Abstract
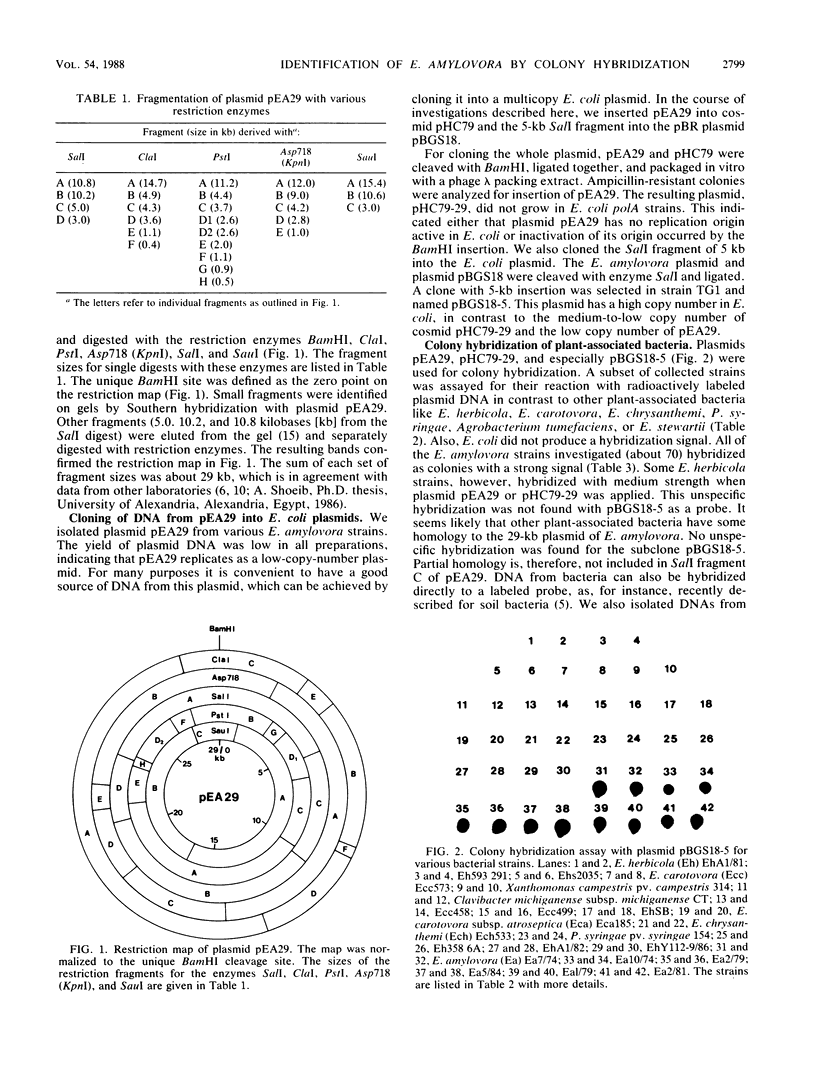
All strains of Erwinia amylovora characterized carry a medium-size plasmid of 29 kilobases (pEA29). We mapped this plasmid with various restriction enzymes, cloned the whole DNA into an Escherichia coli plasmid, and subcloned restriction fragments. These DNA species were used for identification of E. amylovora after handling of strains in the laboratory and also in field isolates. About 70 strains of E. amylovora and 24 strains from nine other species, mainly found in plant habitats, were checked in a colony hybridization test. Virulent and avirulent E. amylovora strains reacted positively, whereas the other species were negative. Apart from the hybridization assay, the positive strains were additionally tested for ooze production on rich agar with 5% sucrose and on immature-pear slices. Unspecific background hybridization of non-E. amylovora strains found for hybridization with the whole E. amylovora plasmid was almost eliminated when a 5-kilobase SalI fragment from pEA29 was used as a probe and when the washes after the hybridization procedure were done with high stringency. Under these conditions, E. amylovora could be readily identified from field isolates.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Grimont P. A., Grimont F., Desplaces N., Tchen P. DNA probe specific for Legionella pneumophila. J Clin Microbiol. 1985 Mar;21(3):431–437. doi: 10.1128/jcm.21.3.431-437.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Holben William E., Jansson Janet K., Chelm Barry K., Tiedje James M. DNA Probe Method for the Detection of Specific Microorganisms in the Soil Bacterial Community. Appl Environ Microbiol. 1988 Mar;54(3):703–711. doi: 10.1128/aem.54.3.703-711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Hedge P. J., te Heesen S., Edelman A., Broome-Smith J. K. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene. 1986;41(2-3):337–342. doi: 10.1016/0378-1119(86)90117-4. [DOI] [PubMed] [Google Scholar]
- Tautz D., Renz M. An optimized freeze-squeeze method for the recovery of DNA fragments from agarose gels. Anal Biochem. 1983 Jul 1;132(1):14–19. doi: 10.1016/0003-2697(83)90419-0. [DOI] [PubMed] [Google Scholar]




