Abstract
The T cell receptor (TCR) α/δ locus is composed of a common, shared set of variable (V) and distinct diversity (D), joining (J), and constant (C) genes. It has been recognized for several years that transcripts of the rearranged VDJδ or VJα genes are spliced to the Cδ or Cα genes, respectively, encoding distinct TCR δ and α proteins. Herein, we describe the discovery of a splicing variation that allows the assembled VDJδ genes to be fused with the Cα gene. This variation is prominent in TCRδ gene-deficient mice but is also detectable in wild-type mice. Furthermore, we show that several in-frame VDJδ rearrangements in TCRδ gene-deficient mice are strikingly underrepresented, suggesting that the alternative transcripts, with protein coding capacity, influence the development of αβ thymocytes. In-frame TCRγ gene rearrangements do not appear underrepresented, indicating that the effect is not mediated by the γ chain. Instead, indirect evidence supports the hypothesis that the δ/α chimeric protein acts in conjunction with the TCRβ chain. These results have implications for the transcriptional control of the TCRα/δ locus and provide a novel insight into the distinct functional capacities of the TCR α and δ proteins during thymocyte development.
T cell receptor (TCR) and Ig (Ig) genes are assembled from separate variable (V), diversity (D) and joining (J) elements by a site-specific recombination mechanism termed V(D)J recombination (1). The assembled V(D)J complex is transcribed and spliced to a separate gene encoding the constant (C) domain of the particular antigen receptor. The TCR α and δ genes form a single complex locus (2), spanning approximately 1 megabase (3) on chromosome 14 (4) in the mouse. The locus is composed of more than 100 V genes (3), followed in the 3′ direction by D/J/Cδ, the 61 Jα and the Cα genes (ref. 5 and see Fig. 1a). Despite the intimate chromosomal context, the α and δ genes encode proteins participating exclusively in the αβ or γδ TCR complexes, which in turn are expressed in the two separate lineages of αβ and γδ T cells, respectively (6). Extensive TCR α gene rearrangements occur predominantly in αβ T cells (7, 8), whereas VDJδ and γ rearrangements have been found in the majority of T cells of both lineages (9–11). Sequence analysis of several TCR γ and δ gene rearrangements revealed that significantly less than the expected 33% random proportion of in-frame VDJ joints are present in αβ T cells (11–13). These results have prompted a model in which αβ and γδ T cells share a common intrathymic precursor and generation of a γδ TCR biases development toward the γδ lineage (11, 12).
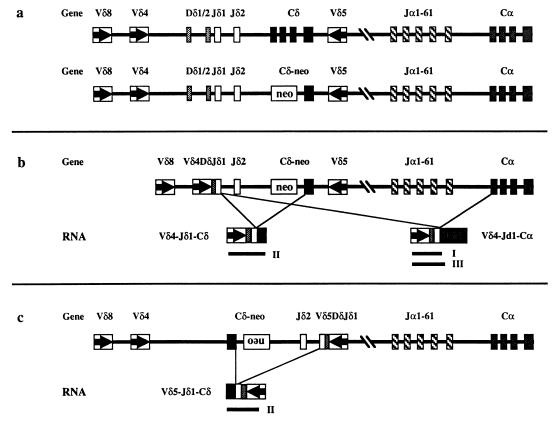
Figure 1.
(a) Schematic map of the TCRα/δ locus (not to scale). Boxes represent the various coding sequences. For the C genes, boxes are used to indicate each of the four exons. Arrows within the V gene boxes indicate transcriptional orientation. The top and bottom lines depict the wild-type and TCRδ-deficient loci, respectively. (b) An example of a V gene (Vδ4) rearrangement that can generate VDJδ1-Cα transcripts on the TCRδ-deficient locus. Lines indicate the two potential splicing events that can give rise to the two distinct mRNA species shown below the locus. Solid lines indicate the three RT–PCR products (marked as I, II, and III) that are detected in Fig. 4. (c) An example of a V gene (Vδ5) rearrangement that cannot generate VDJδ1-Cα transcripts on the TCRδ-deficient locus. Note the reverse transcriptional orientation of the assembled Vδ5DJδ1-Cδ region generated by the chromosomal inversion. Lines indicate the only potential splicing event that can give rise to the Vδ5DJδ1-Cδ mRNA shown below the locus and the solid line indicates the only RT–PCR product (marked as II) that can be detected (data not shown).
Herein, we describe the alternative splicing of VDJδ transcripts to the Cα gene. This phenomenon has been observed in both TCRδ-deficient and, to a lesser extent, in wild-type mice. The putative chimeric protein translated from this novel message would consist of the variable domain of TCRδ and the constant domain of the α chain. We show that, in contrast to the expected random distribution, several in-frame VDJδ joints are strikingly underrepresented in TCRδ-deficient mice, suggesting that these chimeric proteins exert a negative effect on αβ thymocyte development. Finally, we demonstrate that this effect is probably mediated by a novel TCR complex between the chimeric protein and the TCRβ rather than the γ chain.
