Abstract
Basic fibroblast growth factor (FGF2) is a wide-spectrum mitogenic, angiogenic, and neurotrophic factor that is expressed at low levels in many tissues and cell types and reaches high concentrations in brain and pituitary. FGF2 has been implicated in a multitude of physiological and pathological processes, including limb development, angiogenesis, wound healing, and tumor growth, but its physiological role is still unclear. To determine the function of FGF2 in vivo, we have generated FGF2 knockout mice, lacking all three FGF2 isoforms, by homologous recombination in embryonic stem cells. FGF2−/− mice are viable, fertile and phenotypically indistinguishable from FGF2+/+ littermates by gross examination. However, abnormalities in the cytoarchitecture of the neocortex, most pronounced in the frontal motor-sensory area, can be detected by histological and immunohistochemical methods. A significant reduction in neuronal density is observed in most layers of the motor cortex in the FGF2−/− mice, with layer V being the most affected. Cell density is normal in other regions of the brain such as the striatum and the hippocampus. In addition, the healing of excisional skin wounds is delayed in mice lacking FGF2. These results indicate that FGF2, although not essential for embryonic development, plays a specific role in cortical neurogenesis and skin wound healing in mice, which, in spite of the apparent redundancy of FGF signaling, cannot be carried out by other FGF family members.
FGF2 (basic fibroblast growth factor), is the prototype of the FGF family of structurally related proteins, which so far includes 15 members. Although FGFs have been implicated in a variety of physiological and pathological processes and FGF signaling clearly plays an important role in development, the specific function of each FGF in vivo is not yet clear (1–4).
FGF2 is a pleiotropic factor that induces proliferation of most mesoderm- and neuroectoderm-derived cells. FGF2 is also a potent chemotactic factor for fibroblasts and endothelial cells, can promote or inhibit cell differentiation, and is a potent angiogenic and neurotrophic factor (3). The biological activities of FGF2 are mediated by its binding to specific tyrosine kinase transmembrane receptors on the target cell surface (1–4). Among the four FGF receptors identified so far, FGFR1 and FGFR2 bind FGF2 with the highest affinity (5–7). However, there is a high level of redundancy in receptor utilization within the FGF family (7).
In contrast to other FGFs that have a restricted pattern of expression, FGF2 is present in the majority of tissues of both adult and embryonic origin, and it is produced by many cell types. An overwhelming variety of pharmacological effects have been reported for FGF2, both in vitro and in vivo (reviewed in ref. 3). FGF2 acts as a mesoderm inducer when applied to Xenopus embryonic caps, and it can substitute the apical ectodermal ridge and maintain proliferation of limb bud mesenchyme during limb development (8, 9). FGF2 also promotes differentiation of both endothelial cells and hematopoietic cells from dissociated quail epiblasts, which, along with its angiogenic activity, suggests a role for FGF2 in blood vessel development (10). In the hematopoietic system, FGF2 enhances myelopoiesis in long-term bone marrow cultures and is a potent stimulator of megakaryocytopoiesis (11, 12). FGF2 is released by cardiomyocytes in response to mechanical load and causes myocardial hypertrophy (13). Systemic administration of FGF2 in rats decreases arterial blood pressure (14).
The activities of FGF2 in the central nervous system are also multiple. FGF2 promotes quiescent astrocytes to reenter the cell cycle and induces expression of glial fibrillary acidic protein, a marker of astrocyte differentiation (15). FGF2 maintains survival of isolated neurons, promotes neurite outgrowth of hippocampal and cortical neurons (16), and regulates expression of neurotransmitters like neuropeptide Y (17). FGF2 stimulates division of cortical multipotential stem cells and may also act on postmitotic neurons to promote differentiation and survival (18–20).
FGF2 acts as a survival factor in many models of cell and tissue injury (3). Topical application of FGF2 accelerates healing of skin wounds in animal models, as well as of eye, retina, and corneal wounds (3). Finally, FGF2 may also play a role in tumor growth and angiogenesis (2, 3).
This very wide range of biological activities, however, does not identify FGF2 as the natural effector of all these processes, because FGF2 could mimic the effect normally produced by another member of the FGF family, or perhaps by more than one FGF that can act on the same receptor.
FGF2 is also unusual because it lacks a signal peptide and is not secreted by a classical endoplasmic reticulum/Golgi-dependent mechanism, and thus is not released efficiently from producing cells, although it is detected in the extracellular environment in many tissues (3). Furthermore, the FGF2 mRNA contains additional translational start sites (CUG codons) upstream of the canonical initiator AUG. Translation starting at these upstream sites occurs efficiently both in vitro and in vivo and produces N-terminally extended, high molecular mass FGF2 forms that, in contrast to the AUG-initiated 18-kDa form, which is mostly cytosolic or found associated with the extracellular matrix, localize predominantly in the nucleus (3, 21, 22). Although the function of the nuclear FGF2 forms is still unclear, a dual mechanism of action for FGF2 has been proposed (23).
