Abstract
A colony of mice that do not harbor lactobacilli in their digestive tracts but whose intestinal microflora is otherwise functionally similar to that of conventional animals was derived. Methods used to reconstitute the intestinal microflora of the mice included inoculation of the animals with cultures of specific microbes, noncultivable microbes attached to epithelial cells, and cecal contents from conventional mice treated with chloramphenicol. Twenty-six microflora-associated characteristics were monitored by using relatively simple tests to determine the microflora status of the mice.
Full text
PDF


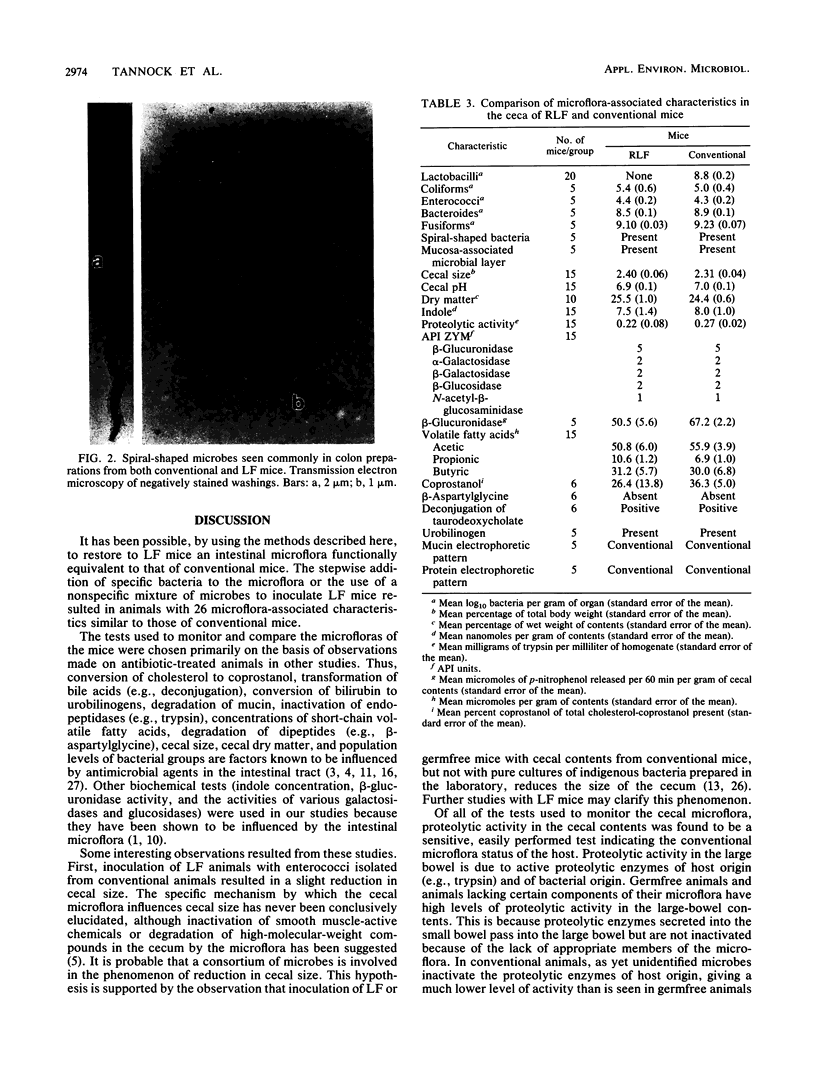

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brockett M., Tannock G. W. Dietary influence on microbial activities in the caecum of mice. Can J Microbiol. 1982 May;28(5):493–499. doi: 10.1139/m82-075. [DOI] [PubMed] [Google Scholar]
- Davis C. P., Savage D. C. Effect of penicillin on the succession, attachment, and morphology of segmented, filamentous microbes in the murine small bowel. Infect Immun. 1976 Jan;13(1):180–188. doi: 10.1128/iai.13.1.180-188.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold S. M. Intestinal microbial changes and disease as a result of antimicrobial use. Pediatr Infect Dis. 1986 Jan-Feb;5(1 Suppl):S88–S90. doi: 10.1097/00006454-198601001-00015. [DOI] [PubMed] [Google Scholar]
- Gordon H. A., Pesti L. The gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol Rev. 1971 Dec;35(4):390–429. doi: 10.1128/br.35.4.390-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B. E., Carlstedt-Duke B. Intestinal water-soluble mucins in germfree, exgermfree and conventional animals. Acta Pathol Microbiol Immunol Scand B. 1984 Oct;92(5):247–252. doi: 10.1111/j.1699-0463.1984.tb02829.x. [DOI] [PubMed] [Google Scholar]
- Hobson P. N., Wallace R. J. Microbial ecology and activities in the rumen: Part II. Crit Rev Microbiol. 1982 May;9(4):253–320. doi: 10.3109/10408418209104492. [DOI] [PubMed] [Google Scholar]
- Hobson P. N., Wallace R. J. Microbial ecology and activities in the rumen: part 1. Crit Rev Microbiol. 1982 Apr;9(3):165–225. doi: 10.3109/10408418209104490. [DOI] [PubMed] [Google Scholar]
- Mallett A. K., Bearne C. A., Rowland I. R. Metabolic activity and enzyme induction in rat fecal microflora maintained in continuous culture. Appl Environ Microbiol. 1983 Sep;46(3):591–595. doi: 10.1128/aem.46.3.591-595.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midtvedt T., Bjørneklett A., Carlstedt-Duke B., Gustafsson B. E., Høverstad T., Lingaas E., Norin K. E., Saxerholt H., Steinbakk M. The influence of antibiotics upon microflora-associated characteristics in man and mammals. Prog Clin Biol Res. 1985;181:241–244. [PubMed] [Google Scholar]
