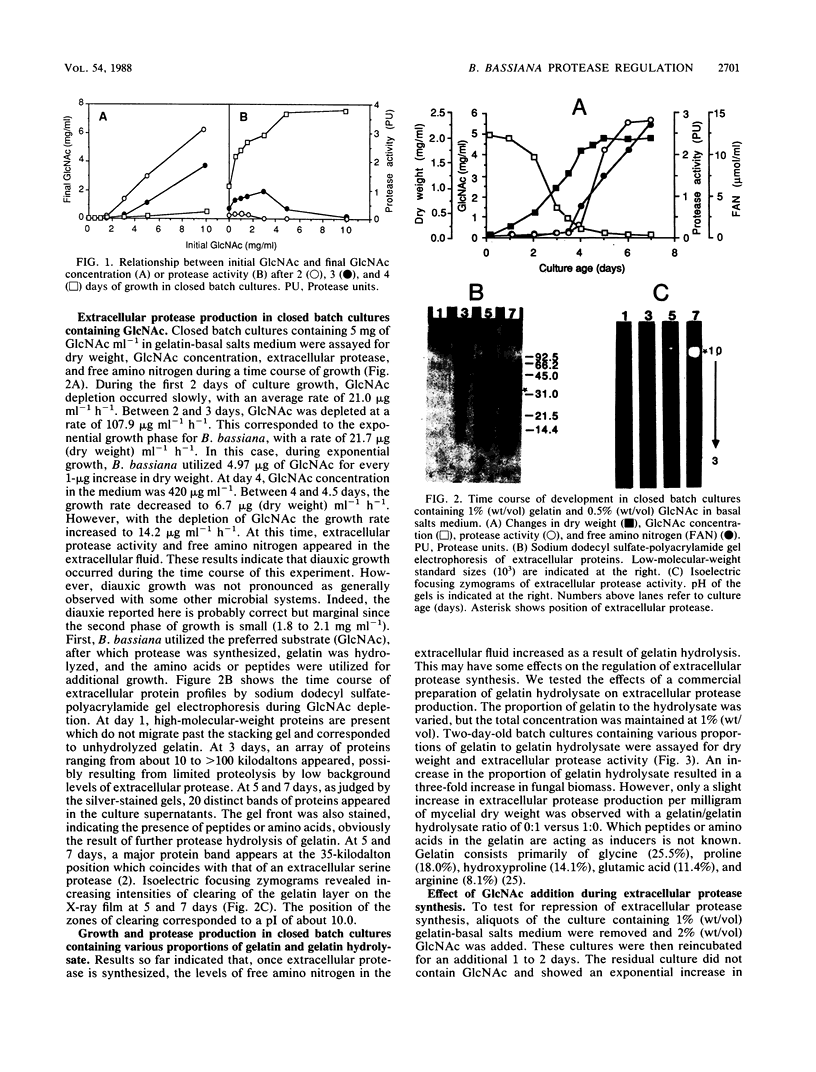
The entomopathogenic fungus Beauveria bassiana GK2016 grown in a liquid medium incorporating gelatin as the sole carbon and nitrogen source produced an extracellular serine protease (molecular weight, 35,000; pI ca. 10). Without gelatin, B. bassiana could utilize N-acetyl-d-glucosamine (GlcNAc; 2-acetamido-2-deoxy-d-glucose) as the sole source of carbon and nitrogen, and GlcNAc availability increased the storage carbohydrate content in mycelia. Synthesis of protease was repressed in gelatin medium containing GlcNAc at levels of >1.07 μmol mg of fungal dry weight−1. At levels below this, protease synthesis was initiated; subsequently, free amino nitrogen appeared in the medium and diauxic growth was observed. Slow feeding with GlcNAc (35.34 μg ml−1 h−1) did not repress protease synthesis nor did GlcNAc accumulate in the medium above 0.5 mg ml−1. Increasing the rate of release of GlcNAc (83.51 μg ml−1 h−1) resulted in the accumulation of GlcNAc in the medium to 2.0 mg ml−1, a 45% increase in growth and a decrease in protease synthesis by about 81%. Free amino acids generated from the hydrolysis of gelatin did not repress protease synthesis. These data are interpreted in terms of known interaction of B. bassiana with insect cuticular components. We suggest that the action of extracellular chitinases synthesized by B. bassiana on insect cuticle, and pursuant release of GlcNAc, may have important consequences on the regulation of other extracellular catabolic enzymes such as the protease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott R. J., Marzluf G. A. Major extracellular protease of Neurospora crassa. J Bacteriol. 1984 Aug;159(2):505–510. doi: 10.1128/jb.159.2.505-510.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidochka M. J., Khachatourians G. G. Purification and Properties of an Extracellular Protease Produced by the Entomopathogenic Fungus Beauveria bassiana. Appl Environ Microbiol. 1987 Jul;53(7):1679–1684. doi: 10.1128/aem.53.7.1679-1684.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Eriksson K. E., Goodell E. W. Pleiotropic mutants of the wood-rotting fungus Polyporus adustus lacking cellulase, mannanase, and xylanase. Can J Microbiol. 1974 Mar;20(3):371–378. doi: 10.1139/m74-057. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Myers M. G., Eberhart B. Regulation of cellulase and cellobiase in Neurospora crassa. Biochem Biophys Res Commun. 1966 Sep 8;24(5):782–785. doi: 10.1016/0006-291x(66)90394-9. [DOI] [PubMed] [Google Scholar]
- Pirt S. J. The diffusion capsule, a novel device for the addition of a solute at a constant rate to a liquid medium. Its application to metabolic regulation. Biochem J. 1971 Jan;121(2):293–297. doi: 10.1042/bj1210293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]