MATERIALS AND METHODS
Animals.
Four- to 8-week-old C57BL/6 wild-type mice, TCRδ- and TCRβxδ-deficient (both on C57BL/6 background) mice were obtained from The Jackson Laboratories and maintained at Yale University animal facilities.
Flow Cytometric Analysis.
Flow cytometric analyses were performed as described (14). For separation of CD4/CD8 double-negative (DN) subsets, cells were stained with a combination of biotinylated anti-mouse CD4 (clone H129.19) and anti-mouse CD8a (clone 53–6.7) antibodies; depleted with magnetic-bead-conjugated anti-rat IgG (PerSeptive Diagnostics, Framingham, MA), stained with streptavidin pallophycocyanin, anti-mouse heat-stable antigen (HSA), anti-mouse CD44, and anti-mouse CD25 (all from PharMingen); and electronically sorted on FACStar Plus (Beckton Dickinson).
DNA PCR and PCR Restriction Fragment Length Polymorphism (PCR RFLP) Analyses.
High molecular weight DNA prepared (15) from various thymocyte populations was amplified for 30 cycles with Taq DNA polymerase. PCR RFLP analysis (14) was performed with reverse primers specific for Jδ1, Jγ1, and Jγ4 genes (11, 14) that were 5′-end-labeled with [γ-32P]ATP and T4 polynucleotide kinase and V gene-specific forward primers (11, 14, 16) in a thermocycler. The purified PCR products were digested with the indicated restriction enzymes and separated on 6% denaturing polyacrylamide gels. The gels were imaged with a PhosphorImager and the relative intensity of the rearranged bands was calculated with imagequant 3.0 software (Molecular Dynamics).
Reverse Transcriptase (RT) PCR Analysis.
Total cellular RNA was prepared from various thymocyte populations by using the Nonidet P-40 detergent lysis method (15). The RNA was reverse-transcribed into cDNA with random hexamers by using Superscript II RT enzyme (GIBCO/BRL) and amplified for 30–35 cycles with Taq DNA polymerase. The forward V gene-specific primers were used in combination with reverse primers of the following sequences: 3′CαJδ1, 5′-GGTTCTGAATTCTGGATGTTTGGTTC (note that the first 19 nucleotides correspond to Cα and the last 7 nucleotides correspond to Jδ1); 3′Cδ, 5′-GCGGATCCATGCTCGCCTCAGGAGA (corresponding to the last exon of the Cδ gene, which remains intact in both wild-type and TCRδ-deficient mice); and 3′Cα, 5′-CGGAATTCTAGAGGCAATGGCCCCATTGCTCTTG. The PCR products were analyzed by Southern blotting (15) and high-stringency hybridization with a radiolabeled oligonucleotide probe corresponding to the Jδ1 gene. Some VDJδ1 PCR products were subcloned into pBluescript (Stratagene) and the bacterial colonies were screened with radiolabeled oligonucleotide probes corresponding to Jδ1 (identical to the 3Jδ1 primer, ref. 11) or Cα (identical to the 3′Cα primer). Control hybridization experiments demonstrated that the probes were specific for the Jδ1 or Cα genes (data not shown). Individual subclones were sequenced by using the Applied Biosystems model 373 automated sequencer. All sequences derived from subclones generated with RT–PCR using the 3′CαJδ1 primer contained the Jδ1 gene rearranged to the expected Vδ gene.
RESULTS
Selection Against In-Frame VDJδ Rearrangements in TCRδ-Deficient Thymocytes.