The generation of mice with homozygous deletion of individual genes has highlighted new and specific roles of individual gene functions, as well as revealed complex redundancies in the function of molecules within given gene families. We have generated FGF2 knockout mice by homologous recombination in embryonic stem (ES) cells in an attempt to elucidate the physiological role of FGF2 in vivo. The FGF2−/− mice are viable, indistinguishable from wild-type littermates by gross examination, survive to adulthood, and are fertile. However, the brains of the FGF2 −/− mice show abnormalities in the cytoarchitecture of the neocortex and a significant reduction in the number of neurons in the motor-sensory area of the cortex. The mice also exhibit a significant delay in the rate of healing of full-thickness excisional skin wounds.
MATERIALS AND METHODS
Generation of Targeted ES Clones.
A genomic clone containing the first coding exon of FGF2 was isolated from a 129SVJ mouse genomic library (Stratagene). The library was screened with fragments of the human FGF2 cDNA, and four positive clones containing the first FGF2 exon were isolated. The targeting vector was constructed in the pPNT plasmid (24). The XhoI and EcoRI sites of pPNT were used to clone the 2.4-kb 5′ arm and 6.6-kb 3′ arm of the targeting vector, respectively. The vector (20 μg) was linearized with NotI and electroporated into E14 ES cells. Colonies were selected in G418 (400 μg/ml) and 1-(2′-deoxy-2′-fluoro-1-β-d-arabinofuranosyl)-5-iodouracil (0.25 μM) on a feeder layer of neomycin-resistant mouse primary embryonic fibroblasts. Colonies were picked after 7 days in selection medium and expanded. Genomic DNA was isolated, and clones were screened by Southern hybridization.
Brain Histology and Immunohistochemistry.
Mice were perfused with 4% paraformaldehyde, pH 7.4. Brains were dissected, postfixed in 4% paraformaldehyde for 2 hr at room temperature, incubated in 30% sucrose at room temperature overnight, embedded in M-1 #1310 matrix (Lipshaw Manufacturing, Detroit), and stored at −70°C. Coronal sections, 40 μm in width, were cut with a cryostat throughout the brain and stored at 4°C in 0.1 M phosphate buffer containing 0.01% sodium azide. For immunohistochemistry, floating sections were incubated in 0.25% Triton X-100 in TS (0.1 M Tris⋅HCl, pH 7.4/150 mM NaCl) 15 min at room temperature, blocked in 3% goat serum in TS, and incubated with the primary antibody overnight at room temperature. Anti-calbindin and anti-parvalbumin mAbs (Sigma) were used at 1/200 and 1/1,000 dilutions, respectively in TS containing 1% goat serum. Sections were incubated 30 min with biotinylated anti-mouse IgG and stained with the Vectastain kit of Vector Laboratories.
Wound Healing.
Adult mice, between 2 and 3 months of age, were anesthetized with an intraperitoneal injection of 2.5% avertin (15 μl/g). The back was shaved and disinfected with 70% ethanol. A circle, 6 mm in diameter, was drawn on the skin of the mid-dorsal region, and a full-thickness wound was created by excision of the area within with curved scissors. Wounds were considered healed when the wound area was completely closed, the epithelial covering was restored, and the surface of the wound was smooth, homogeneous in color, and without residual defects.
For histology, mice were sacrificed by CO2 inhalation. Skin biopsies (including 1 cm of normal skin around the wound) were fixed overnight in 4% paraformaldehyde, pH 7.4, at room temperature and placed in 30% sucrose for up to 24 hr. Wounds were bisected transversally through the center, and each half was embedded in M-1 #1310 embedding matrix (Lipshaw Manufacturing) and stored frozen at −70°C. Central 10-μm-thick sections were cut perpendicular to the surface of the wounds mounted on glass slides and stained with hematoxylin-eosin.
The wound diameter was measured with an optical micrometer from the first intact hair follicle on each side of the wound, and the scab thickness was measured in the center of the wound. The percentage of reepithelialization was calculated from the size of the residual ulcer and the wound diameter. Collagen deposition was assessed in the dermis in the center of the wound and graded semiquantitatively as 0 (no collagen deposition), 1+ (slight deposition), 2+ (moderate deposition), and 3+ (heavy deposition). Wounds were considered healed if they were completely reepithelialized, without scabs, and the only evidence of wounding was a dermal scar.
Western Blot.
Protein extracts were prepared by homogenizing the frozen tissues in 20 mM Tris⋅HCl buffer (pH 7.4) containing 5 mM EDTA, 1% Nonidet P-40, 0.5% deoxycholate, 2 μg/ml leupeptin, 5 μg/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride and clarified by centrifugation.
Equal amounts of total protein (10–20 mg) were incubated with heparin-Sepharose CL-6B beads preswollen in 20 mM Tris⋅HCl/2.5 mM EDTA/100 mM NaCl (100 μl of heparin-Sepharose beads/10 mg of protein) for 2 hr at 4°C. Beads were washed three times, boiled 5 min in loading buffer containing 100 mM DTT and 0.1% SDS, and loaded on 15% polyacrylamide/SDS gels. Proteins were transferred to nitrocellulose filters and analyzed by Western blotting by using an anti-FGF2 mAb (Transduction Laboratories, Lexington, KY) or a polyclonal antibody (Santa Cruz Biotechnology), and the enhanced chemiluminescence detection system (Amersham).
RESULTS
Generation of FGF2−/− Mice.
