Abstract
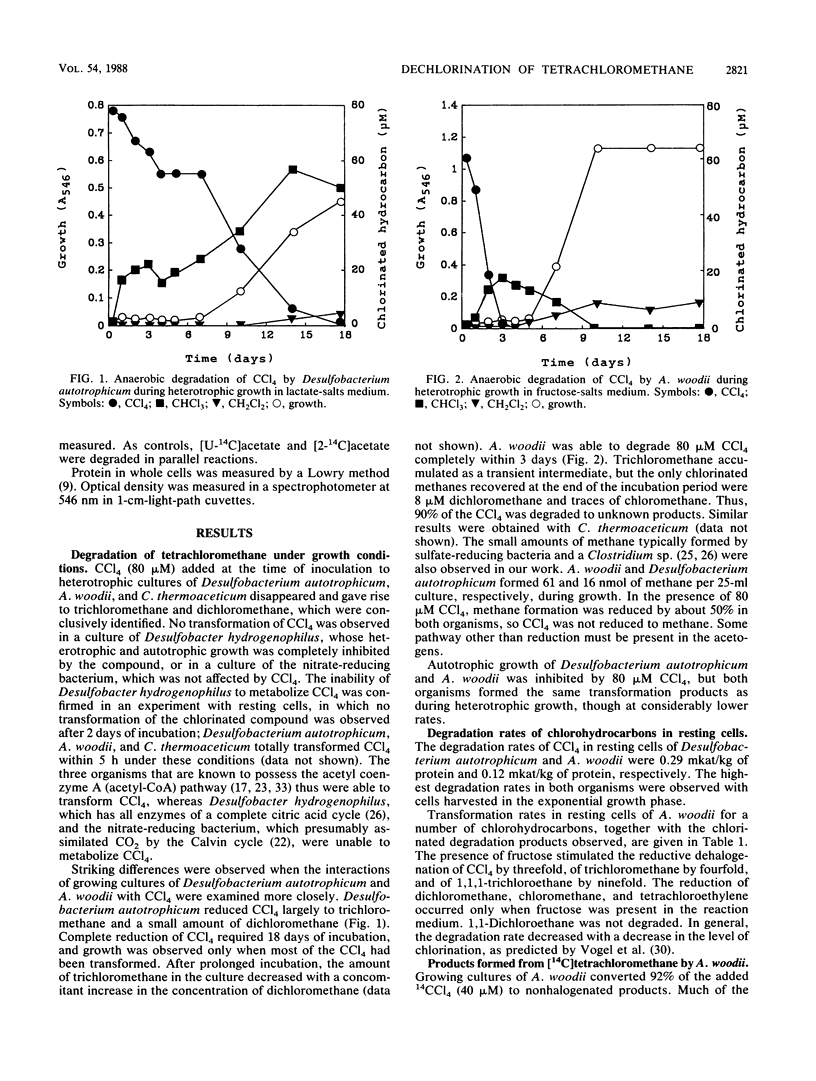
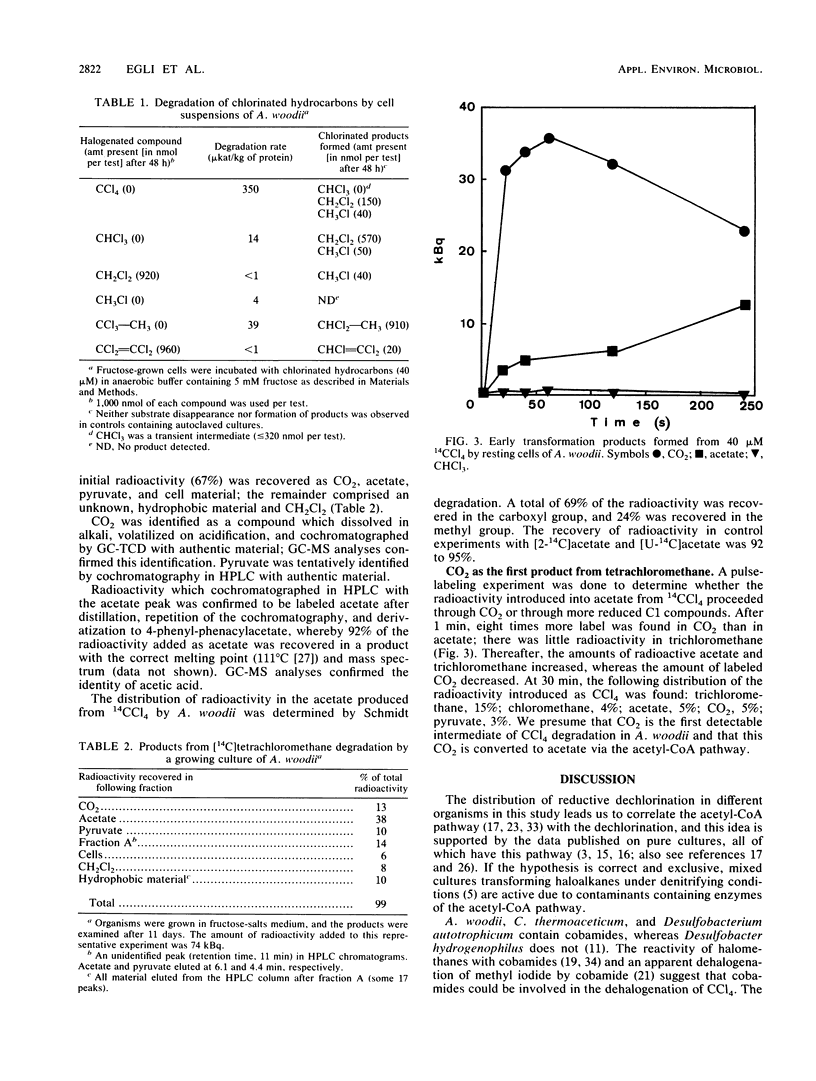
Five anaerobic bacteria were tested for their abilities to transform tetrachloromethane so that information about enzymes involved in reductive dehalogenations of polychloromethanes could be obtained. Cultures of the sulfate reducer Desulfobacterium autotrophicum transformed some 80 microM tetrachloromethane to trichloromethane and a small amount of dichloromethane in 18 days under conditions of heterotrophic growth. The acetogens Acetobacterium woodii and Clostridium thermoaceticum in fructose-salts and glucose-salts media, respectively, degraded some 80 microM tetrachloromethane completely within 3 days. Trichloromethane accumulated as a transient intermediate, but the only chlorinated methanes recovered at the end of the incubation were 8 microM dichloromethane and traces of chloromethane. Desulfobacter hydrogenophilus and an autotrophic, nitrate-reducing bacterium were unable to transform tetrachloromethane. Reduction of chlorinated methanes was thus observed only in the organisms with the acetyl-coenzyme A pathway. Experiments with [14C]tetrachloromethane were done to determine the fate of this compound in the acetogen A. woodii. Radioactivity in an 11-day heterotrophic culture was largely (67%) recovered in CO2, acetate, pyruvate, and cell material. In experiments with cell suspensions to which [14C]tetrachloromethane was added, 14CO2 appeared within 20 s as the major transformation product. A. woodii thus catalyzes reductive dechlorinations and transforms tetrachloromethane to CO2 by a series of unknown reactions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahr H. J., King L. J., Nastainczyk W., Ullrich V. The mechanism of chloroform and carbon monoxide formation from carbon tetrachloride by microsomal cytochrome P-450. Biochem Pharmacol. 1980 Oct 15;29(20):2855–2861. doi: 10.1016/0006-2952(80)90022-2. [DOI] [PubMed] [Google Scholar]
- Belay N., Daniels L. Production of ethane, ethylene, and acetylene from halogenated hydrocarbons by methanogenic bacteria. Appl Environ Microbiol. 1987 Jul;53(7):1604–1610. doi: 10.1128/aem.53.7.1604-1610.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwer E. J., McCarty P. L. Transformations of halogenated organic compounds under denitrification conditions. Appl Environ Microbiol. 1983 Apr;45(4):1295–1299. doi: 10.1128/aem.45.4.1295-1299.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro C. E., Wade R. S., Belser N. O. Biodehalogenation: reactions of cytochrome P-450 with polyhalomethanes. Biochemistry. 1985 Jan 1;24(1):204–210. doi: 10.1021/bi00322a029. [DOI] [PubMed] [Google Scholar]