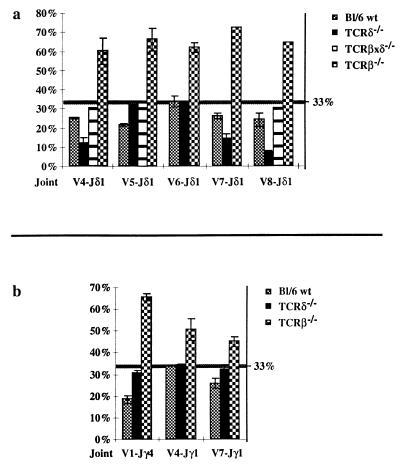
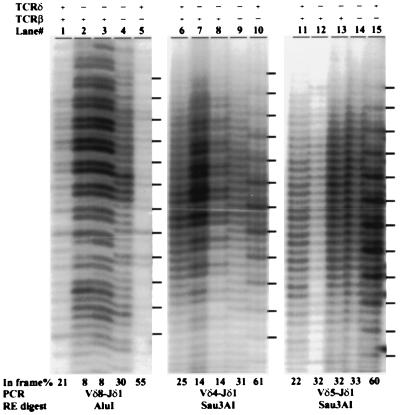
We have been studying selection events mediated by the TCR during thymocyte development with a PCR RFLP technique (12, 14, 17). With this method it is possible to quantitatively determine the extent of selection of a particular TCR in a given cell population by comparing the relative proportion of in-frame and out of frame V(D)J joints. If no selection occurs, the proportion of in-frame V(D)J joints should theoretically be 33%. If development of the cell population requires the expression of the given TCR, in-frame joints should represent more than 33% (typically 60–70%) of the analyzed V(D)J rearrangements (17). In contrast, if expression of the TCR negatively affects lineage choice (11–13) or development (14) of the given cell population, a percentage of in-frame joints of less than 33% can be observed. As part of our study of αβ/γδ T cell lineage commitment, we have been analyzing TCR γ and δ gene rearrangements in various thymocyte populations. As one control for random rearrangements of these loci, we used TCRδ gene-deficient mice, in which no VDJ-Cδ protein can be produced due to the targeted deletion of all three coding exons of the Cδ gene (ref. 18 and see Fig. 1a). Because in these mice, no TCRγδ immunoreactivity has been detected, it has been assumed that no functional γδ TCR can be produced despite the fact that both TCR γ and δ locus rearrangements proceed normally (ref. 18 and data not shown). To our surprise, however, we found significant depletion of in-frame Vδ4, Vδ7, and Vδ8 to Jδ1 rearrangements from total thymus DNA of several TCRδ-deficient mice (Fig. 2a; for representative samples see Fig. 3, lanes 2, 3, 7, and 8). The level of depletion was consistently more severe than that observed in wild-type thymocytes (12% vs. 25% for Vδ4, 15% vs. 26% for Vδ7, and 8% vs. 22% for Vδ8; see Fig. 2a). In contrast, Vδ5 and Vδ6 to Jδ1 joints showed no deviation from the expected random 33% proportion of in-frame joints in TCRδ-deficient samples (Fig. 2a; and see Fig. 3, lanes 12 and 13). In-frame Vδ5 (but not Vδ6) rearrangements are depleted in wild-type mice (Fig. 2a and refs. 11, 12, and 14).
Figure 2.
Graphical representation of quantitative analyses of PCR RFLP reactions on various Vδ-DJδ1 (a) and Vγ-Jγ (b) rearrangements in total thymus samples from C57BL/6 wild-type (Bl/6 wt), TCRδ-deficient (δ−/−), TCRβxδ-deficient (βxδ−/−, only for Vδ-DJδ1), and TCRβ-deficient (β−/−) mice. The percent of in-frame rearrangements is shown on the vertical axes. The position of the 33% random level is marked with a horizontal line. The error bars indicate the standard deviation from the mean for each joint where at least two independent samples were analyzed. Some individual samples were measured several times on different gels.
Figure 3.
PCR RFLP analysis of Vδ4, Vδ5, and Vδ8 to DJδ1 rearrangements. DNA samples from total thymi with the indicated genotype (+/+ or −/− for the TCRδ and/or TCRβ loci) were PCR-amplified and digested with the indicated restriction enzymes. Dashes on the right mark the position of the in-frame joints as determined from TCRβ-deficient mice which have only γδ TCR selected cells (14). The percentage of in-frame rearrangements is shown below each lane. Two individual TCRδ samples are shown.
Some TCRγ gene rearrangements also exhibit a depletion of in-frame rearrangements in total wild-type thymus DNA (12, 13), due to the requirement of both TCR γ and δ proteins to form a functional γδ TCR complex (11, 12). However, when we analyzed TCRγ gene rearrangements in TCRδ-deficient mice, we observed no significant depletion of in-frame Vγ1-Jγ4 and Vγ4 or Vγ7 to Jγ1 joints (31%, 34%, and 32%, respectively; see Fig. 2b). These results suggest that the TCRγ locus, which is intact in TCRδ-deficient mice, does not significantly contribute to the observed depletion of in-frame TCRδ rearrangements. This in turn implies that there is indeed no functional γδ TCR complex formed in these mice (18) and that the unexpected behavior of the TCRδ locus is due either to an intrinsic property of the locus itself or to a TCR complex formed with another TCR chain.
To test the role of the only available candidate, the TCRβ protein, we studied CD25+, HSAhi, and CD4/CD8 DN immature thymocytes from wild-type and TCRδ-deficient mice and unseparated thymocytes from TCRβxδ-deficient mice (in which essentially all thymocytes are CD25+ DN). PCR RFLP analysis of Vδ4 and Vδ8 to Jδ1 rearrangements showed no significant deviation from the expected random 33% proportion of in-frame joints in any of DN cell populations (data not shown for wild-type and TCRδ-deficient cells; see Figs. 2 and 3 for TCRβxδ-deficient thymus). Because no thymocytes in the TCRβxδ-deficient mice (19) and only a small fraction of CD25+ HSAhi DN thymocytes in wild-type or TCRδ-deficient mice are expected to express the TCRβ protein (20), the above results are consistent with the hypothesis that depletion of in-frame TCRδ rearrangements in TCRδ-deficient mice is dependent on or at least coincident with expression of the TCRβ protein.