The murine FGF2 gene is a single-copy gene localized on chromosome 3 (human chromosome 4). The gene is approximately 40 kb in length and consists of three coding exons separated by two large introns. The murine FGF2 gene encodes three different proteins of 22-, 21-, and 18-kDa molecular mass, respectively, that result from alternative translation initiation codons (3). We designed a replacement-type vector to create a 200-bp deletion in the FGF2 gene that eliminates exon I sequences. The deletion extends from the NcoI site in exon I to the first XbaI site in the first intron and includes 59 codons of the FGF2 coding sequence, starting at the codon immediately downstream of the ATG initiation codon. The deletion also eliminates the splicing donor site of exon I, preventing the correct splicing of the FGF2 mRNA. The deleted sequences are replaced by the neomycin-resistance gene expressed under the control of the PGK-1 promoter and polyadenylation signal.
The targeting vector was electroporated into E14-ES cells (25). Colonies were selected in the presence of G418 (positive selection) and 1-(2′-deoxy-2′-fluoro-1-β-d-arabinofuranosyl)-5-iodouracil (negative selection). A total of 120 colonies were expanded and screened for homologous recombination by Southern blotting by using initially a 5′ internal probe (Probe I). Homologous recombinants were confirmed by subsequent screening with 5′ external and 3′ internal probes (Fig. 1 A and B). Out of 120 colonies, 2 were homologous recombinants. Targeted ES cells were injected into C57BL/6 blastocysts. One of the clones contributed to the germ line as assessed by transmission of the agouti coat color to the offspring after breeding male chimeras to C57BL/6 females. As expected, 50% of the agouti offspring contained one mutated FGF2 allele. The heterozygous FGF2+/− mice were indistinguishable from their FGF2+/+ littermates and were bred to generate homozygous offspring (Fig. 1C). Homozygous FGF2−/− mutant mice were born at the expected Mendelian frequency of 1 in 4, indicating no embryonic lethality.
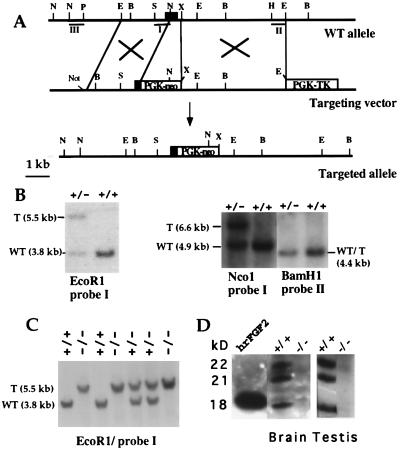
Figure 1.
FGF2 gene targeting. (A) Partial map of the FGF2 gene (Top), gene-targeting vector (Middle), and FGF2-targeted allele (Bottom). The first exon of the FGF2 gene is shown as a black box. The neo- and hsvTK-expressing cassettes, containing the PGK-1 promoter and polyadenylation sequences, are shown as white boxes. The position of the relevant restriction sites for the enzymes BamHI (B), EcoRI (E), XbaI (X), NcoI (N), SalI (S), HindIII (H), and PstI (P) is marked. The NotI site was used to linearize the targeting vector. DNA fragments I, II, and III were used as probes in Southern blot hybridization. (B) Genomic DNA from ES cells was digested with EcoRI, NcoI, and BamHI and hybridized to the probes indicated. T, targeted allele. (C) Southern blot of tail DNA from the offspring of heterozygous FGF2+/− mutant parents. DNA was digested with EcoRI and probed with probe I. (D) Absence of FGF2 in protein extracts from brain and testis of FGF2 −/− mice. Western blots of protein extracts concentrated on heparin-Sepharose beads. Blots were probed with a monoclonal anti-FGF-2 antibody (Transduction Laboratories). The same result was obtained with polyclonal antibodies for FGF2. The position of the three isoforms of FGF2 is indicated; 25 ng of human recombinant FGF2 was loaded as a control.
Western blot analysis was performed on protein extracts of various organs to confirm the absence of FGF2 protein in the FGF2−/− mice. As expected, all three FGF2 isoforms were absent in these tissues (Fig. 1D). Reverse transcription–PCR analysis confirmed the absence of FGF2 mRNA in brain and embryonic fibroblasts of FGF2−/− mice. We tested whether expression of the RNA for FGFR1 and FGFR2, the highest-affinity FGF2 receptors, or that of FGF1 RNA and protein was up-regulated as a result of FGF2 gene inactivation. We found no changes in the levels of expression of these molecules in the brain, testis, liver, heart, and bone marrow of FGF2−/− vs. wild-type mice (data not shown).
The FGF2−/− mice appeared normal and indistinguishable from the FGF+/+ littermates. No significant differences were observed in the size or the weight of the homozygous mutants versus the wild-type littermates. Homozygous mutants reached sexual maturity at 6–8 weeks of age and when interbred were fertile and produced normal-sized litters, indicating no major defects in the male or female reproductive system of the FGF2−/− mice. The offspring born from FGF2−/− homozygous parents also was normal. Histopathological examination of all organs and tissues of the FGF2−/− mice did not initially reveal any striking abnormalities, consistent with the normal and healthy appearance of the FGF2−/− mice. Thus, the absence of FGF2 has no obvious effect on embryonic development.
