Abstract
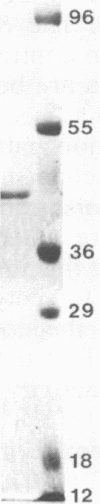
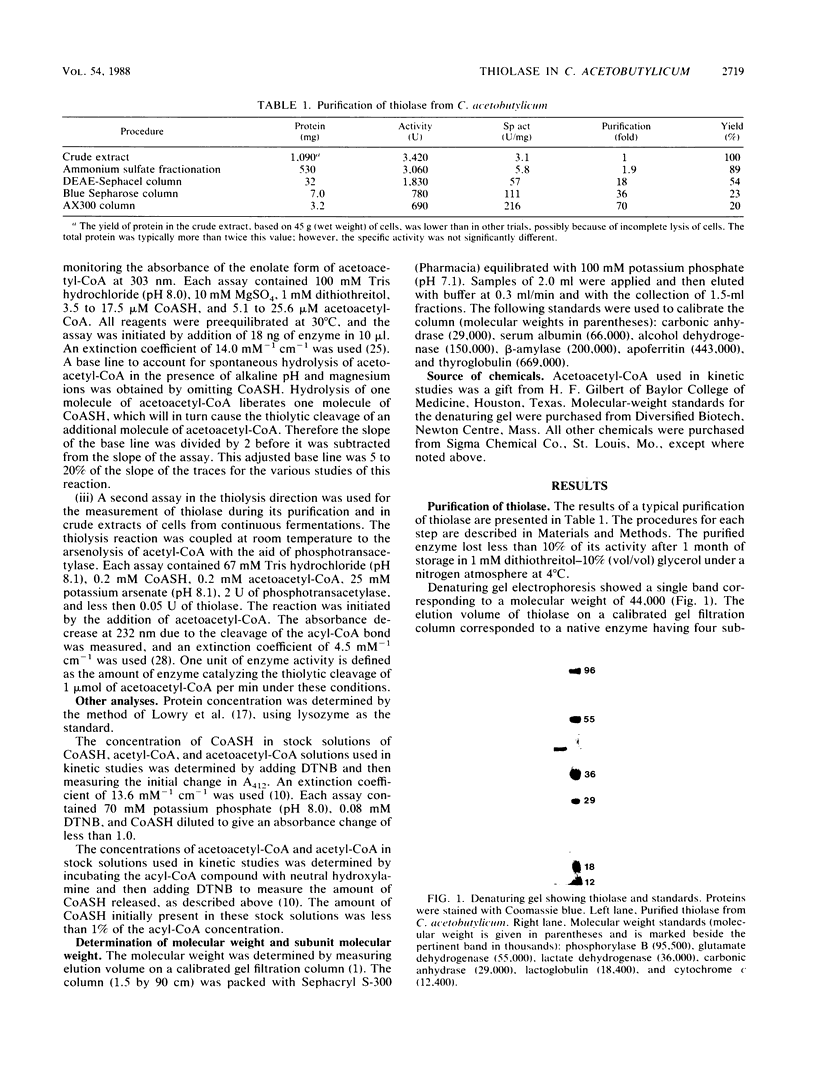
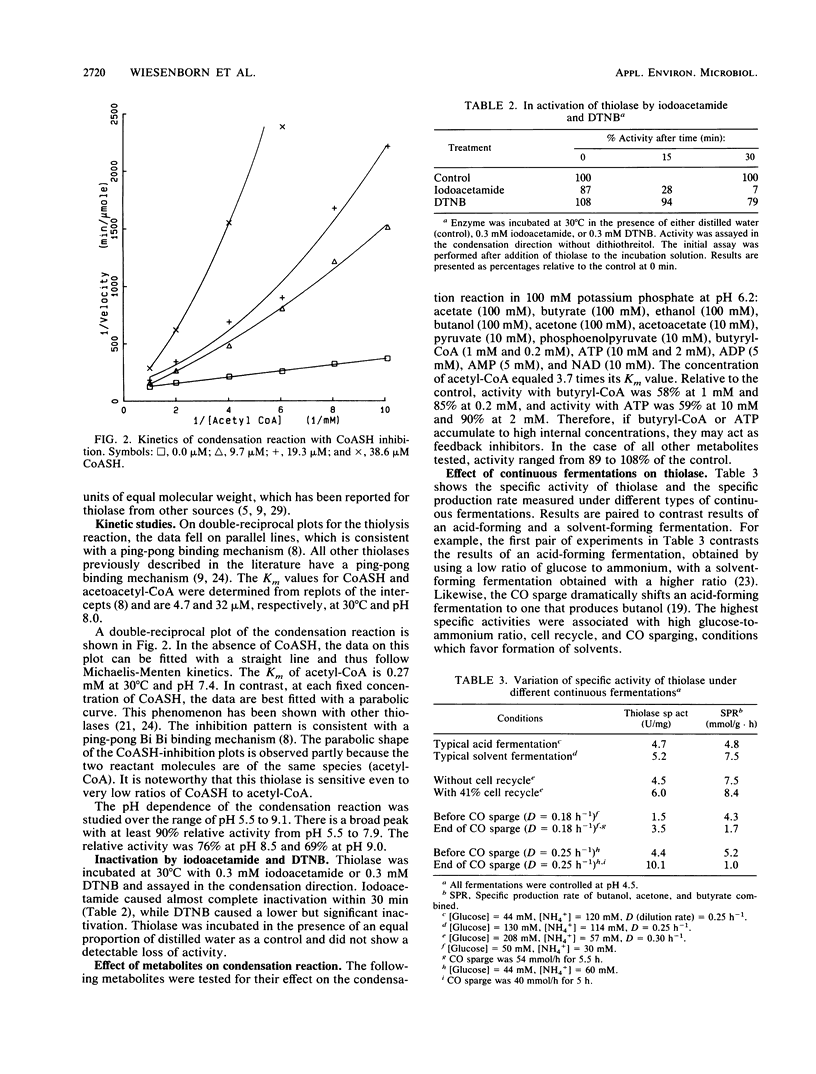
Thiolase (acetyl-coenzyme A [CoA] acetyltransferase, E.C. 2.3.1.19) from Clostridium acetobutylicum ATCC 824 has been purified 70-fold to homogeneity. Unlike the thiolase in Clostridium pasteurianum, this thiolase has high relative activity throughout the physiological range of internal pH of 5.5 to 7.0, indicating that change in internal pH during acid production is not an important factor in the regulation of this thiolase. In the condensation direction, the thiolase is inhibited by micromolar levels of CoA, and this may be an important factor in modulating the net condensation of acetyl-CoA to acetoacetyl-CoA. Other cofactors and metabolites that were tested and shown to be inhibitors are ATP and butyryl-CoA. The native enzyme consists of four 44,000-molecular-weight subunits. The kinetic binding mechanism is ping-pong. The Km value for acetyl-CoA is 0.27 mM at 30°C and pH 7.4. The Km values for sulfhydryl-CoA and acetoacetyl-CoA are, respectively, 0.0048 and 0.032 mM at 30°C and pH 8.0. The active site apparently contains a sulfhydryl group, but unlike other thiolases, this thiolase is relatively stable in the presence of 5,5′-dithiobis(2-nitrobenzoic acid). Studies of thiolase specific activity under various types of continuous fermentations show that regulation of this enzyme at both the genetic and enzyme levels is important.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt H., Schlegel H. G. Kinetics and properties of beta-ketothiolase from Clostridium pasteurianum. Arch Microbiol. 1975 Mar 12;103(1):21–30. doi: 10.1007/BF00436325. [DOI] [PubMed] [Google Scholar]
- Crabbendam P. M., Neijssel O. M., Tempest D. W. Metabolic and energetic aspects of the growth of Clostridium butyricum on glucose in chemostat culture. Arch Microbiol. 1985 Sep;142(4):375–382. doi: 10.1007/BF00491907. [DOI] [PubMed] [Google Scholar]
- Davis J. T., Moore R. N., Imperiali B., Pratt A. J., Kobayashi K., Masamune S., Sinskey A. J., Walsh C. T., Fukui T., Tomita K. Biosynthetic thiolase from zoogloea ramigera. I. Preliminary characterization and analysis of proton transfer reaction. J Biol Chem. 1987 Jan 5;262(1):82–89. [PubMed] [Google Scholar]
- Gilbert H. F., Lennox B. J., Mossman C. D., Carle W. C. The relation of acyl transfer to the overall reaction of thiolase I from porcine heart. J Biol Chem. 1981 Jul 25;256(14):7371–7377. [PubMed] [Google Scholar]
