Abstract
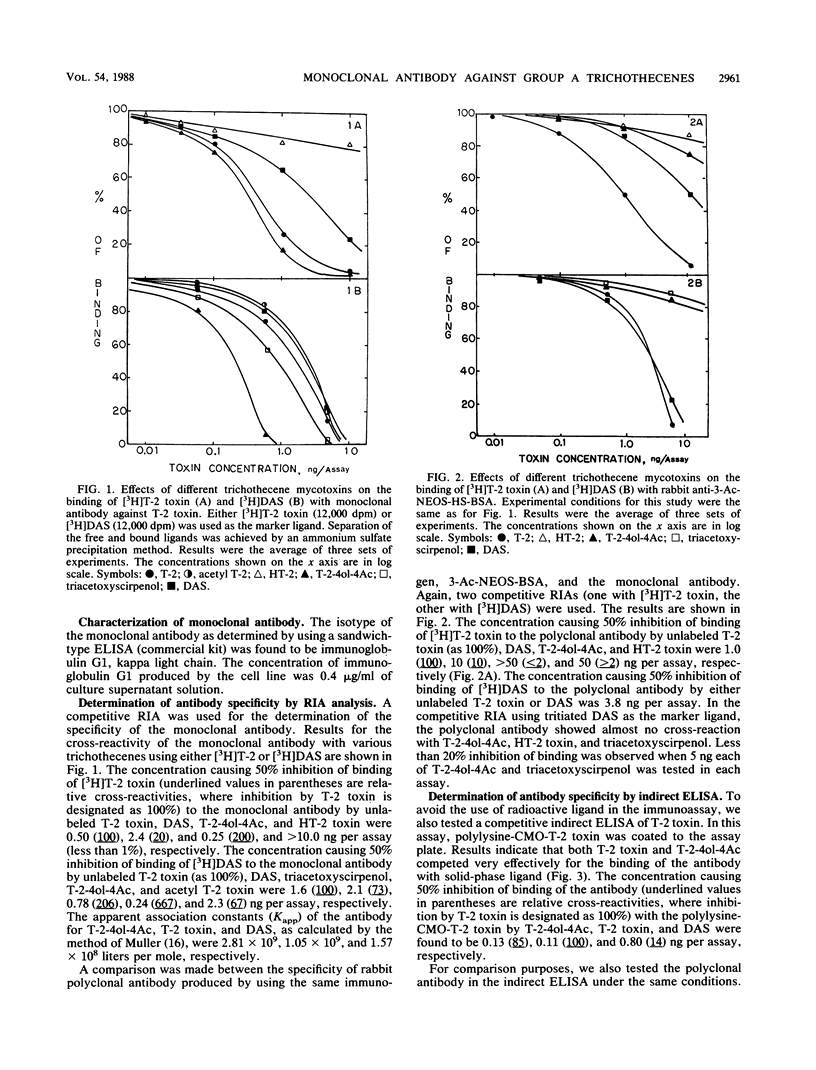
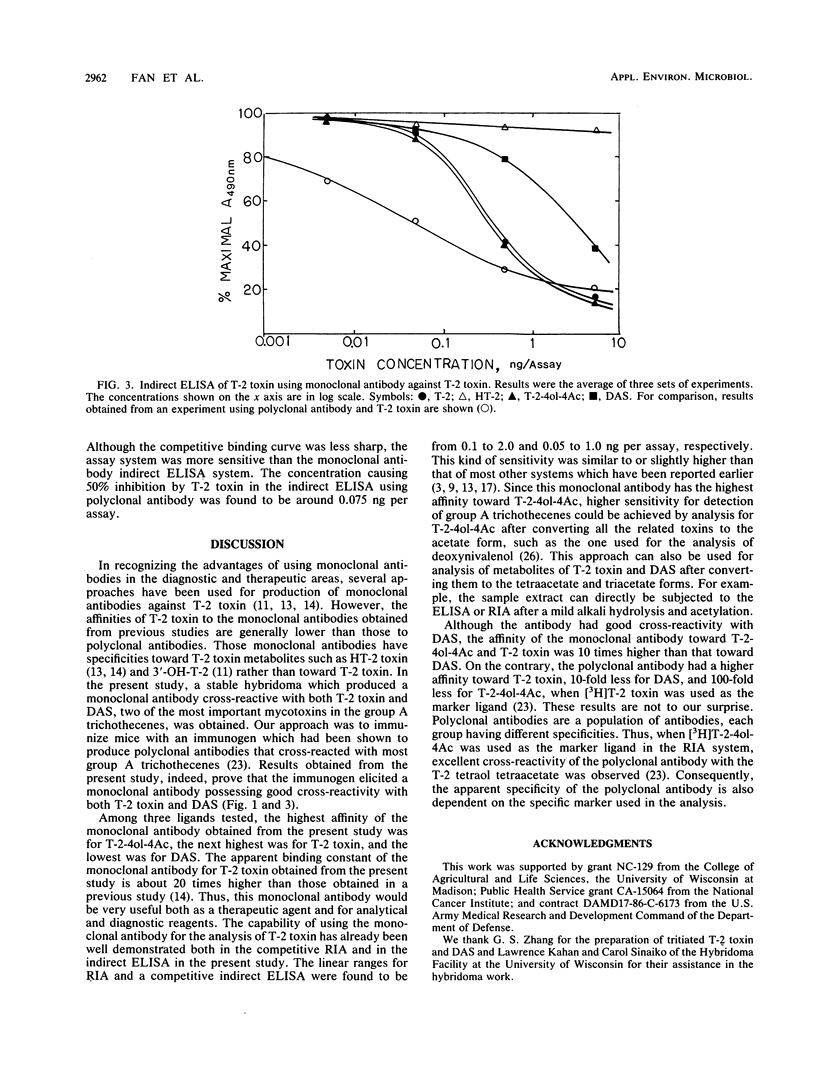
A monoclonal antibody cross-reactive with most group A trichothecenes was produced by fusion of P3/NS-1/1-AG4-1 myeloma cells with spleen cells isolated from a BALB/c mouse that had been immunized with 3-acetyl-neosolaniol-hemisuccinate conjugated to bovine serum albumin. One stable clone, H159B1D5, which produced monoclonal antibody that bound with both T-2 toxin and diacetoxyscirpenol (DAS) was obtained after subcloning. Enzyme-linked immunosorbent assay (ELISA) revealed that the antibody belongs to the immunoglobulin G1 (kappa chain) isotype and had binding constants of 2.81 x 10(9), 1.05 x 10(9), and 1.57 x 10(8) liters per mole for T-2 tetraol tetraacetate, T-2 toxin, and DAS, respectively. The relative cross-reactivities of the antibody with T-2 tetraol tetraacetate, T-2 toxin, and DAS were 200, 100, and 20, respectively, with tritiated T-2 toxin as the marker ligand. The relative cross-reactivities for the above toxins were 667, 100, and 73, respectively, with tritiated DAS as the marker ligand. No cross-reaction with HT-2 and deoxynivalenol triacetate was observed in either system. By using this monoclonal antibody, an indirect ELISA for analysis of T-2 toxin was also developed. The linear portion of the standard curve for analysis of T-2 toxin in each analysis by radioimmunoassay and ELISA was in the range of 0.1 to 2 ng and 0.05 to 1.0 ng, respectively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu F. S., Grossman S., Wei R. D., Mirocha C. J. Production of antibody against T-2 toxin. Appl Environ Microbiol. 1979 Jan;37(1):104–108. doi: 10.1128/aem.37.1.104-108.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F. S., Liang M. Y., Zhang G. S. Production and characterization of antibody against diacetoxyscirpenol. Appl Environ Microbiol. 