Abstract
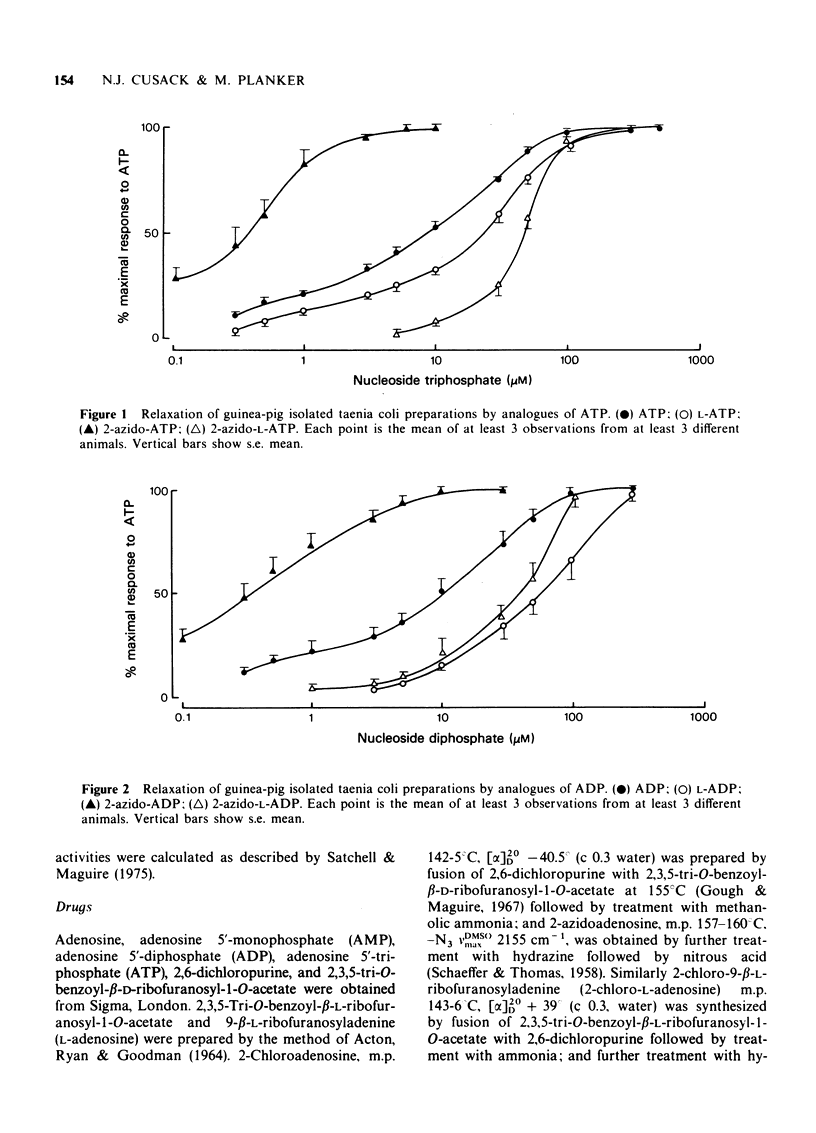
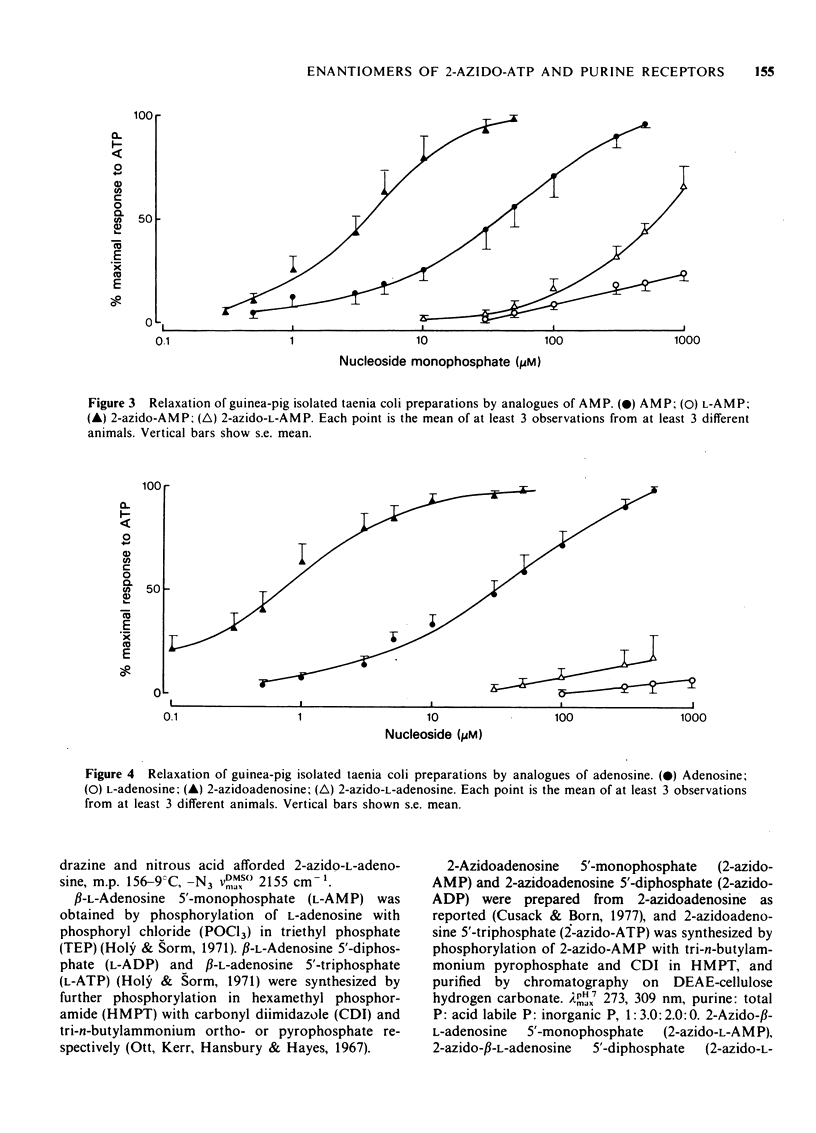
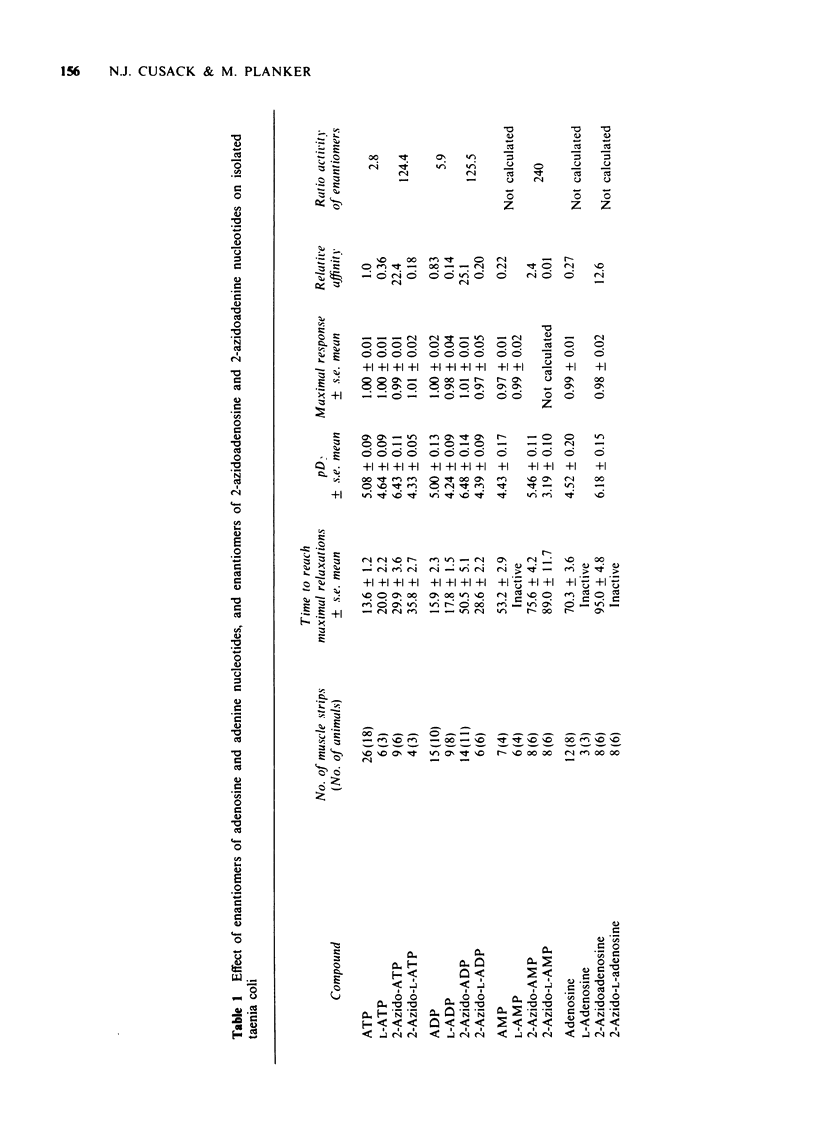
1 2-Azido photoaffinity analogues of adenosine 5'triphosphate (ATP), adenosine 5'-diphosphate (ADP), adenosine 5'-monophosphate (AMP), and adenosine have been synthesized and tested on guinea-pig taenia coli. 2 2-Azido-ATP and 2-azido-ADP were approximately 20 times more potent than ATP as relaxants of taenia coli, and required prolonged washout times before recovery of the muscle. 3 2-Azido-AMP and 2-azidoadenosine were 2 to 12 times more potent than ATP, but took much longer (up to 100 s) to reach maximal relaxation. This behaviour is different from that of AMP and adenosine which were much less potent than ATP. 4 L-Enantiomers of adenosine and adenine nucleotides were also tested. L-ATP and L-ADP were 3 to 6 times less potent than ATP and ADP, and L-AMP and L-adenosine were inactive. 