Abstract
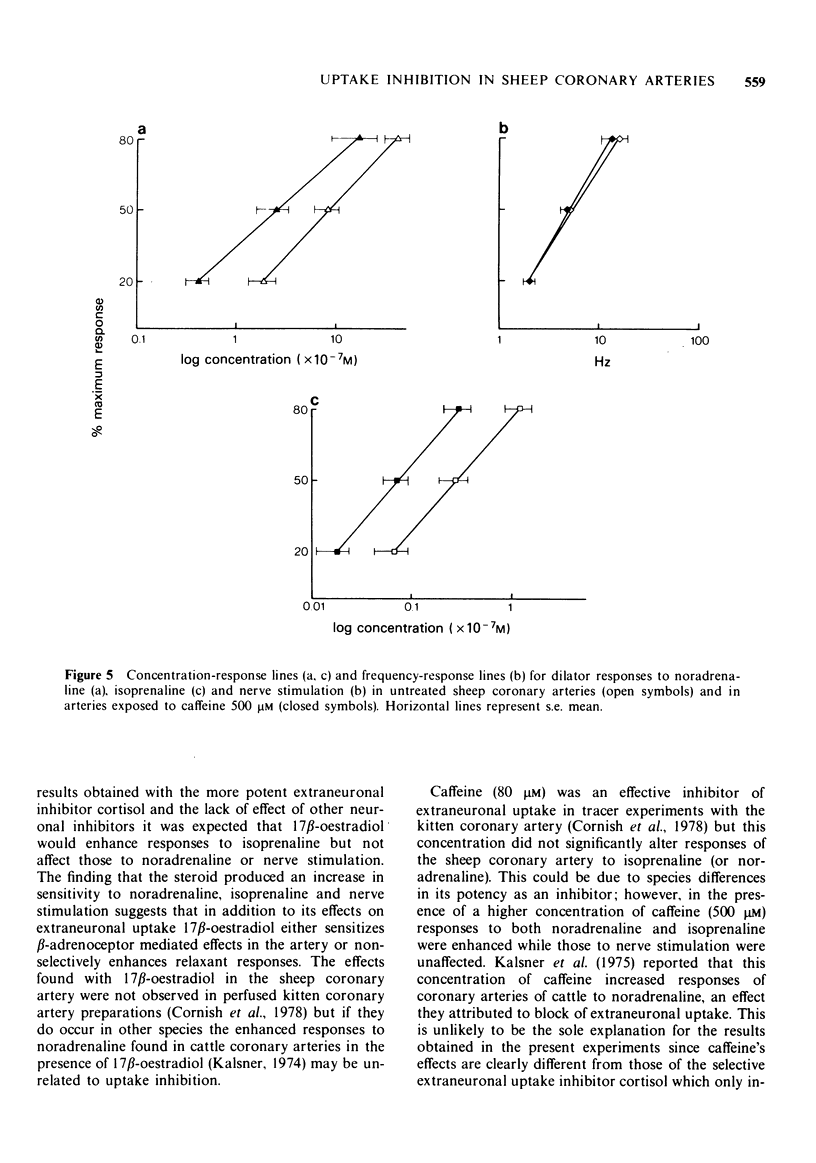
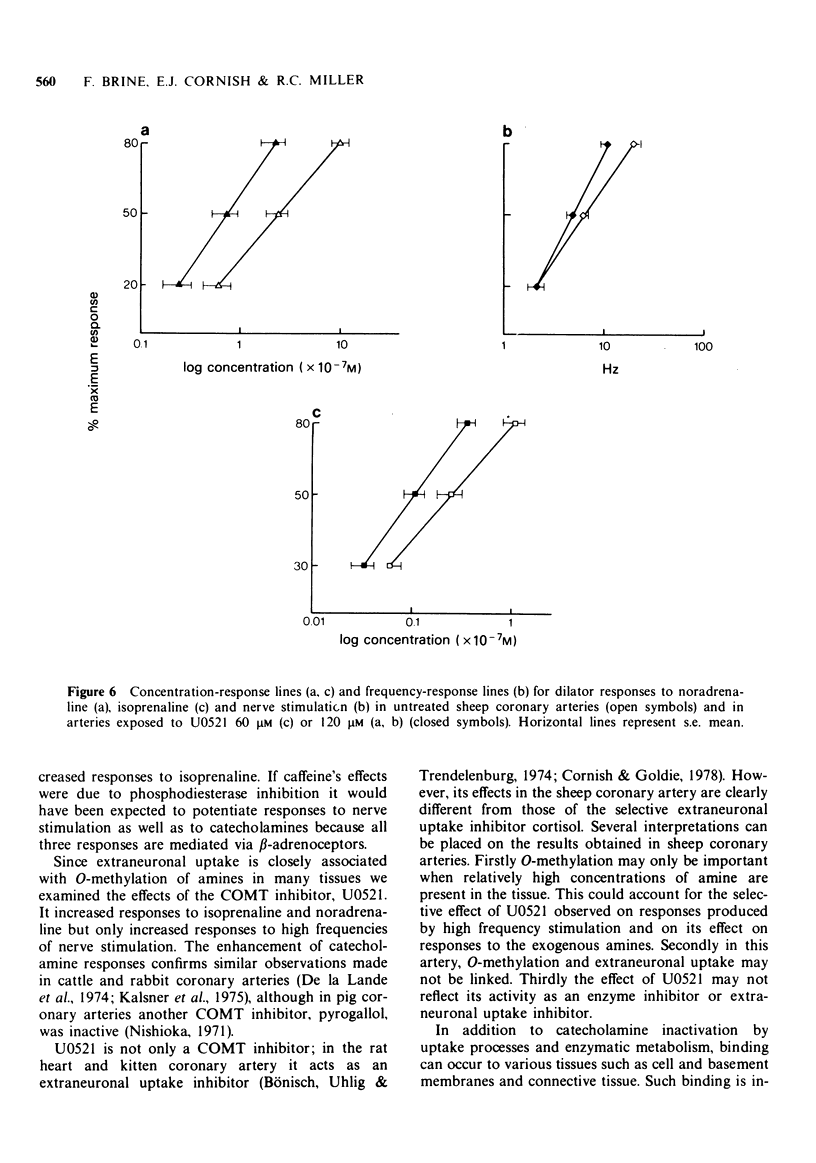
1. Transmural stimulation of intrinsic sympathetic nerves and exogenous catecholamines produce beta 1-adrenoceptor mediated relaxant responses in strips of contracted sheep coronary artery. 2. The neuronal uptake inhibitors, metaraminol, cocaine and desipramine and the extraneuronal uptake inhibitor, cortisol, failed to potentiate responses to noradrenaline or sympathetic stimulation; responses to isoprenaline were enhanced by cortisol. 3. Oxytetracycline, which inhibits binding to connective tissue fibres, did not affect responses to noradrenaline or nerve stimulation. 4. 17 beta-Oestradiol, caffeine and U0521 proved to be unsuitable compounds for studying catecholamine inactivation since they non-selectively potentiated responses to noradrenaline and isoprenaline. 5. It is concluded that catecholamine inactivation processes do not modify transmitter function in sheep coronary arteries.
Full text
PDF

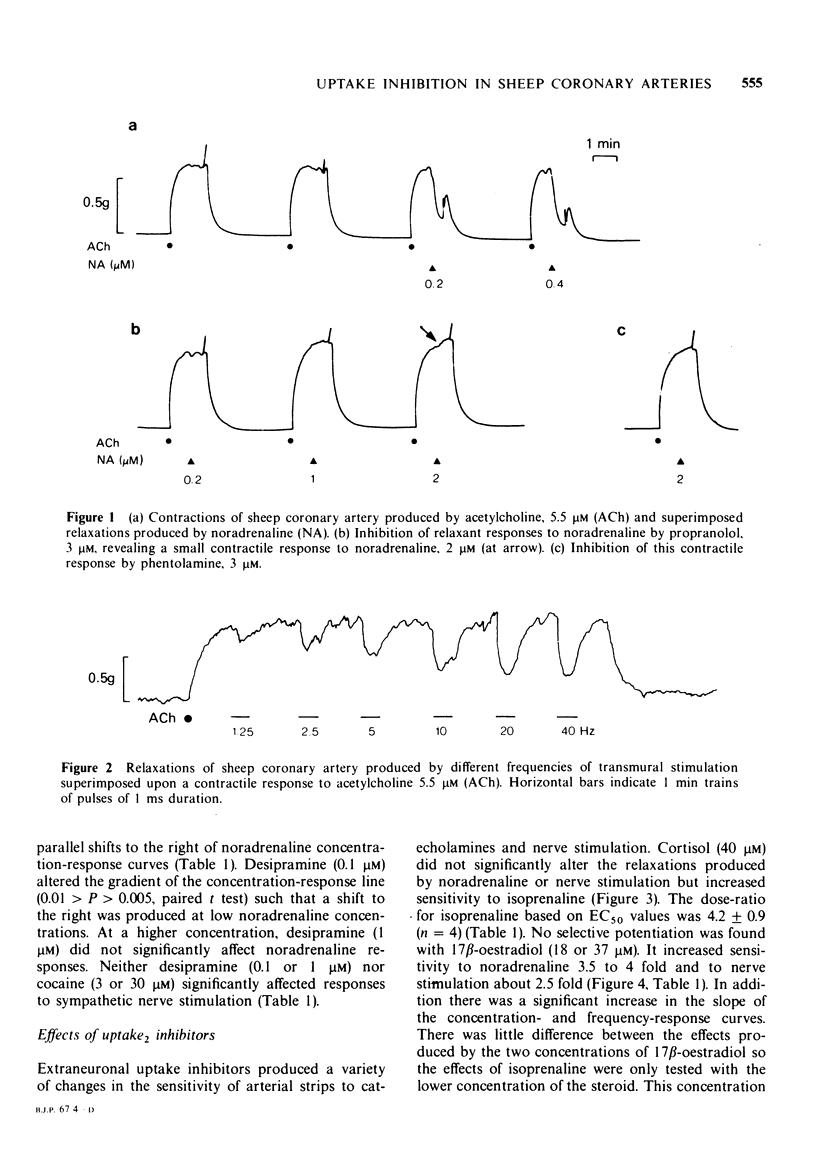