1984 Oct;48(4):777–780. doi: 10.1128/aem.48.4.777-780.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen D., Smalley E. B., Caldwell R. W. New process for T-2 toxin production. Appl Environ Microbiol. 1982 Aug;44(2):371–375. doi: 10.1128/aem.44.2.371-375.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T. S., Xu Y. C., Chu F. S. Simultaneous analysis of T-2 toxin and HT-2 toxin by an indirect enzyme-linked immunosorbent assay. J Assoc Off Anal Chem. 1987 Jul-Aug;70(4):657–661. [PubMed] [Google Scholar]
- Fan T. S., Zhang G. S., Chu F. S. Production and characterization of antibodies against HT-2 toxin and T-2 tetraol tetraacetate. Appl Environ Microbiol. 1987 Jan;53(1):17–21. doi: 10.1128/aem.53.1.17-21.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontelo P. A., Beheler J., Bunner D. L., Chu F. S. Detection of T-2 toxin by an improved radioimmunoassay. Appl Environ Microbiol. 1983 Feb;45(2):640–643. doi: 10.1128/aem.45.2.640-643.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendloff E. H., Pestka J. J., Swanson S. P., Hart L. P. Detection of T-2 toxin in Fusarium sporotrichioides-infected corn by enzyme-linked immunosorbent assay. Appl Environ Microbiol. 1984 May;47(5):1161–1163. doi: 10.1128/aem.47.5.1161-1163.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K. W., Jr, Brimfield A. A., Miller M., Finkelman F. D., Chu S. F. Preparation and characterization of monoclonal antibodies to the trichothecene mycotoxin T-2. Appl Environ Microbiol. 1985 Jan;49(1):168–172. doi: 10.1128/aem.49.1.168-172.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Chu F. S. Radioimmunoassay of T-2 toxin in biological fluids. J Assoc Off Anal Chem. 1981 May;64(3):684–688. [PubMed] [Google Scholar]
