Abstract
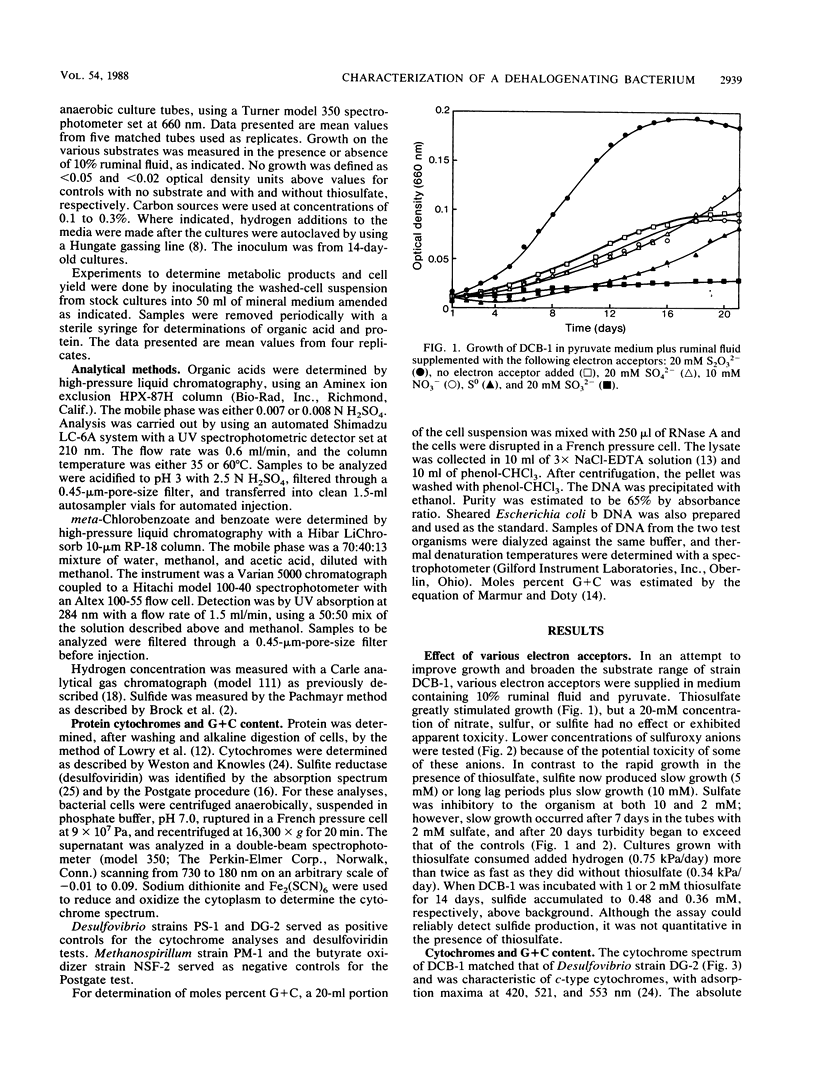
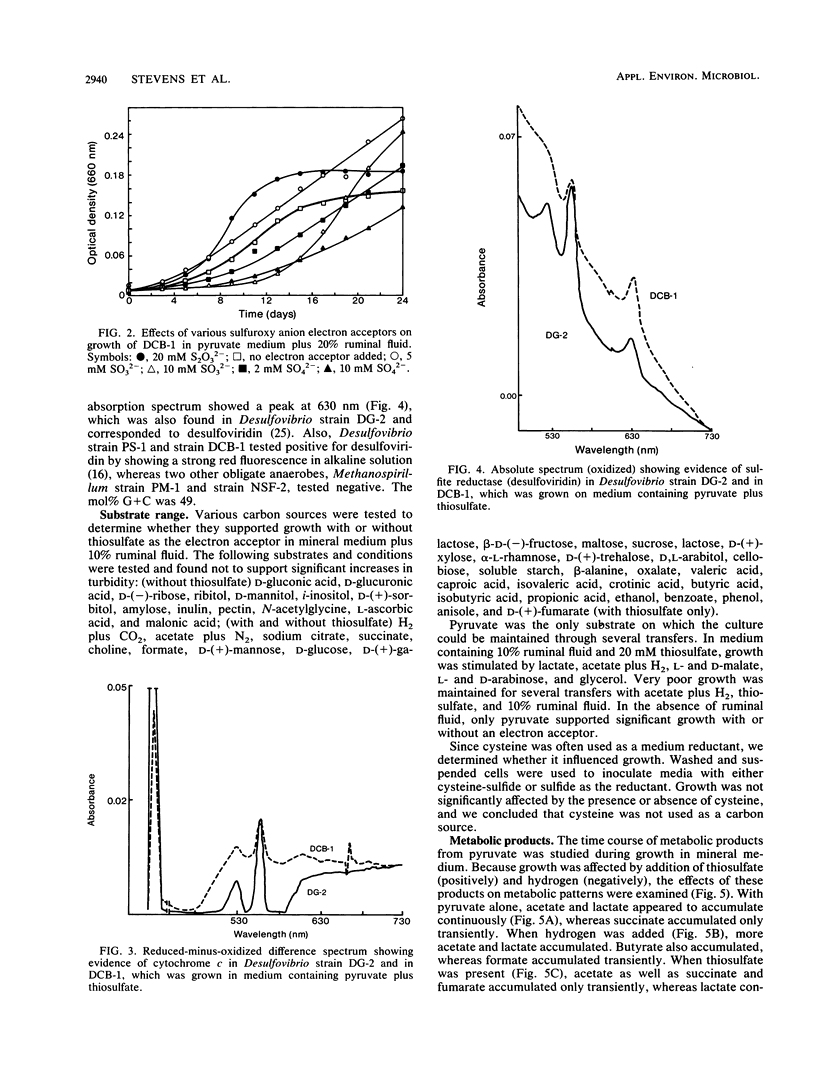
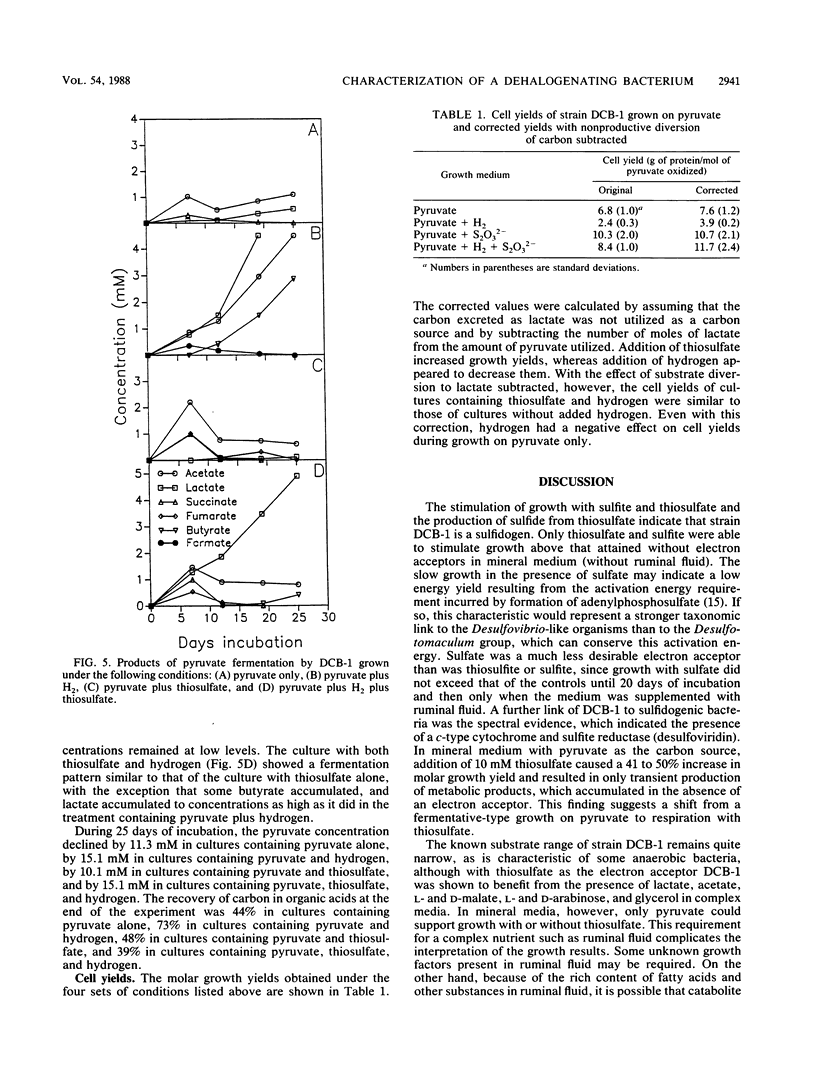
Strain DCB-1 is an obligately anaerobic bacterium which carries out the reductive dehalogenation of halobenzoates and was previously known to grow only on pyruvate plus 20% ruminal fluid. When various electron acceptors were supplied, thiosulfate and sulfite were found to stimulate growth. Sulfide was produced from thiosulfate. Cytochrome c and desulfoviridin were detected. The mol% G+C was 49 (at the thermal denaturation temperature). Of 55 carbon sources tested, only pyruvate supported growth as the sole carbon source in mineral medium. Lactate, acetate, L- and D-malate, glycerol, and L- and D-arabinose stimulated growth when supplemented with 10% ruminal fluid and 20 mM thiosulfate. In mineral medium, pyruvate was converted to acetate and lactate, with small amounts of succinate and fumarate accumulating transiently. During growth with thiosulfate, all of these products accumulated transiently. Addition of excess hydrogen to pyruvate-grown cultures resulted in diversion of carbon to formate, lactate, and butyrate, which caused a decrease in cell yield. We conclude that strain DCB-1 is a new type of sulfidogenic bacterium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock T. D., Brock M. L., Bott T. L., Edwards M. R. Microbial life at 90 C: the sulfur bacteria of Boulder Spring. J Bacteriol. 