Morphological Defects and Reduced Number of Neurons in the Neocortex of FGF2−/− Mice.
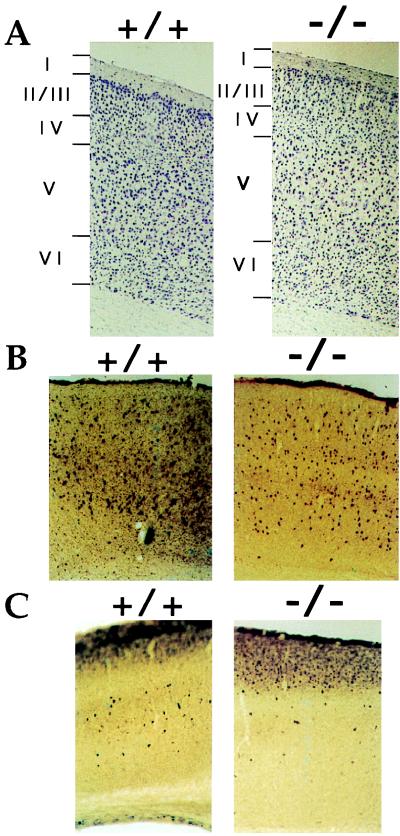
The brains of FGF2−/− mice were grossly similar to those of their wild-type littermates in size or in weight. However, when FGF2−/− mice were studied in detail, abnormalities could be detected. Cresyl violet (Nissl) staining of matched coronal sections throughout the brain of littermate FGF2−/− and FGF2+/+ 8-week-old mice revealed differences in the cytoarchitecture of the neocortex that were more obvious in the frontal than in the occipital part of the brain. In particular, the motor area of the frontal cortex (Fig. 2) showed some thickening, with less defined layers in the FGF2−/− mice. There was a slight expansion of all the cortical layers that was more pronounced in layers V and VI. In the barrel field of the S1 area, the typical barrel structure of layer IV, consisting of a series of cylindrical hollow areas surrounded by dense rings of granular neurons, was reduced in the FGF2−/− mice (data not shown). Most important, the cell density was reduced in most layers of the motor cortex, with more space between cresyl violet-stained cells (Fig. 2). Table 1 shows the number of cresyl violet-stained cells (per area unit) of the different layers of the motor cortex. A statistically significant reduction in neuronal density in layers II–VI was observed in FGF2−/− mice. This reduction was most pronounced in layers V and VI but affected also layers II–IV.
Figure 2.
Defects in the motor area of the neo-cortex of FGF2−/− mice. Coronal sections of the FGF2−/− mutant cortex compared with those of wild-type FGF2+/+ cortex after cresyl violet (NISSL) staining (A), parvalbumin (B), and calbindin (C) immunohistochemistry. Reduced cell density is evident in layers V and VI in A–C.
Table 1.
Neuronal cell density in the motor cortex of FGF2 null mice
| Layer | Number of cells/area unit
|
Δ, % | Significance | |
|---|---|---|---|---|
| FGF2+/+ | FGF2−/− | |||
| I | 5.42 ± 1.38, n = 20 (103) | 5.68 ± 1.70, n = 20 (108) | P > 0.1 | |
| II | 30.70 ± 5.51, n = 20 (614) | 24.78 ± 6.37, n = 20 (471) | 20 | 0.001 < P < 0.005 |
| III | 15.98 ± 4.26, n = 60 (991) | 14.43 ± 3.73, n = 56 (823) | 10 | 0.01 < P < 0.05 |
| IV | 17.50 ± 4.50, n = 64 (1103) | 15.54 ± 4.37, n = 64 (979) | 12 | 0.005 < P < 0.01 |
| V | 15.97 ± 2.90, n = 44 (719) | 10.55 ± 2.18, n = 36 (388) | 34 | P < 0.0005 |
| VI | 18.27 ± 3.80, n = 64 (1151) | 14.41 ± 4.15, n = 64 (894) | 22 | P < 0.0005 |
Data show cresyl violet-positive cells per area unit of layers I–VI of the motor cortex of FGF2−/− compared with FGF2+/+ mice. Mean values ± SD are shown. n, total number of areas counted. The total numbers of cells counted are shown in parenthesis. Stained cells were counted with a Zeiss Axioplan-2 microscope by using a 10 × 10 micrometer grid and a ×40 objective. Three different depths of field were counted, and each count included only cells that were in sharp focus. Counts were obtained from four sets of matching sections, corresponding to four FGF2+/+ and four FGF2−/− animals, respectively. Each animal was equally represented in the counting. Δ, decrease in cell density in the FGF−/− sections. The data were analyzed statistically by using a two-sample (pooled) t test.
To further investigate alterations in cell number, we examined the distribution of specific neuronal phenotypes. The calcium-binding proteins parvalbumin and calbindin are expressed in distinct subpopulations of neurons that therefore may be distinguished by specific calcium-dependent processes. In the cerebral cortex these calcium-binding proteins are prominent only in a small number of neurons that are nonpyramidal cells containing GABA and/or peptide neuromodulators, and there is only very limited overlap of calbindin- and parvalbumin-positive cells; parvalbumin occurs mainly in chandelier and basquet cells, and calbindin-D28K occurs mainly in double-bouquet cells (30).
