Abstract
1 The relative sensitivities to aspartate and glutamate of neurones receiving a corticofugal innervation were examined by microiontophoresis, and compared with the relative sensitivities of neurones not appearing to receive such an input.
2 On all the cells tested, glutamate appeared to be a more potent excitant than aspartate in terms of neuronal response size or effective dose.
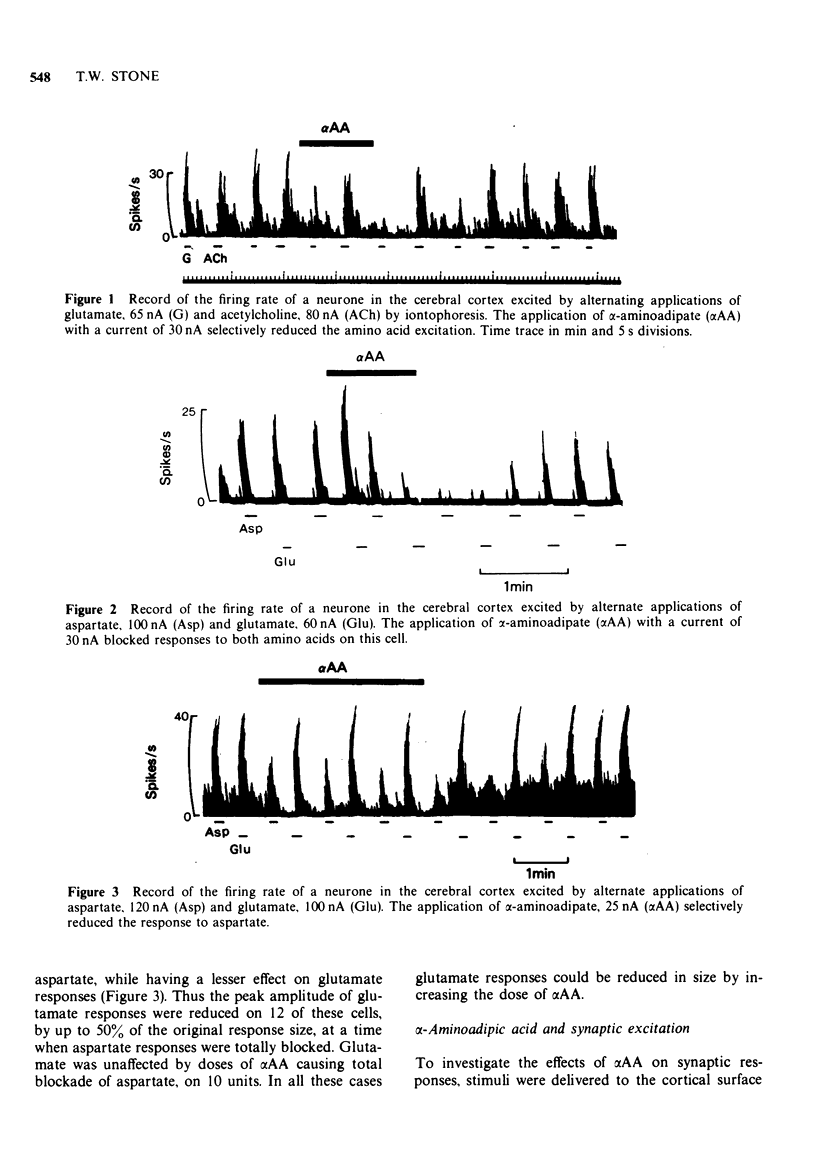
3 DL-α-Aminoadipate (αAA) reduced the excitatory amino acid responses on all the neurones tested. On many of these cells a control excitation could be produced, by acetylcholine or hydrogen ions, which was in most cases unaffected by doses of αAA producing antagonism of amino acid excitation.
4 On 70% of the cells, aminoadipate showed no selectivity for aspartate compared with glutamate but a differential action, involving blockade of aspartate but not glutamate, was apparent on the other 30%.