Detection of VDJδ Transcripts Spliced to the Cα Gene.
Depletion of in-frame VDJδ joints in thymocytes of TCRδ-deficient mice strongly suggests selection against TCRδ locus-derived protein products. Because no Cδ domain can be produced in these mice (18), we propose that a different protein must be translated from the rearranged TCRδ messages during thymocyte differentiation. One potential protein product, which can be predicted from the structure of the locus, would be generated by translation of transcripts of rearranged VDJδ sequences spliced to the first exon of the Cα gene (see Fig. 1b). One appealing feature of this hypothesis is that it provides an explanation for the lack of depletion of in-frame Vδ5 gene rearrangements in TCRδ-deficient mice (Fig. 2a and 3). Because Vδ5, in contrast to all other Vδ genes, rearranges to Jδ1 with inversion (21, 22), the transcriptional orientation of Vδ5-Jδ1 is incompatible with splicing to Cα (Fig. 1c).
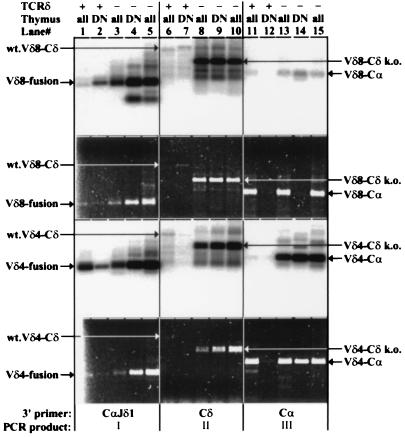
We used an RT–PCR assay to attempt to identify alternatively spliced transcripts containing VDJδ and Cα sequences (Fig. 4). Three reverse primers (described below) were used in combination with forward Vδ gene-specific primers. The RT–PCR products were visualized with ethidium bromide staining (Fig. 4, second and fourth panels from the top) and by high-stringency hybridization to an oligonucleotide probe that specifically detects Jδ1 sequences (Fig. 4, first and third panels from the top).
Figure 4.
RT–PCR analysis of VDJδ transcripts from wild-type (TCRδ+) and TCRδ-deficient (TCRδ−) total (all) or sorted HSAhi, CD25+, CD4/CD8 double negative (DN) thymocytes. The first and third panels from the top show hybridization to a Jδ1 gene-specific oligonucleotide probe. The second and fourth panels show the corresponding gels stained with ethidium bromide and visualized with UV illumination. Two independent TCRδ total thymus samples are shown. The top two panels show results obtained with a Vδ8-specific forward primer, and the bottom two show results with a Vδ4-specific forward primer. Lanes: 1–5, PCRs performed with the 3′CαJδ1 hybrid reverse primer; 6–10, PCRs performed with a Cδ reverse primer; 11–15, PCRs performed with a Cα reverse primer. The predominant PCR product (I, II, or III, as defined in Fig. 1) for each group of five lanes is indicated at the bottom. The discrepancy observed between the hybridization and ethidium staining signals for Vδ8 RT–PCR reactions in lanes 1 vs. 2, lanes 4 vs. 5, and lanes 13 and 15 vs. 14 is probably due to mispriming (lanes 1–5) or priming (lanes 13–15) of RT–PCR from VJα-Cα templates abundant in total thymus but absent from DN thymus templates. Note the difference in the size of the Cδ-specific RT–PCR products between wild-type and TCRδ-deficient mice due to the targeted deletion of the Cδ sequences. The identity of these bands was assigned based on their expected sizes and for the Vδ4-Cδ products from TCRδ-deficient samples was also confirmed by DNA sequencing.
To detect the potential Jδ1-Cα hybrid transcripts with high sensitivity, we devised a reverse primer (3′CαJδ1) that spans the first 19 nucleotides of Cα and the last 7 nucleotides of Jδ1. Only rearranged VDJδ1 transcripts correctly spliced to the first exon of Cα should be amplified with this primer. With this reverse primer and forward primers specific for Vδ4 or Vδ8, PCR products were readily detected from reverse-transcribed RNA from total thymocytes and purified CD25+ HSAhi DN thymocytes from TCRδ-deficient mice (Fig. 4, lanes 3–5; note ethidium bromide-stained visible bands). Furthermore, at least two independent wild-type thymus samples had Vδ4 to Jδ1-Cα hybrid transcripts (Fig. 4, lanes 1 and 2, and data not shown) and one sample had Vδ8 to Jδ1-Cα hybrid transcripts (Fig. 4, lanes 1 and 2). In contrast, and as expected, no hybrid transcripts were detected in the same RNA samples with the Vδ5-specific forward primer and the 3′CαJδ1 reverse primer (data not shown). This demonstrates that the 3′CαJδ1 reverse primer does not inadvertently amplify products from transcripts in which Jδ1 is spliced to sequences other than Cα. Control PCRs performed without addition of RT produced no detectable products in the same hybridization experiments, demonstrating that the RT–PCR products are derived from RNA molecules.