1971 Jul;107(1):303–314. doi: 10.1128/jb.107.1.303-314.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolfing J., Tiedje J. M. Growth yield increase linked to reductive dechlorination in a defined 3-chlorobenzoate degrading methanogenic coculture. Arch Microbiol. 1987;149(2):102–105. doi: 10.1007/BF00425073. [DOI] [PubMed] [Google Scholar]
- Dwyer D. F., Tiedje J. M. Metabolism of polyethylene glycol by two anaerobic bacteria, Desulfovibrio desulfuricans and a Bacteroides sp. Appl Environ Microbiol. 1986 Oct;52(4):852–856. doi: 10.1128/aem.52.4.852-856.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis A. J., Miller J. D. The tricarboxylic and acid pathway in Desulfovibrio. Can J Microbiol. 1977 Jul;23(7):916–921. doi: 10.1139/m77-135. [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl Environ Microbiol. 1982 Mar;43(3):552–560. doi: 10.1128/aem.43.3.552-560.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. A diagnostic reaction of Desulphovibrio desulphuricans. Nature. 1959 Feb 14;183(4659):481–482. doi: 10.1038/183481b0. [DOI] [PubMed] [Google Scholar]
- Peck H. D., Jr, LeGall J. Biochemistry of dissimilatory sulphate reduction. Philos Trans R Soc Lond B Biol Sci. 1982 Sep 13;298(1093):443–466. doi: 10.1098/rstb.1982.0091. [DOI] [PubMed] [Google Scholar]