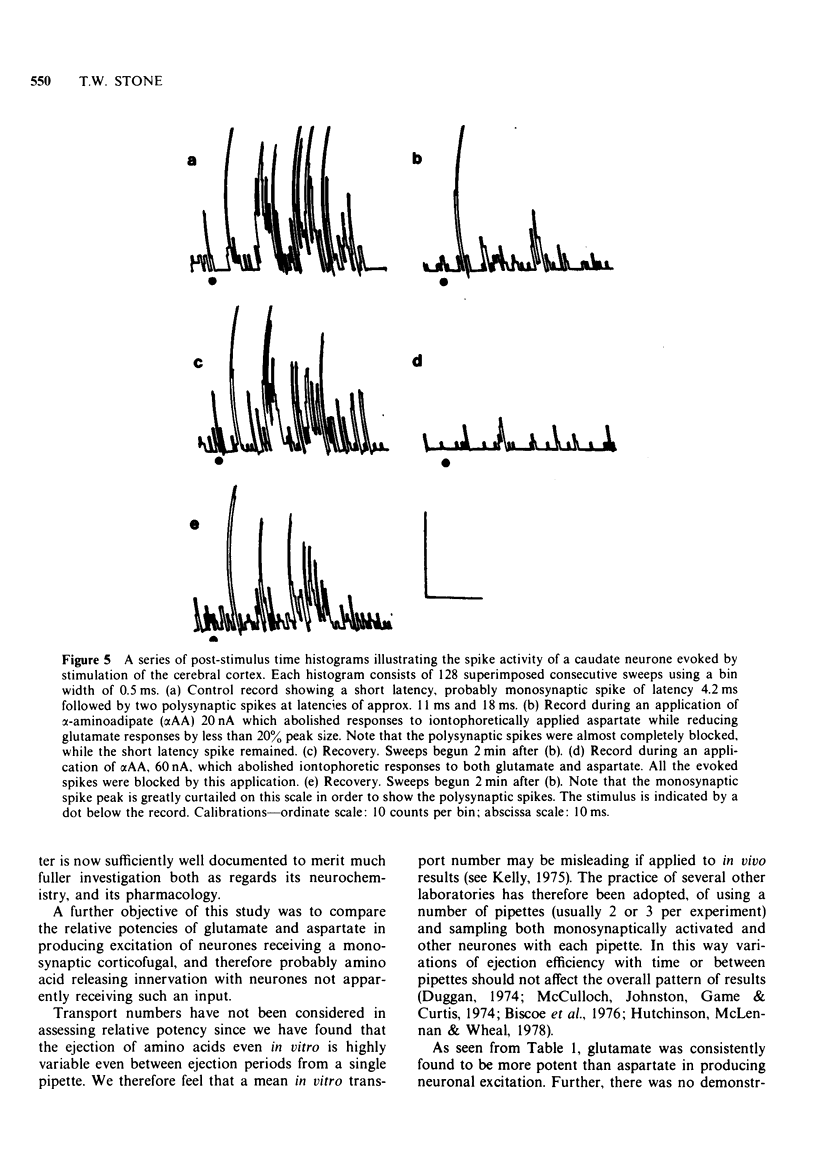
5 Doses of αAA which selectively reduced responses to aspartate had no effect on short latency evoked spikes, but doses which also reduced responses to glutamate reduced the short-latency synaptic excitation induced by electrical stimulation of either the surface of the cerebral cortex, or of the pyramidal tracts in the medulla.
6 These findings suggest that corticofugal neurones having an excitatory action on cells in various parts of the brain may use an amino acid, probably glutamate, as a common neurotransmitter.
7 As no significant difference could be demonstrated in the potency ratios of glutamate:aspartate on monosynaptically activated cells compared with other cells, doubt is cast on the validity of drawing conclusions about transmitter identity from potency ratios alone, without the support of antagonist studies.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biscoe T. J., Davies J., Dray A., Evans R. H., Francis A. A., Martin M. R., Watkins J. C. Depression of synaptic excitation and of amino acid induced excitatory responses of spinal neurones by D-alpha-aminoadipate, alpha,epsilon-diaminopimelic acid and HA-966. Eur J Pharmacol. 1977 Oct 1;45(3):315–316. doi: 10.1016/0014-2999(77)90017-6. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Evans R. H., Francis A. A., Martin M. R., Watkins J. C., Davies J., Dray A. D-alpha-Aminoadipate as a selective antagonist of amino acid-induced and synaptic excitation of mammalian spinal neurones. Nature. 1977 Dec 22;270(5639):743–745. doi: 10.1038/270743a0. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Headley P. M., Lodge D., Martin M. R., Watkins J. C. The sensitivity of rat spinal interneurones and renshaw cells to L-glutamate and L-aspartate. Exp Brain Res. 1976 Dec 22;26(5):547–551. doi: 10.1007/BF00238827. [DOI] [PubMed] [Google Scholar]
- Buchwald N. A., Price D. D., Vernon L., Hull C. D. Caudate intracellular response to thalamic and cortical inputs. Exp Neurol. 1973 Feb;38(2):311–323. doi: 10.1016/0014-4886(73)90155-6. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A. Amino acid transmitters in the mammalian central nervous system. Ergeb Physiol. 1974;69(0):97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Microelectrophoretic studies on the depressant action of HA-966 on chemically and synaptically excited neurones in the cat cerebral cortex and cuneate nucleus. Brain Res. 1973 Sep 14;59:311–322. doi: 10.1016/0006-8993(73)90269-2. [DOI] [PubMed] [Google Scholar]
- Divac I., Fonnum F., Storm-Mathisen J. High affinity uptake of glutamate in terminals of corticostriatal axons. Nature. 1977 Mar 24;266(5600):377–378. doi: 10.1038/266377a0. [DOI] [PubMed] [Google Scholar]
- Duggan A. W. The differential sensitivity to L-glutamate and L-aspartate of spinal interneurones and Renshaw cells. Exp Brain Res. 1974 Mar 29;19(5):522–528. doi: 10.1007/BF00236115. [DOI] [PubMed] [Google Scholar]