2-Azido-L-ATP and 2-azido-L-ADP were approximately 120 times less potent than 2-Azido-ATP and 6 times less potent than ATP as relaxants of taenia coli. 2-Azido-L-AMP and 2-azidio-L-adenosine were almost inactive. 5 2-Azido derivatives are photolysed by u.v. irradiation to reactive intermediates. 2-Azido-ATP and 2-azidoadenosine might be suitable photoaffinity ligands for labelling putative P2 and P1 purine receptors respectively. 2-Azido-L-ATP and 2-azido-L-adenosine could be useful controls for nonspecific labelling.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULBRING E. Measurements of oxygen consumption in smooth muscle. J Physiol. 1953 Oct;122(1):111–134. doi: 10.1113/jphysiol.1953.sp004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Neural nomenclature. Nature. 1971 Jan 22;229(5282):282–283. doi: 10.1038/229282d0. [DOI] [PubMed] [Google Scholar]
- Cusack N. J., Born G. V. Effects of photolysable 2-azido analogues of adenosine, AMP and ADP on human platelets. Proc R Soc Lond B Biol Sci. 1977 Jul 20;197(1129):515–520. doi: 10.1098/rspb.1977.0084. [DOI] [PubMed] [Google Scholar]
- Cusack N. J., Born G. V. Inhibition of adenosine deaminase and of platelet aggregation by 2-azidoadenosine, a photolysable analogue of adenosine. Proc R Soc Lond B Biol Sci. 1976 May 18;193(1112):307–311. doi: 10.1098/rspb.1976.0048. [DOI] [PubMed] [Google Scholar]
- Ott D. G., Kerr V. N., Hansbury E., Hayes F. N. Chemical synthesis of nucleoside triphosphates. Anal Biochem. 1967 Dec;21(3):469–472. doi: 10.1016/0003-2697(67)90323-5. [DOI] [PubMed] [Google Scholar]
- Satchell D. G., Maguire M. H. Inhibitory effects of adenine nucleotide analogs on the isolated guinea-pig taenia coli. J Pharmacol Exp Ther. 1975 Dec;195(3):540–548. [PubMed] [Google Scholar]