By using a reverse primer (3′Cδ) located within the last exon of Cδ (which is intact in both wild-type and TCRδ-deficient mice), we were able to demonstrate the alternative splicing of several VDJδ complexes to the last exon of Cδ in TCRδ-deficient mice (Fig. 4, lanes 8–10 and data not shown). Notably, the Vδ5 gene-specific primer also detected these products efficiently in TCRδ-deficient mice (data not shown), indicating that transcription and splicing of this V gene is not different from other Vδ genes within the modified Cδ region. In wild-type mice, the same forward primers amplify a larger product, with lower efficiency, corresponding to the VDJ-Cδ transcripts spliced normally to the first exon of Cδ (Fig. 4, lane 6, and data not shown).
Finally, we analyzed the putative VDJδ-Cα hybrid transcripts with a Cα gene-specific reverse primer (3′Cα). In contrast to 3′CαJδ1, use of the 3′Cα primer avoids any bias in favor of amplification of Jδ1-Cα hybrid sequences. Because most Vδ genes, except Vδ5, are also used as Vα genes (ref. 23 and references therein), this reverse primer detects primarily genuine VJα-Cα transcripts from total thymus samples of wild-type and TCRδ-deficient mice. Ethidium bromide staining of Vδ4- and Vδ8-specific RT–PCR reactions confirms the high abundance of V-Cα transcripts in total thymocytes (Fig. 4, lanes 11, 13, and 15). In CD25+, HSAhi DN thymocytes, Vδ8-Cα transcripts are only weakly detected in wild-type and TCRδ-deficient mice, whereas Vδ4-Cα transcripts are abundant in TCRδ-deficient but not wild-type mice (Fig. 4, lanes 12 and 14). Because V-Jα gene rearrangements have not yet begun in this population (7), these products almost certainly derive from VDJδ1-Cα hybrid transcripts (confirmed by the hybridization experiments described below). As expected, no Vδ5-Cα RT–PCR products were detected from any of the RNA samples with the 3′Cα reverse primer (data not shown).
Hybridization of the RT–PCR products to a Jδ1 gene-specific oligonucleotide probe demonstrated the presence of Vδ4DJδ1-Cα hybrid transcripts in TCRδ-deficient mice and, at lower levels, in wild-type mice (Fig. 4, lanes 11–15, third panel from the top). CD25+ HSAhi DN thymocytes from TCRδ-deficient mice also express Vδ8DJδ1-Cα hybrid transcripts (Fig. 4, lane 14). To confirm the specificity of the Jδ1 oligonucleotide hybridization, RT–PCR products for Vδ4 and Vδ8 rearrangements from total thymus and CD25+ DN thymocyte samples were amplified by using the 3′Cα reverse primer (Fig. 4, lanes 11, 12, 13, and 15), subcloned, and screened with the same Jδ1 gene-specific oligonucleotide probe (Table 1). Again, the results indicate that a significant proportion of Vδ4-Cα transcripts from total thymus in TCRδ-deficient mice (13–48%), and a small fraction in wild-type mice (0.2%), represent Vδ4DJδ1-Cα hybrid transcripts. Although very few Jδ1+ Vδ8Jα-Cα transcripts were detected in either wild-type or TCRδ-deficient total thymus samples, wild-type DN thymocytes contained significant proportions of Vδ8- (and fewer Vδ4-) DJδ1-Cα transcripts (Table 1). Direct sequence analysis confirmed the presence of Jδ1 sequences in all 50 of the oligonucleotide-hybridization-positive clones sequenced, and the presence of various Jα sequences in all 12 of the negative RT–PCR clones sequenced (data not shown). This confirms that the Jδ1 oligonucleotide probe is indeed specific for Jδ1 and does not recognize Jα sequences. Importantly, the sequences demonstrated the correct splicing of the last Jδ1 nucleotide to the first nucleotide of the Cα gene in the Jδ1 hybridization positive clones (data not shown).
Table 1.