- Robinson J. A., Tiedje J. M. Kinetics of hydrogen consumption by rumen fluid, anaerobic digestor sludge, and sediment. Appl Environ Microbiol. 1982 Dec;44(6):1374–1384. doi: 10.1128/aem.44.6.1374-1384.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. Isolation and partial characterization of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic Acid. Appl Environ Microbiol. 1984 Oct;48(4):840–848. doi: 10.1128/aem.48.4.840-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin Iu I. Istochniki energii i igleroda dlia biosinteza u sul'fatredutsiruiushchikh bakterii. Mikrobiologiia. 1966 Sep-Oct;35(5):761–766. [PubMed] [Google Scholar]
- Stevens T. O., Tiedje J. M. Carbon dioxide fixation and mixotrophic metabolism by strain DCB-1, a dehalogenating anaerobic bacterium. Appl Environ Microbiol. 1988 Dec;54(12):2944–2948. doi: 10.1128/aem.54.12.2944-2948.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedje J. M., Stevens T. O. The ecology of an anaerobic dechlorinating consortium. Basic Life Sci. 1988;45:3–14. doi: 10.1007/978-1-4899-0824-7_1. [DOI] [PubMed] [Google Scholar]
- Weston J. A., Knowles C. J. A soluble CO-binding c-type cytochrome from the marine bacterium Beneckea natriegens. Biochim Biophys Acta. 1973 Apr 27;305(1):11–18. doi: 10.1016/0005-2728(73)90226-0. [DOI] [PubMed] [Google Scholar]