- Rinderknecht H., Geokas M. C., Silverman P., Haverback B. J. A new ultrasensitive method for the determination of proteolytic activity. Clin Chim Acta. 1968 Aug;21(2):197–203. doi: 10.1016/0009-8981(68)90127-7. [DOI] [PubMed] [Google Scholar]
- Roach S., Tannock G. W. Indigenous bacteria influence the number of Salmonella typhimurium in the ileum of gnotobiotic mice. Can J Microbiol. 1979 Dec;25(12):1352–1358. doi: 10.1139/m79-213. [DOI] [PubMed] [Google Scholar]
- Rod T. O., Midtvedt T. Origin of intestinal beta-glucuronidase in germfree, monocontaminated and conventional rats. Acta Pathol Microbiol Scand B. 1977 Aug;85(4):271–276. doi: 10.1111/j.1699-0463.1977.tb01973.x. [DOI] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R. J. The fecal flora of various strains of mice. Its bearing on their susceptibility to endotoxin. J Exp Med. 1962 Jun 1;115:1149–1160. doi: 10.1084/jem.115.6.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R., COSTELLO R. THE DEVELOPMENT OF THE BACTERIAL FLORA IN THE GASTROINTESTINAL TRACT OF MICE. J Exp Med. 1965 Jul 1;122:59–66. doi: 10.1084/jem.122.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., Dubos R. Alterations in the mouse cecum and its flora produced by antibacterial drugs. J Exp Med. 1968 Jul 1;128(1):97–110. doi: 10.1084/jem.128.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- Tannock G. W., Archibald R. D. The derivation and use of mice which do not harbour lactobacilli in the gastrointestinal tract. Can J Microbiol. 1984 Jun;30(6):849–853. doi: 10.1139/m84-131. [DOI] [PubMed] [Google Scholar]
- Tannock G. W. Demonstration of mucosa-associated microbial populations in the colons of mice. Appl Environ Microbiol. 1987 Aug;53(8):1965–1968. doi: 10.1128/aem.53.8.1965-1968.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Savage D. C. Indigenous microorganisms prevent reduction in cecal size induced by Salmonella typhimurium in vaccinated gnotobiotic mice. Infect Immun. 1976 Jan;13(1):172–179. doi: 10.1128/iai.13.1.172-179.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W. The normal microflora: new concepts in health promotion. Microbiol Sci. 1988 Jan;5(1):4–8. [PubMed] [Google Scholar]
- Welling G. W. Comparison of methods for the determination of beta-aspartylglycine in fecal supernatants of leukemic patients treated with antimicrobial agents. J Chromatogr. 1982 Oct 8;232(1):55–62. doi: 10.1016/s0378-4347(00)86007-7. [DOI] [PubMed] [Google Scholar]
- Whitt D. D., Demoss R. D. Effect of microflora on the free amino acid distribution in various regions of the mouse gastrointestinal tract. Appl Microbiol. 1975 Oct;30(4):609–615. doi: 10.1128/am.30.4.609-615.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton D. P., Savage D. C. Influence of certain indigenous gastrointestinal microorganisms on duodenal alkaline phosphatase in mice. Appl Environ Microbiol. 1976 Jun;31(6):880–888. doi: 10.1128/aem.31.6.880-888.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]