Fraction of VDJδ1 sequences among RT–PCR-amplified V-Jα transcripts
| Lane no. | Vδ8 RT–PCR subclones
|
Vδ4 RT–PCR subclones
|
||||||
|---|---|---|---|---|---|---|---|---|
| 11 | 12 | 13 | 15 | 11 | 12 | 13 | 15 | |
| Sample | B6 total | B6 DN | δ−/− 1 | δ−/− 2 | B6 total | B6 DN | δ−/− 1 | δ−/− 2 |
| Cα+ | 57 | 71 (90) | 104 | 60 | 156 (165) | 157 (164) | 160 (167) | 233 (233) |
| Jδ1+ | 0 | 25 (44) | 2 | 0 | 3 (12) | 3 (10) | 21 (28) | 111 (111) |
| Ratio, % | <1.8 | 35 | 1.9 | <1.7 | 1.9 | 1.9 | 13 | 48 |
Vδ8-Cα and Vδ4-Cα cDNA sequences from one wild-type (B6 total and B6 DN) and two independent TCRδ deficient (δ−/−) mice were PCR-amplified, subcloned, and screened separately with Cα- and Jδ1-gene-specific oligonucleotide probes under high-stringency conditions. The samples correspond to those shown in Fig. 4, lanes 11, 12, 13 and 15. The number of unique positive, the total number of positive (in parentheses), and the ratio of unique Jδ1+ to Cα+ clones, expressed in percentage, are shown. The number of unique Jδ1+ clones was extrapolated from direct sequencing of 10 B6 total, 16 B6 DN (for Vδ4 and Vδ8 products), and 12 δ−/− 1 and 12 δ−/− 2 individual clones.
DISCUSSION
In the present study, we have identified an alternative splicing event in the TCRα/δ locus that can potentially generate a VDJδ-Cα hybrid polypeptide. We have found that Vδ4, Vδ8 (Fig. 4), and Vδ6 (data not shown) to DJδ1 rearrangements can be transcribed and spliced to the first exon of Cα. Subcloning and colony hybridization experiments demonstrated that 20–50% of Vδ4-Cα transcripts in unseparated TCRδ-deficient mice contain the Jδ1 gene (Table 1) and hence derive from Vδ4DJδ1 rearrangements. In wild-type DN thymocytes only 2% of Vδ4-Cα RT–PCR products, but about one-third of Vδ8-Cα RT–PCR products, contain Jδ1 sequences (Table 1). These observations indicate that large pre-mRNA molecules, approximately 85 kb long, can be transcribed across the TCRα/δ locus and can be spliced precisely to the first exon of Cα. Our data also indicate that hybrid VDJδ1-Cα transcripts are detectable in wild-type mice, especially in sorted DN thymocytes, where V-Jα transcripts are far less abundant. VDJδ1-Cα transcripts are less prevalent in wild-type than in TCRδ-deficient mice. Whether this is due to preferential splicing to the intact first exon of Cδ or to a lower level of primary transcription through the TCRα/δ locus in wild-type mice will need to be addressed in future studies.
It has been established that transcriptional activation (24, 25) and rearrangement (26) of the TCRδ locus precedes that of the TCRα locus (7, 27). Thus it has been assumed that in DN thymocytes, only the D-J-Cδ region is transcriptionally active and the J-Cα portion of the locus is silent. Furthermore, the TCRδ locus becomes transcriptionally inactive after thymocytes progress into the CD4/CD8 double-positive stage, concomitant with the activation of the J-Cα genes (14, 24). Our results indicate, however, that mutually exclusive transcriptional activity of the TCRδ and J-Cα genes is not necessarily the case. (i) The alternative splicing of VDJδ sequences to Cα indicates that both regions of the locus can be accessible simultaneously, at least to the RNA polymerase II complex. (ii) We detect these alternative spliced transcripts in HSAhi, CD25+, DN thymocytes in both wild-type and TCRδ-deficient mice, at a stage when germ-line transcription (24) and rearrangement (7) of the TCRα locus are not observed despite the fact that these cells are actively engaged in VDJ recombination (20). From these data we suggest that the entire TCRα/δ locus can be transcriptionally accessible during early thymocyte differentiation even in the absence of TCRα gene rearrangement. This conclusion is in agreement with accumulating evidence that transcription of a locus per se does not ensure that the locus is accessible to the recombination machinery (see ref. 28).
Another striking observation of our studies is the dramatic under representation of in-frame Vδ4, Vδ7, and Vδ8 to DJδ1 rearrangements in TCRδ-deficient mice (Fig. 2a). This indicates that the variable domain of these joints must have participated in some protein-associated function, the exact biochemical nature of which remains to be determined. We suggest that it is the translation product of the VDJδ-Cα hybrid transcripts we have identified. The Cα gene has the same translational reading frame as the Cδ gene; therefore, splicing of Jδ1 to Cα would result in the same reading frame utilization as with the intact Cδ gene. Another splice acceptor site available in TCRδ-deficient mice is in the last untranslated exon of Cδ; however, splicing of this exon to VDJδ would result in the addition of only 3 amino acids to an in-frame VDJδ protein before reaching a termination codon. We did not attempt to identify the translation product of the VDJδ-Cα hybrid transcripts because the only expected difference from a genuine TCRα protein would be the short internal DJδ1 peptide, which cannot readily be identified with current reagents.