- Hartmanis M. G., Stadtman T. C. Isolation of a selenium-containing thiolase from Clostridium kluyveri: identification of the selenium moiety as selenomethionine. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4912–4916. doi: 10.1073/pnas.79.16.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Gibbins L. N., Forsberg C. W. Transmembrane pH gradient and membrane potential in Clostridium acetobutylicum during growth under acetogenic and solventogenic conditions. Appl Environ Microbiol. 1985 Oct;50(4):1043–1047. doi: 10.1128/aem.50.4.1043-1047.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., Woods D. R. Acetone-butanol fermentation revisited. Microbiol Rev. 1986 Dec;50(4):484–524. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nishimura T., Saito T., Tomita K. Purification and properties of beta-ketothiolase from Zoogloea ramigera. Arch Microbiol. 1978 Jan 23;116(1):21–27. doi: 10.1007/BF00408729. [DOI] [PubMed] [Google Scholar]
- Oeding V., Schlegel H. G. Beta-ketothiolase from Hydrogenomonas eutropha H16 and its significance in the regulation of poly-beta-hydroxybutyrate metabolism. Biochem J. 1973 May;134(1):239–248. doi: 10.1042/bj1340239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior P. J., Dawes E. A. The regulation of poly-beta-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem J. 1973 May;134(1):225–238. doi: 10.1042/bj1340225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwkowski M. X., Hartmanis M. G. Simultaneous single-step purification of thiolase and NADP-dependent 3-hydroxybutyryl-CoA dehydrogenase from Clostridium kluyveri. Anal Biochem. 1984 Sep;141(2):344–347. doi: 10.1016/0003-2697(84)90052-6. [DOI] [PubMed] [Google Scholar]
- Sliwkowski M. X., Stadtman T. C. Incorporation and distribution of selenium into thiolase from Clostridium kluyveri. J Biol Chem. 1985 Mar 10;260(5):3140–3144. [PubMed] [Google Scholar]
- Sramek S. J., Frerman F. E. Purification and properties of Escherichia coli coenzyme A-transferase. Arch Biochem Biophys. 1975 Nov;171(1):14–26. doi: 10.1016/0003-9861(75)90002-8. [DOI] [PubMed] [Google Scholar]
- Suzuki F., Zahler W. L., Emerich D. W. Acetoacetyl-CoA thiolase of Bradyrhizobium japonicum bacteroids: purification and properties. Arch Biochem Biophys. 1987 Apr;254(1):272–281. doi: 10.1016/0003-9861(87)90103-2. [DOI] [PubMed] [Google Scholar]