By immunostaining with anti-calbindin and anti-parvalbumin antibodies we also observed a 30% reduction in the number of calbindin- and parvalbumin-positive cells in the motor cortex (Fig. 2). Therefore, the reduction in cell number is not specific for a particular subtype of neurons. Furthermore, the decreased neuronal density detected in 8-week-old animals did not become significantly more pronounced in older mice. The number of calbindin- or parvalbumin-stained neurons in the striatum and cerebellum (Purkinje cells) was similar in both FGF2−/− and FGF2+/+ mice (not shown).
Delayed Wound Healing in the FGF2−/− Mice.
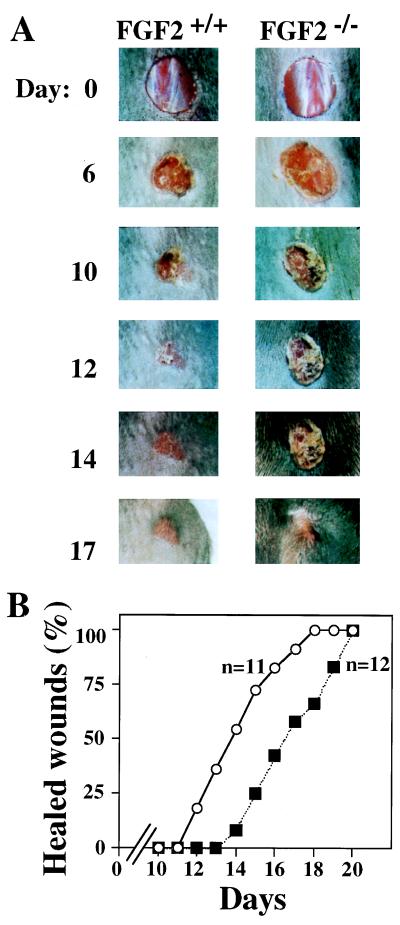
To study the role of FGF2 in skin wound healing, we created single full-thickness excisional skin wounds in the mid-dorsal region of the mice. The healing rate of age-matched FGF2−/− or FGF2+/+ mice was monitored in various ways. Initially, 11 FGF2+/+ and 12 FGF2−/− adult mice, 8–11 weeks of age, were examined macroscopically and photographed every other day to analyze the healing progress (Fig. 3). No significant difference between FGF2+/+ and FGF2−/− mice was observed in the gross appearance of the wounds between days 1 and 7. By day 7 all wounds were covered with a dry scab that remained adherent to the wound of FGF2 −/− mice until day 10, when it began to detach in FGF2+/+ mice. After 12 days approximately 20% of the FGF2+/+ wounds were completely healed, with no sign of residual skin defect, and by day 14 more than 50% of the wounds were closed (Fig. 3B). However, by day 14 only 10% of the FGF2−/− wounds were healed, and 50% healing was not achieved until day 17. Thus, there is a 3-day delay in the time required by the FGF2−/− mice for complete healing of excisional wounds.
Figure 3.
Skin wound healing of FGF2−/− mice. (A) Example of the healing progress in an FGF2−/− mouse and an FGF+/+ mouse. Wounds were photographed at the time indicated. Day 0 picture was taken immediately after wounding. All wounds were photographed at the same distance. (B) The fraction of completely healed wounds (determined as described in Materials and Methods) is plotted versus the time after wounding. n, number of mice of each genotype analyzed. Wounds were performed in a genotype-blind fashion. Mice were caged separately throughout the experiment. ○, FGF2+/+; ▪, FGF2−/−.
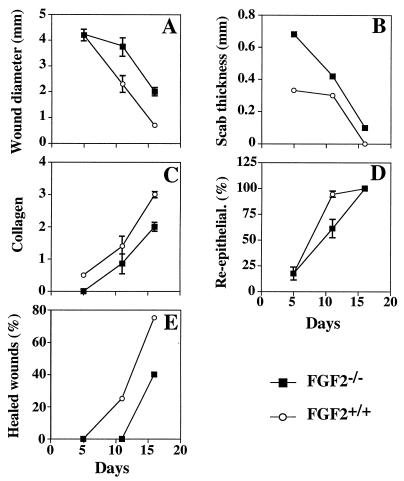
In an independent experiment, wound healing was analyzed histologically at different times after wounding in a genotype-blind study (Fig. 4). Mice were sacrificed at 5, 11, and 16 days, and frozen sections were prepared through the center of the wounded area. Wound diameter, scab thickness, percentage of reepithelialization, and collagen deposition were assessed as described in Materials and Methods. During the wound healing the wound diameter and scab thickness progressively decrease while the epithelium is reconstituted so that the wound is completely reepithelialized. This is accompanied by deposition of collagen to reconstitute the dermis as a scar. All of these processes were delayed in the FGF2−/− mice (Fig. 4). At 11 days after wounding the mean wound diameter in the FGF2−/− mice was almost twice that of controls, the scab thickness was 30% greater, the percentage of reepithelialization was only 60% of controls, and the collagen deposition was only 50% that of controls. There was an approximately 3-day delay in the healing process of knockout mice as assessed histologically, which is quite similar to the results of the experiments in which wound healing was assessed by gross examination.
Figure 4.