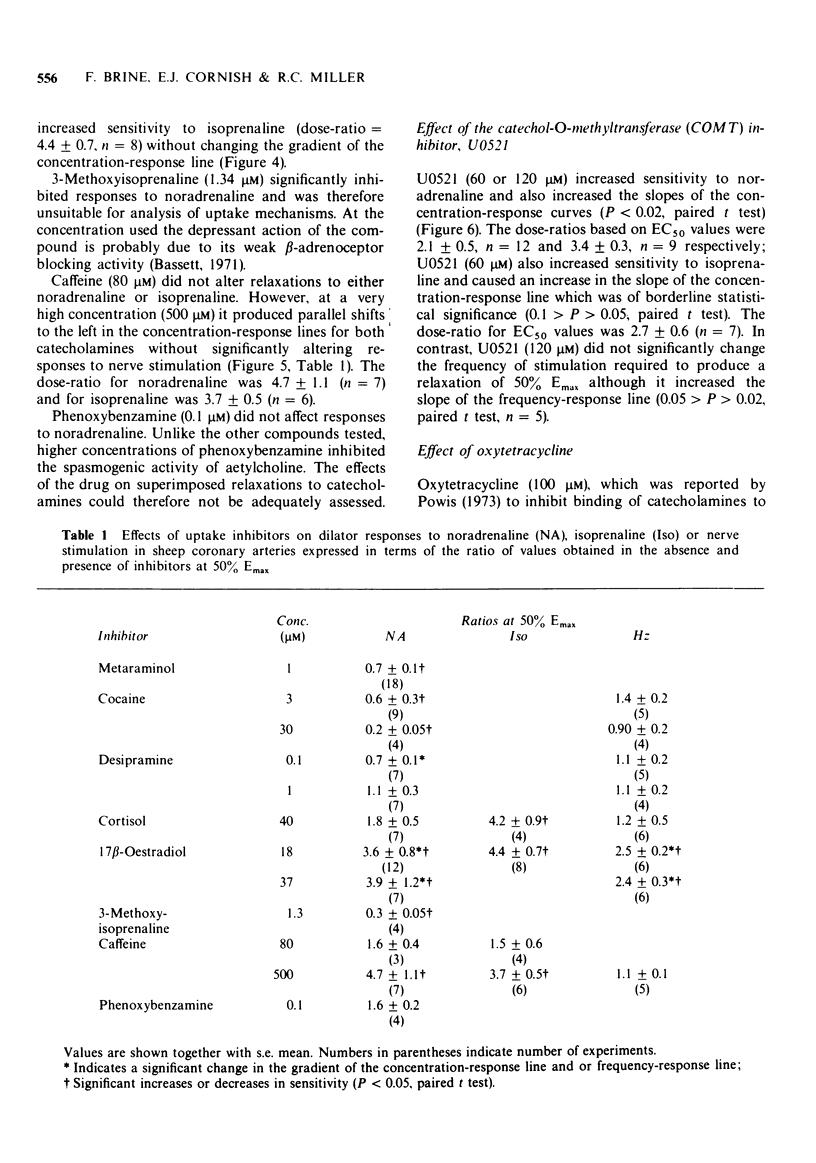
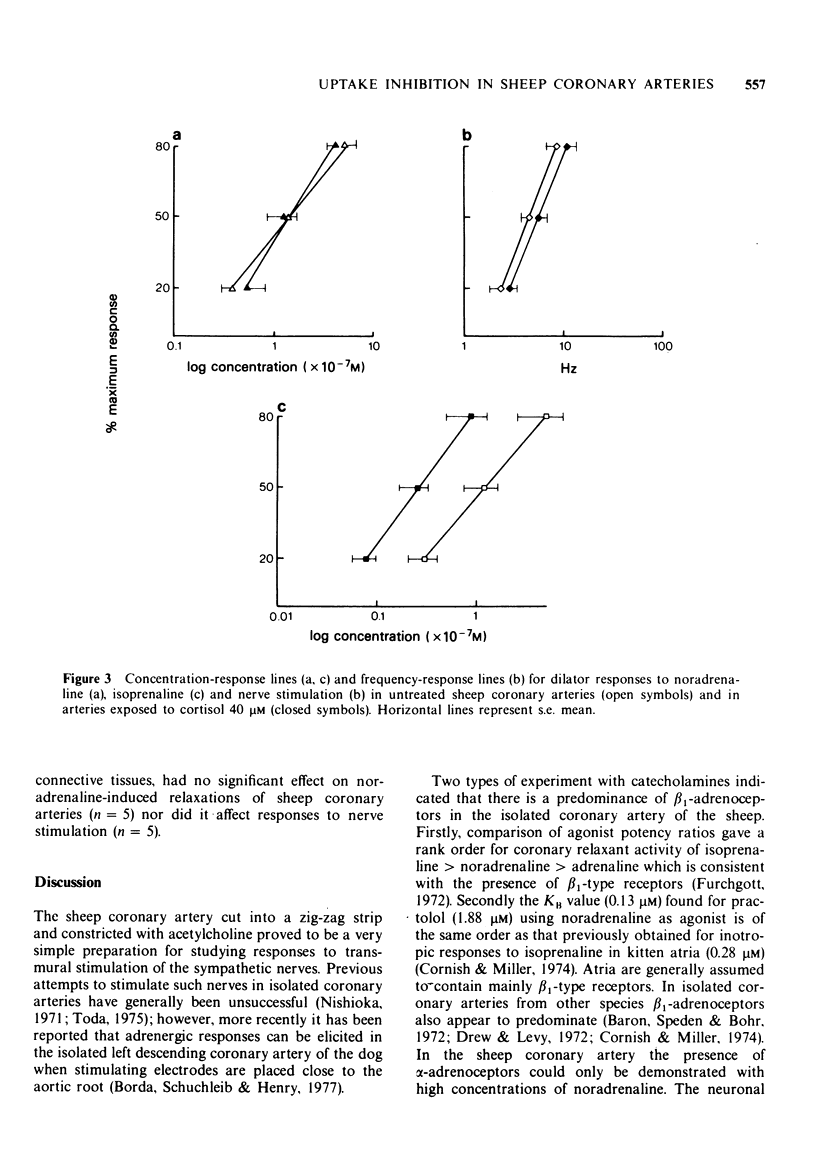
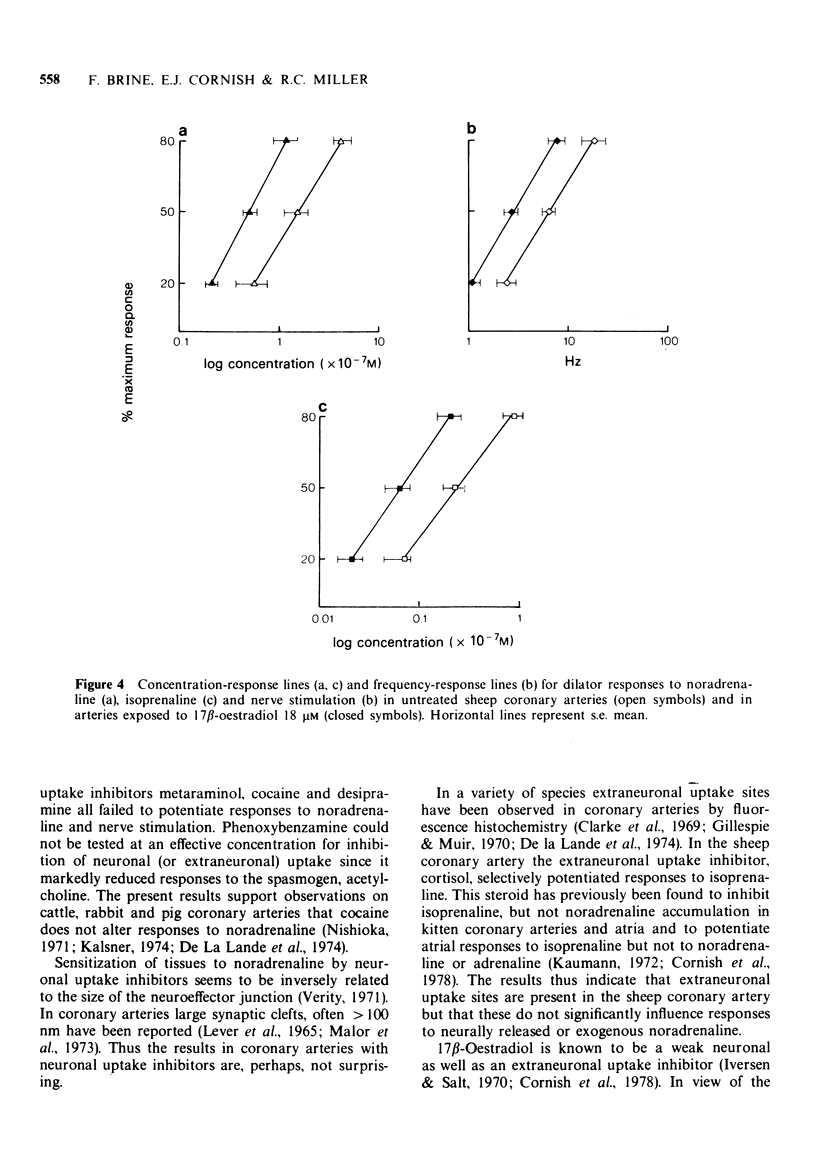

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron G. D., Speden R. N., Bohr D. F. Beta-adrenergic receptors in coronary and skeletal muscle arteries. Am J Physiol. 1972 Oct;223(4):878–881. doi: 10.1152/ajplegacy.1972.223.4.878. [DOI] [PubMed] [Google Scholar]
- Bassett J. R. Beta-adrenoceptor antagonist activity of 3-methoxyisoprenaline. Br J Pharmacol. 1971 Jan;41(1):113–121. doi: 10.1111/j.1476-5381.1971.tb09941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borda L., Shuchleib R., Henry P. D. Effects of potassium on isolated canine coronary arteries. Modulation of adrenergic responsiveness and release of norepinephrine. Circ Res. 1977 Dec;41(6):778–786. doi: 10.1161/01.res.41.6.778. [DOI] [PubMed] [Google Scholar]
- Bönisch H., Uhlig W., Trendelenburg U. Analysis of the compartments involved in the extraneuronal storage and metabolism of isoprenaline in the perfused heart. Naunyn Schmiedebergs Arch Pharmacol. 1974;283(3):223–244. doi: 10.1007/BF00499185. [DOI] [PubMed] [Google Scholar]