It is likely that different mechanisms are responsible in wild-type and TCRδ-deficient mice for depletion of in frame TCRδ rearrangements in αβ lineage thymocytes. In wild-type mice, both TCRδ and TCRγ in-frame rearrangements are depleted and this has been ascribed to the effect of the γδTCR on T cell lineage commitment (11–13) or on TCRβ rearrangement (14). In TCRδ-deficient mice, however, no depletion of in-frame TCRγ rearrangements is observed (Fig. 2b), arguing that depletion of productive VDJδ joints is TCRγ-independent. One mechanism by which the hybrid VDJδ-Cα TCR might exert a negative effect on αβ lineage precursors is by pairing with TCRβ. That this is possible is suggested by the finding that TCR β and δ polypeptides can pair and be transported to the cell surface with CD3 (29). Pairing of the VDJδ-Cα chimeric protein with TCRβ would be expected to displace the pre-Tα chain from the pre-TCR complex, perhaps leading to a failure of αβ precursors to proliferate or to survive the DN to DP transition (20). This in turn would allow the preferential expansion of αβ lineage cells containing nonproductive VDJδ joints. Whatever the mechanism, the negative effect of the chimeric TCR on thymocyte development must be imposed before or at the time of expansion of DP precursors. Interestingly, it has been suggested that premature expression of transgenic TCRs can also reduce total thymocyte numbers (30).
Despite the fact that Vδ6 rearrangements are common in total thymus of both wild-type (11) and TCRδ-deficient mice (data not shown) and are also frequently used in γδ T cells (31, 32), in-frame Vδ6-DJδ1 rearrangements are randomly distributed both in wild-type (14, 24) and TCRδ-deficient mice (Fig. 2a). One hypothetical way to explain differential depletion of productive VDJδ joints would be if different Vδ domains confer different ligand specificities in the thymus. Alternatively, Vδ6 proteins may not be able to associate with the TCRβ chain and thus would not exert a negative effect on αβ thymocyte development in TCRδ-deficient mice.
A prediction of our results is that transgenic expression of an appropriate hybrid VDJδ-Cα protein would interfere with normal αβ thymocyte development. Because VJα-Cα and VDJδ-Cα proteins differ in only a small region, TCRα transgenic mice provide an initial test of this prediction. Consistent with the prediction, TCRα-only transgenic mice prematurely expressing the transgene in DN thymocytes display a 4- to 5-fold reduction in the total number of thymocytes compared with nontransgenic littermates (D. Sant’Angelo, personal communication). Further studies of chimeric TCRδ/α proteins in TCRδ-deficient mice or in transgenic models should contribute to our understanding of differential developmental effects of TCRδ and TCRα chains.
Acknowledgments
We thank A. Hayday, D. Sant’Angelo, and B. Sleckman for providing valuable comments on the manuscript and T. Taylor and L. Eynon with assistance with the cell sorting. The oligonucleotides used in this study were synthesized by the W. M. Keck Foundation Biotechnology Resource Laboratory at Yale University. D.G.S. was supported by the Howard Hughes Medical Institute. This work was supported in part by a Presidential Faculty Fellows Award to D.G.S. from the National Science Foundation.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: DN, CD4/CD8 double negative; RT, reverse transcriptase; TCR, T-cell receptor; HSA, heat-stable antigen; RFLP, restriction fragment length polymorphism.