Histologic analysis of wound healing in FGF2−/− and FGF2+/+ mice. Wound diameter (A), scab thickness (B), collagen deposition (C), percentage of reepithelialization (D), and percentage of completely healed wounds (E) are plotted versus time after wounding. The number of animals was five FGF2−/− and five FGF2+/+ at 5 days and 16 days after wounding, respectively, and seven FGF2−/− and seven FGF2+/+ at day 11. Each point represents the mean ± SEM, except in E. Wounds that were completely healed were not considered in the calculation of the mean. The histologic parameters were determined as described in Materials and Methods.
DISCUSSION
The absence of any dramatic phenotype in FGF2−/− mice was quite surprising in view of the multitude of functions that have been ascribed to this growth factor (reviewed in the Introduction). Many of these effects were observed after administration of FGF2 to tissue culture cells and to particular tissues in vivo and thus might reflect the interaction of multiple FGFs with the same receptors, rather than identify FGF2 as the specific ligand relevant to the process studied. However, some of the phenotypes that we did not observe merit discussion.
It has been suggested that FGF2 could play a role in the growth and patterning of the limb (9). FGF2 is present together with FGF8 and FGF4 in the limb bud (4), but the results presented here clearly show that FGF2 does not participate in limb development or that its function can be replaced by FGF8 and FGF4. Similarly, although FGF2 is expressed by endothelial cells and is angiogenic, it does not appear to be essential for the development of the vascular system, in dramatic contrast to another angiogenic factor, vascular endothelial growth factor (27).
To further probe the role of FGF2 in angiogenesis, we injected a recombinant retrovirus (NTR-mT) that expresses the polyoma middle T antigen and causes a very high frequency of hemangiomas into wild-type and FGF2−/− mice (28). We did not detect any significant difference in the occurrence or the growth of these tumors between the two types of mice (data not shown).
FGF signaling has been shown to be important for the development and maintenance of retinal cells and of the eye lens (29, 30). We could not detect any impairment of vision by electroretinography in 2- to 3-month-old FGF2−/− mice, and no eye defects were obvious by histological examination. It cannot be excluded, however, that, with age, some deficiencies may become apparent.
Considerable evidence both from human and mouse genetics shows that FGF signaling is important for harmonious bone development. Unregulated activation of FGF signaling produces bone morphogenetic defects such as achondroplasia and various craniosynostosis syndromes in humans (4, 31). In mice, a syndrome similar to achondroplasia has been shown to occur in transgenic mice expressing FGF2 under the control of a constitutive promoter (32). Knockout of the FGFR3 gene, which is highly expressed in chondrocytes, results in excessive growth and deformity of the long bones, and it has been proposed that FGF2 or FGF1 could be the ligand regulating FGF signaling in bone (33, 34). Our FGF2 −/− mice do not show any gross abnormality of the bones, but more precise studies will have to be carried out to rule out or identify subtle bone formation defects.
On the other hand, we detected abnormalities in our FGF2−/− mice in two processes in which FGF2 had been suggested to play a role. We have detected a significant reduction in the number of neurons in the brain neocortex, particularly in the motor area, of the FGF2−/− mice. The neuronal defect was not found in striatum, hippocampus, or cerebellum. The observed reduction in neuron density in the neocortex does not appear to be caused by increased neuronal cell death, because we did not observe an accentuation of the phenotype in older mice (>1 year old), and the youngest mice examined (3 weeks of age) showed essentially the same pattern as did the 8-week-old mice. Moreover, staining for glial fibrillary acidic protein, which is indicative of ongoing neuronal degeneration, was similar in FGF2−/− and FGF2+/+ mice. Thus, the reduced cell density seems rather to be the consequence of an early defect, possibly limiting the extent of proliferation, differentiation, or migration of neuronal progenitor cells.
FGF2 has been shown to stimulate in vitro proliferation of telencephalic neuroectodermal cells with characteristics of multipotential stem cells (35, 36). Specifically, cortical progenitor cells also have been shown to proliferate in vitro in response to FGF2. It has been proposed that FGF2 and NT-3 cooperate in the regulation of cortical neurogenesis in vivo (18). Qian et al. (19) also have shown that FGF2 influences the development of cortical progenitor cells into neuronal or glial lineages in a dose-dependent manner. FGF2 mRNA is present in the mouse neuroepithelium as early as day E9.5 and is the only FGF detected in the neuroepithelium at that early stage (37).
These observations are consistent with the hypothesis that the neuronal deficiency observed in our FGF2−/− mice is the consequence of an early developmental defect. It is also possible that the dramatic wave of apoptosis that occurs in the brain of newborn animals is increased or prolonged in FGF2−/− mice because of the absence of this antiapoptotic factor.
FGF2 has long been thought to play a role in tissue regeneration and skin wound healing. FGF2 is present in normal skin together with other members of the FGF family, FGF1, FGF5, and FGF7, and its expression level increases upon injury (38). Moreover, it has been shown that the local application of FGF2 to skin wounds accelerates both dermal as well as epidermal wound healing (39–41). A mechanism has been proposed by which stored endogenous FGF2, bound to heparin sulfate proteoglycans in the extracellular matrix, could be released by matrix-degrading proteases present at the wound site and could participate in wound healing (42).