- Callingham B. A., Burgen A. S. The uptake of isoprenaline and noradrenaline by the perfused rat heart. Mol Pharmacol. 1966 Jan;2(1):37–42. [PubMed] [Google Scholar]
- Clarke D. E., Jones C. J., Linley P. A. Histochemical fluorescence studies on noradrenaline accumulation by Uptake 2 in the isolated rat heart. Br J Pharmacol. 1969 Sep;37(1):1–9. doi: 10.1111/j.1476-5381.1969.tb09515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish E. J., Goldie R. G., Miller R. C. Catecholamine uptake by isolated coronary arteries and atria of the kitten. Br J Pharmacol. 1978 Jul;63(3):445–456. doi: 10.1111/j.1476-5381.1978.tb07796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denn M. J., Stone H. L. Automic innervation of dog coronary arteries. J Appl Physiol. 1976 Jul;41(1):30–35. doi: 10.1152/jappl.1976.41.1.30. [DOI] [PubMed] [Google Scholar]
- Drew G. M., Levy G. P. Characterization of the coronary vascular -adrenoceptor in the pig. Br J Pharmacol. 1972 Oct;46(2):348–350. doi: 10.1111/j.1476-5381.1972.tb06880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Muir T. C. Species and tissue variation in extraneuronal and neuronal accumulation of noradrenaline. J Physiol. 1970 Mar;206(3):591–604. doi: 10.1113/jphysiol.1970.sp009032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Salt P. J. Inhibition of catecholamine Uptake-2 by steroids in the isolated rat heart. Br J Pharmacol. 1970 Nov;40(3):528–530. doi: 10.1111/j.1476-5381.1970.tb10637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsner S., Frew R. D., Smith G. M. Mechanism of methylxanthine sensitization of norepinephrine responses in a coronaryartery. Am J Physiol. 1975 Jun;228(6):1702–1707. doi: 10.1152/ajplegacy.1975.228.6.1702. [DOI] [PubMed] [Google Scholar]
- Kalsner S. Sensitization of noradrenaline responses by inhibitors of extraneuronal uptake in a coronary artery preparation. Br J Pharmacol. 