- Hutchinson G. B., McLennan H., Wheal H. V. The responses of Renshaw cells and spinal interneurones of the rat to L-glutamate and L-aspartate. Brain Res. 1978 Feb 3;141(1):129–136. doi: 10.1016/0006-8993(78)90622-4. [DOI] [PubMed] [Google Scholar]
- Kitai S. T., Kocsis J. D., Wood J. Origin and characteristics of the cortico-caudate afferents: an anatomical and electrophysiological study. Brain Res. 1976 Dec 10;118(1):137–141. doi: 10.1016/0006-8993(76)90848-9. [DOI] [PubMed] [Google Scholar]
- Lodge D., Headley P. M., Curtis D. R. Selective antagonism by D-alpha-aminoadipate of amino acid and synaptic excitation of cat spinal neurons. Brain Res. 1978 Sep 8;152(3):603–608. doi: 10.1016/0006-8993(78)91117-4. [DOI] [PubMed] [Google Scholar]
- McCulloch R. M., Johnston G. A., Game C. J., Curtis D. R. The differential sensitivity of spinal interneurones and Renshaw cells to Kainate and N-methyl-D-aspartate. Exp Brain Res. 1974;21(5):515–518. doi: 10.1007/BF00237169. [DOI] [PubMed] [Google Scholar]
- McGeer E. G., McGeer P. L., Singh K. Kainate-induced degeneration of neostriatal neurons: dependency upon corticostriatal tract. Brain Res. 1978 Jan 13;139(2):381–383. doi: 10.1016/0006-8993(78)90941-1. [DOI] [PubMed] [Google Scholar]
- McLennan H., Hall J. G. The action of D-alpha-aminoadipate on excitatory amino acid receptors of rat thalamic neurones. Brain Res. 1978 Jun 30;149(2):541–545. doi: 10.1016/0006-8993(78)90501-2. [DOI] [PubMed] [Google Scholar]
- Schultz W., Ungerstedt U. A method to detect and record from striatal cells of low spontaneous activity by stimulating the corticostriatal pathway. Brain Res. 1978 Feb 24;142(2):357–362. doi: 10.1016/0006-8993(78)90643-1. [DOI] [PubMed] [Google Scholar]
- Spencer H. J. Antagonism of cortical excitation of striatal neurons by glutamic acid diethyl ester: evidence for glutamic acid as an excitatory transmitter in the rat striatum. Brain Res. 1976 Jan 30;102(1):91–101. doi: 10.1016/0006-8993(76)90577-1. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Blockade by amino acid antagonists of neuronal excitation mediated by the pyramidal tract. J Physiol. 1976 May;257(1):187–198. doi: 10.1113/jphysiol.1976.sp011363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T. W. Cortical pyramidal tract interneurones and their sensitivity to L-glutamic acid. J Physiol. 1973 Aug;233(1):211–225. doi: 10.1113/jphysiol.1973.sp010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T. W. Cortical responses to pyramidal tract stimulation in the rat. Exp Neurol. 1972 Jun;35(3):492–502. doi: 10.1016/0014-4886(72)90119-7. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Glutamate as the neurotransmitter of cerebellar granule cells in the rat: electrophysiological evidence. Br J Pharmacol. 1979 Jun;66(2):291–296. doi: 10.1111/j.1476-5381.1979.tb13678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T. W. Selective antagonism of amino acids by alpha-aminoadipate on pyramidal tract neurones but not Purkinje cells. Brain Res. 1979 Apr 20;166(1):217–220. doi: 10.1016/0006-8993(79)90668-1. [DOI] [PubMed] [Google Scholar]