In line with these hypotheses, we have detected a small but significant defect in skin wound healing of FGF2−/− mice. This resulted in a temporary delay for the complete healing of skin wounds and appeared to affect several parameters of wound healing, rather than a single, specific process. It is interesting to note that mice which do not produce FGF7, another FGF that is up-regulated in wounds and is specific for keratinocytes, do not have wound-healing defects (43). It will be also interesting in the future to determine whether in pathological situations leading to wound healing defects, such as diabetes, the requirement for FGF2 is more pronounced than in normal animals.
In conclusion, the results presented here show that FGF2 is not essential for embryonic development and its absence only causes a modest defect in wound healing and a decrease in neuronal density in the motor cortex, whose functional importance remains to be determined. Because FGF signaling has been shown to be essential for many processes of development and tissue formation, these findings probably do not imply that FGF2 is a superfluous gene product, but rather that its function can be fulfilled by other members of the FGF family. A variety of considerations (lack of a signal peptide and inefficient secretion, production of nuclear forms, widespread expression) suggest that the FGF most likely to duplicate FGF2 function is FGF1. FGF1 has a very broad affinity for FGF receptors and is present in many tissues and cells where FGF2 is also expressed (2). The generation of FGF1 knockout mice, which is now in progress in our laboratory, as well as the breeding of heterozygous FGF1 and FGF2 knockout mice to produce doubly homozygous mutants, will verify whether widespread developmental and adult defects are produced by the simultaneous absence of both of these growth factors.
As this manuscript was being completed, Zhou et al. (44) also reported the creation of FGF2 null mutant mice. Although these authors also showed that mice lacking FGF2 are morphologically normal and fertile, they focused their investigation on the cardiovascular system and observed low blood pressure accompanied by a reduction in vein spontaneous contractility. We had also observed a slight decrease in blood pressure in our FGF2−/− mice, but we did not pursue this observation further.
Acknowledgments
We thank Dr. P. Mombaerts for providing us with the E14-ES cells, Dr. P. Gouras for help in electroretinography experiments, Dr. S. Goff for advice on ES cell culture and knockout, Dr. E. Wagner for the NTK mT retrovirus, and Drs. D. Rifkin and A. Mansukhani for the critical reading of this manuscript. This investigation was supported by Public Health Service Grant CA42568 from the National Cancer Institute.
ABBREVIATIONS
- FGF
fibroblast growth factor
- ES
embryonic stem
References
- 1.Baird A, Bohlen P. In: Peptide Growth Factors and Their Receptors. Sporn M B, Roberts A B, editors. New York: Springer; 1991. pp. 369–418. [Google Scholar]
- 2.Basilico C, Moscatelli D. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- 3.Bikfalvi A, Klein S, Pintucci G, Rifkin D B. Endocr Rev. 1997;18:26–45. doi: 10.1210/edrv.18.1.0292. [DOI] [PubMed] [Google Scholar]
- 4.Goldfarb M. Cytokine Growth Factor Rev. 1996;7:311–325. doi: 10.1016/s1359-6101(96)00039-1. [DOI] [PubMed] [Google Scholar]
- 5.Mansukhani A, Dell’Era P, Moscatelli D, Kornbluh S, Hanafusa H, Basilico C. Proc Natl Acad Sci USA. 1992;89:3305–3309. doi: 10.1073/pnas.89.8.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansukhani A, Moscatelli D, Talarico D, Levytska V, Basilico C. Proc Natl Acad Sci USA. 1990;87:4378–4382. doi: 10.1073/pnas.87.11.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ornitz D M, Xu J, Colvin J S, McEwen D G, MacArthur C A, Coulier F, Gao G, Goldfarb M. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- 8.Slack J M W, Darlington B G, Heath J K, Godsave S F. Nature (London) 1987;326:197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- 9.Fallon J F, Lopez A, Ros M A, Savage M P, Olwin B B, Simandl B K. Science. 1994;264:104–107. doi: 10.1126/science.7908145. [DOI] [PubMed] [Google Scholar]
- 10.Flamme I, Risau W. Development. 1992;116:435–439. doi: 10.1242/dev.116.2.435. [DOI] [PubMed] [Google Scholar]
- 11.Avraham M, Banu N, Scadden D T, Abraham J, Groopman J E. Blood. 1994;83:2126–2132. [PubMed] [Google Scholar]
- 12.Wilson E L, Rifkin D B, Kelly F, Hannocks M J, Gabrilove J L. Blood. 1991;77:954–959. [PubMed] [Google Scholar]
- 13.Clarke M S, Caldwell R W, Chiao H, Miyake K, McNeil P L. Circ Res. 1995;76:927–934. doi: 10.1161/01.res.76.6.927. [DOI] [PubMed] [Google Scholar]