1974 Jul;51(3):453–455. doi: 10.1111/j.1476-5381.1974.tb10682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaumann A. J. Potentiation of the effects of isoprenaline and noradrenaline by hydrocortisone in cat heart muscle. Naunyn Schmiedebergs Arch Pharmacol. 1972;273(1):134–153. doi: 10.1007/BF00508086. [DOI] [PubMed] [Google Scholar]
- Lever J. D., Ahmed M., Irvine G. Neuromuscular and intercellular relationships in the coronary arterioles. A morphological and quantitative study by light and electron microscopy. J Anat. 1965 Oct;99(Pt 4):829–840. [PMC free article] [PubMed] [Google Scholar]
- MCEWEN L. M. The effect on the isolated rabbit heart of vagal stimulation and its modification by cocaine, hexamethonium and ouabain. J Physiol. 1956 Mar 28;131(3):678–689. doi: 10.1113/jphysiol.1956.sp005493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malor R., Griffin C. J., Taylor S. Innervation of the blood vessels in guinea-pig atria. Cardiovasc Res. 1973 Jan;7(1):95–104. doi: 10.1093/cvr/7.1.95. [DOI] [PubMed] [Google Scholar]
- Mylecharane E. J., Raper C. Influence of N-alkyl substitution on antagonism at 1 - and 2 -receptor sites. Eur J Pharmacol. 1973 Mar;21(3):375–378. doi: 10.1016/0014-2999(73)90142-8. [DOI] [PubMed] [Google Scholar]
- Nishioka M. Pharmacological responses of the smooth muscle of the pig's excised coronary artery, especially in relation to Ca. Kobe J Med Sci. 1971 Sep;17(3):129–159. [PubMed] [Google Scholar]
- Powis G. Binding of catecholamines to connective tissue and the effect upon the responses of blood vessels to noradrenaline and to nerve stimulation. J Physiol. 1973 Oct;234(1):145–162. doi: 10.1113/jphysiol.1973.sp010339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda N. Regional differences in the response to nicotine in isolated canine arteries. Eur J Pharmacol. 1976 Jan;35(1):151–160. doi: 10.1016/0014-2999(76)90310-1. [DOI] [PubMed] [Google Scholar]
- de la Lande I., Harvey J. A., Holt S. Response to the rabbit coronary arteries to autonomic agents. Blood Vessels. 1974;11(5-6):319–337. doi: 10.1159/000158025. [DOI] [PubMed] [Google Scholar]