- Müller R. Determination of affinity and specificity of anti-hapten antibodies by competitive radioimmunoassay. Methods Enzymol. 1983;92:589–601. doi: 10.1016/0076-6879(83)92046-3. [DOI] [PubMed] [Google Scholar]
- Nagayama S., Kawamura O., Ohtani K., Ryu J. C., Latus D., Sudheim L., Ueno Y. Application of an enzyme-linked immunosorbent assay for screening of T-2 toxin-producing Fusarium spp. Appl Environ Microbiol. 1988 May;54(5):1302–1303. doi: 10.1128/aem.54.5.1302-1303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H., Dierich M. P., Dose K. Enzyme-linked immunosorbent assay for detection of T-2 toxin. Hoppe Seylers Z Physiol Chem. 1982 Dec;363(12):1437–1441. doi: 10.1515/bchm2.1982.363.2.1437. [DOI] [PubMed] [Google Scholar]
- Wallace E. M., Pathre S. V., Mirocha C. J., Robison T. S., Fenton S. W. Synthesis of radiolabeled T-2 toxin. J Agric Food Chem. 1977 Jul-Aug;25(4):836–838. doi: 10.1021/jf60212a058. [DOI] [PubMed] [Google Scholar]
- Wei R. D., Chu F. S. Production and characterization of a generic antibody against group A trichothecenes. Anal Biochem. 1987 Feb 1;160(2):399–408. doi: 10.1016/0003-2697(87)90067-4. [DOI] [PubMed] [Google Scholar]
- Wei R., Strong F. M., Smalley E. B., Schnoes H. K. Chemical interconversion of T-2 and HT-2 toxins and related compounds. Biochem Biophys Res Commun. 1971 Oct 15;45(2):396–401. doi: 10.1016/0006-291x(71)90832-1. [DOI] [PubMed] [Google Scholar]
- Woychik N. A., Hinsdill R. D., Chu F. S. Production and characterization of monoclonal antibodies against aflatoxin M1. Appl Environ Microbiol. 1984 Dec;48(6):1096–1099. doi: 10.1128/aem.48.6.1096-1099.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G. S., Schubring S. L., Chu F. S. Improved method for production of antibodies against T-2 toxin and diacetoxyscirpenol in rabbits. Appl Environ Microbiol. 1986 Jan;51(1):132–137. doi: 10.1128/aem.51.1.132-137.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