References
- 1.Schatz D G, Oettinger M A, Schlissel M S. Annu Rev Immunol. 1992;10:359–383. doi: 10.1146/annurev.iy.10.040192.002043. [DOI] [PubMed] [Google Scholar]
- 2.Chien Y, Iwashima M, Kaplan K B, Elliot J F, Davis M M. Nature (London) 1987;327:677–682. doi: 10.1038/327677a0. [DOI] [PubMed] [Google Scholar]
- 3.Wang K, Klotz J L, Kiser G, Bristol G, Hays E, Lai E, Gese E, Kronenberg M, Hood L. Genomics. 1994;20:419–428. doi: 10.1006/geno.1994.1196. [DOI] [PubMed] [Google Scholar]
- 4.Kronenberg M, Siu G, Hood L, Shastri N. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- 5.Koop B F, Wilson R K, Wang K, Vernooij B, Zallwer D, Kuo C, Seto D, Toda M, Hood L. Genomics. 1992;13:1209–1230. doi: 10.1016/0888-7543(92)90039-u. [DOI] [PubMed] [Google Scholar]
- 6.Davis M M, Bjorkman P J. Nature (London) 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 7.Petrie H T, Livák F, Burtrum D, Mazel S. J Exp Med. 1995;182:121–127. doi: 10.1084/jem.182.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malissen M, Trucy J, Jouvinmarche E, Cazenave P A, Scollay R, Malissen B. Immunol Today. 1992;13:315–322. doi: 10.1016/0167-5699(92)90044-8. [DOI] [PubMed] [Google Scholar]
- 9.Haas W, Tonegawa S. Curr Opin Immunol. 1992;4:147–155. doi: 10.1016/0952-7915(92)90004-x. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima P B, Menetski J P, Roth D B, Gellert M, Bosma M J. Immunity. 1995;3:609–621. doi: 10.1016/1074-7613(95)90132-9. [DOI] [PubMed] [Google Scholar]
- 11.Livák F, Petrie H T, Crispe I N, Schatz D G. Immunity. 1995;2:617–627. doi: 10.1016/1074-7613(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 12.Dudley E C, Girardi M, Owen M J, Hayday A C. Curr Biol. 1995;5:659–669. doi: 10.1016/s0960-9822(95)00131-x. [DOI] [PubMed] [Google Scholar]
- 13.Kang J, Baker J, Raulet D H. Eur J Immunol. 1995;25:2706–2709. doi: 10.1002/eji.1830250946. [DOI] [PubMed] [Google Scholar]
- 14.Livák F, Wilson A, MacDonald H R, Schatz D G. Eur J Immunol. 1997;27:2948–2958. doi: 10.1002/eji.1830271130. [DOI] [PubMed] [Google Scholar]
- 15.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Greene & Wiley; 1989. [Google Scholar]
- 16.Sant’Angelo D B, Waterbury G, Preston-Hurlburt P, Yoon S T, Medzhitov R, Hong S, Janeway C A. Immunity. 1996;4:367–376. doi: 10.1016/s1074-7613(00)80250-2. [DOI] [PubMed] [Google Scholar]
- 17.Mallick C A, Dudley E C, Viney J L, Owen M J, Hayday A C. Cell. 1993;73:513–519. doi: 10.1016/0092-8674(93)90138-g. [DOI] [PubMed] [Google Scholar]
- 18.Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, Clarke A R, Hooper M L, Farr A, Tonegawa S. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 19.Mombaerts P, Clarke A R, Rudnicki M A, Iacomini J, Itohara S, Lafaille J J, Wang L, Ichikawa Y, Jaenisch R, Hooper M L, Tonegawa S. Nature (London) 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 20.Tourigny M R, Mazel S, Burtrum D B, Petrie H T. J Exp Med. 1997;185:1549–1557. doi: 10.1084/jem.185.9.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korman A J, Maruyama J, Raulet D H. Proc Natl Acad Sci USA. 1989;86:267–271. doi: 10.1073/pnas.86.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwashima M, Green A, Davis M M, Chien Y. Proc Natl Acad Sci USA. 1988;85:8161–8165. doi: 10.1073/pnas.85.21.8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arden B, Clark S P, Kabelitz D, Mak T W. Immunogenetics. 1995;42:501–530. doi: 10.1007/BF00172177. [DOI] [PubMed] [Google Scholar]
- 24.Wilson A, de Villartay J P, MacDonald H R. Immunity. 1996;4:37–45. doi: 10.1016/s1074-7613(00)80296-4. [DOI] [PubMed] [Google Scholar]
- 25.Wilson A, Held W, Macdonald H R. J Exp Med. 1994;179:1355–1360. doi: 10.1084/jem.179.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauzurica P, Krangel M S. J Exp Med. 1994;179:43–55. doi: 10.1084/jem.179.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godfrey D I, Kennedy J, Mombaerts P, Tonegawa S, Zlotnik A. J Immunol. 1994;152:4783–4792. [PubMed] [Google Scholar]
- 28.Sleckman B P, Gorman J R, Alt F W. Ann Rev Immunol. 1996;14:459–481. doi: 10.1146/annurev.immunol.14.1.459. [DOI] [PubMed] [Google Scholar]
- 29.Hochstenbach F, Brenner M B. Nature (London) 1989;340:562–565. doi: 10.1038/340562a0. [DOI] [PubMed] [Google Scholar]
- 30.Fehling H J, Iritani B M, Krotkova A, Forbush K A, Laplace C, Perlmutter R M, von Boehmer H. Immunity. 1997;6:703–714. doi: 10.1016/s1074-7613(00)80446-x. [DOI] [PubMed] [Google Scholar]
- 31.Happ M P, Kubo R T, Palmer E, Born W K, O’Brien R L. Nature (London) 1989;342:696–698. doi: 10.1038/342696a0. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien R L, Fu Y X, Cranfill R, Dallas A, Ellis C, Reardon C, Lang J, Carding S R, Kubo R, Born W. Proc Natl Acad Sci USA. 1992;89:4348–4352. doi: 10.1073/pnas.89.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]