- Dolfing J., Tiedje J. M. Growth yield increase linked to reductive dechlorination in a defined 3-chlorobenzoate degrading methanogenic coculture. Arch Microbiol. 1987;149(2):102–105. doi: 10.1007/BF00425073. [DOI] [PubMed] [Google Scholar]
- Drake H. L., Hu S. I., Wood H. G. Purification of carbon monoxide dehydrogenase, a nickel enzyme from Clostridium thermocaceticum. J Biol Chem. 1980 Aug 10;255(15):7174–7180. [PubMed] [Google Scholar]
- Durst H. D., Milano M., Kikta E. J., Jr, Connelly S. A., Grushka E. Phenacyl esters of fatty acids via crown ether catalysts for enhanced ultraviolet detection in liquid chromatography. Anal Chem. 1975 Sep;47(11):1797–1801. doi: 10.1021/ac60361a025. [DOI] [PubMed] [Google Scholar]
- Ghambeer R. K., Wood H. G., Schulman M., Ljungdahl L. Total synthesis of acetate from CO2. 3. Inhibition by alkylhalides of the synthesis from CO2, methyltetrahydrofolate, and methyl-B12 by Clostridium thermoaceticum. Arch Biochem Biophys. 1971 Apr;143(2):471–484. doi: 10.1016/0003-9861(71)90232-3. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L., COLLINS J. F., BIGLEY D. The influence of growth substrates on metabolic pathways in Micrococcus denitrificans. Biochim Biophys Acta. 1960 Mar 25;39:9–24. doi: 10.1016/0006-3002(60)90117-7. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- Postgate J. R. Methane as a minor product of pyruvate metabolism by sulphate-reducing and other bacteria. J Gen Microbiol. 1969 Aug;57(3):293–302. doi: 10.1099/00221287-57-3-293. [DOI] [PubMed] [Google Scholar]
- Wackett L. P., Gibson D. T. Degradation of trichloroethylene by toluene dioxygenase in whole-cell studies with Pseudomonas putida F1. Appl Environ Microbiol. 1988 Jul;54(7):1703–1708. doi: 10.1128/aem.54.7.1703-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M., Kennedy F. S., Wolfe R. S. The reaction of multihalogenated hydrocarbons with free and bound reduced vitamin B 12. Biochemistry. 1968 May;7(5):1707–1713. doi: 10.1021/bi00845a013. [DOI] [PubMed] [Google Scholar]
- Zehnder A. J., Brock T. D. Methane formation and methane oxidation by methanogenic bacteria. J Bacteriol. 1979 Jan;137(1):420–432. doi: 10.1128/jb.137.1.420-432.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