- 14.Cuevas P, Carceller F, Ortega S, Zazo M, Nieto I, Gimenez-Gallego G. Science. 1991;254:1208–1210. doi: 10.1126/science.1957172. [DOI] [PubMed] [Google Scholar]
- 15.Kniss D A, Burry R W. Brain Res. 1988;439:281–288. doi: 10.1016/0006-8993(88)91485-0. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda S, Saito H, Nishiyama N. Brain Res. 1990;520:310–316. doi: 10.1016/0006-8993(90)91720-2. [DOI] [PubMed] [Google Scholar]
- 17.Barnes A, Cho G. Endocrinology. 1993;133:1895–1898. doi: 10.1210/endo.133.4.8104779. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh A, Greenberg M E. Neuron. 1995;15:89–103. doi: 10.1016/0896-6273(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 19.Qian X, Davis A A, Goderie S K, Temple S. Neuron. 1997;18:81–93. doi: 10.1016/s0896-6273(01)80048-9. [DOI] [PubMed] [Google Scholar]
- 20.Temple S, Qian X. Neuron. 1995;15:249–252. doi: 10.1016/0896-6273(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 21.Bugler B, Amalric F, Prats H. Mol Cell Biol. 1991;11:573–577. doi: 10.1128/mcb.11.1.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Florkiewicz R Z, Sommer A. Proc Natl Acad Sci USA. 1989;86:3978–3981. doi: 10.1073/pnas.86.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bikfalvi A, Klein S, Pintucci G, Quarto N, Mignatti P, Rifkin D B. J Cell Biol. 1995;129:233–243. doi: 10.1083/jcb.129.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartzberg P L, Stall A M, Hardin J D, Bowdish K S, Humaran T, Boast S, Harbison M L, Robertson E J, Goff S P. Cell. 1991;65:1165–1175. doi: 10.1016/0092-8674(91)90012-n. [DOI] [PubMed] [Google Scholar]
- 25.Hooper M, Hardy K, Handyside A, Hunter S, Monk M. Nature (London) 1987;326:292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- 26.Baimbridge K G, Celio M R, Rogers J H. Trends Neurosci. 1992;15:303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- 27.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Nature (London) 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 28.Williams R L, Courtneidge S A, Wagner E F. Cell. 1988;52:121–131. doi: 10.1016/0092-8674(88)90536-3. [DOI] [PubMed] [Google Scholar]
- 29.Chow R L, Roux G D, Roghani M, Palmer M A, Rifkin D B, Moscatelli D A, Lang R A. Development. 1995;121:4383–4393. doi: 10.1242/dev.121.12.4383. [DOI] [PubMed] [Google Scholar]
- 30.Campochiaro P A, Chang M, Ohsato M, Vinores S A, Nie Z, Hjelmeland L, Mansukhani A, Basilico C, Zack D J. J Neurosci. 1996;16:1679–1688. doi: 10.1523/JNEUROSCI.16-05-01679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muenke M, Schell V. Trends Genet. 1995;11:308–313. doi: 10.1016/s0168-9525(00)89088-5. [DOI] [PubMed] [Google Scholar]
- 32.Coffin J D, Florkiewicz R Z, Neumann J, Mort-Hopkins T, Dorn G W, II, Lightfoot P, German R, Howles P N, Kier A, O’Toole B A, et al. Mol Biol Cell. 1995;6:1861–1873. doi: 10.1091/mbc.6.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colvin J S, Bohne B A, Harding G W, McEwen D G, Ornitz D M. Nat Genet. 1996;12:390–397. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- 34.Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Cell. 1996;84:911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 35.Kilpatrick T J, Bartlett P F. J Neurosci. 1995;15:3635–3661. doi: 10.1523/JNEUROSCI.15-05-03653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gensburger C, Labourdette G, Sensenbrenner M. FEBS Lett. 1987;217:1–5. doi: 10.1016/0014-5793(87)81230-9. [DOI] [PubMed] [Google Scholar]
- 37.Nurcombe V, Ford M D, Wildschut J A, Bartlett P F. Science. 1993;260:103–106. doi: 10.1126/science.7682010. [DOI] [PubMed] [Google Scholar]
- 38.Gibran N S, Isik F F, Heimbach D M, Gordon D. J Surg Res. 1994;56:226–234. doi: 10.1006/jsre.1994.1036. [DOI] [PubMed] [Google Scholar]
- 39.McGee G S, Davidson J M, Buckley A, Sommer A, Woodward S C, Aquino A M, Barbour R, Demetriou A A. J Surg Res. 1988;45:145–153. doi: 10.1016/0022-4804(88)90034-0. [DOI] [PubMed] [Google Scholar]
- 40.Hebda P A, Klingbeil C K, Abraham J A, Fiddes J C. J Inv Dermatol. 1990;95:626–631. doi: 10.1111/1523-1747.ep12513528. [DOI] [PubMed] [Google Scholar]
- 41.Tsuboi R, Rifkin D B. J Exp Med. 1990;172:245–251. doi: 10.1084/jem.172.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flaumenhaft R, Moscatelli D, Saksela O, Rifkin D B. J Cell Physiol. 1989;140:75–81. doi: 10.1002/jcp.1041400110. [DOI] [PubMed] [Google Scholar]
- 43.Guo L, Degenstein L, Fuchs E. Genes Dev. 1996;10:165–175. doi: 10.1101/gad.10.2.165. [DOI] [PubMed] [Google Scholar]
- 44.Zhou M, Sutliff R L, Paul R J, Lorenz J N, Hoying J B, Haudenschild C C, Yin M, Coffin J D, Kong L, Kranias E G, et al. Nat Med. 1998;4:201–207. doi: 10.1038/nm0298-201. [DOI] [PMC free article] [PubMed] [Google Scholar]