Abstract
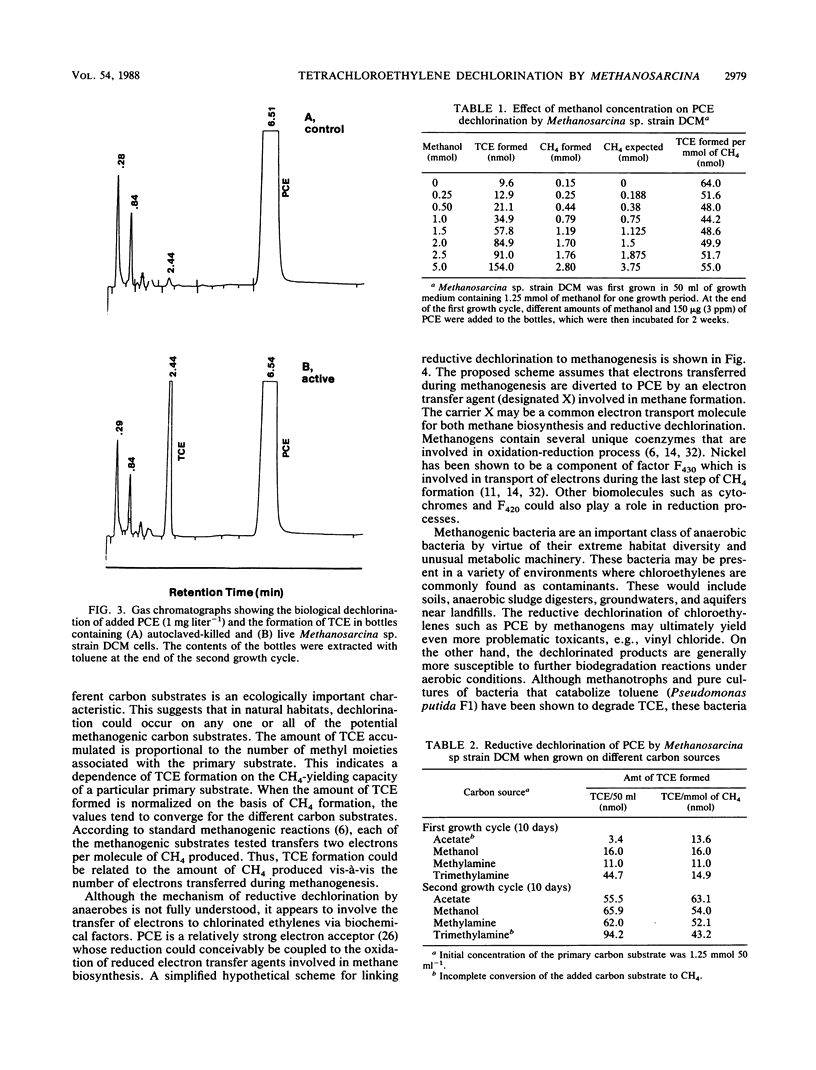
Tetrachloroethylene (perchloroethylene, PCE) is a suspected carcinogen and a common groundwater contaminant. Although PCE is highly resistant to aerobic biodegradation, it is subject to reductive dechlorination reactions in a variety of anaerobic habitats. The data presented here clearly establish that axenic cultures of Methanosarcina sp. strain DCM dechlorinate PCE to trichloroethylene and that this is a biological reaction. Growth on methanol, acetate, methylamine, and trimethylamine resulted in PCE dechlorination. The reductive dechlorination of PCE occurred only during methanogenesis, and no dechlorination was noted when CH4 production ceased. There was a clear dependence of the extent of PCE dechlorination on the amount of methanogenic substrate (methanol) consumed. The amount of trichloroethylene formed per millimole of CH4 formed remained essentially constant for a 20-fold range of methanol concentrations and for growth on acetate, methylamine, and trimethylamine. These results suggest that the reducing equivalents for PCE dechlorination are derived from CH4 biosynthesis and that the extent of chloroethylene dechlorination can be enhanced by stimulating methanogenesis. It is proposed that electrons transferred during methanogenesis are diverted to PCE by a reduced electron carrier involved in methane formation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwer E. J., McCarty P. L. Transformations of 1- and 2-carbon halogenated aliphatic organic compounds under methanogenic conditions. Appl Environ Microbiol. 1983 Apr;45(4):1286–1294. doi: 10.1128/aem.45.4.1286-1294.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway de Macario E., Macario A. J., Wolin M. J. Specific antisera and immunological procedures for characterization of methanogenic bacteria. J Bacteriol. 1982 Jan;149(1):320–328. doi: 10.1128/jb.149.1.320-328.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L., Sparling R., Sprott G. D. The bioenergetics of methanogenesis. Biochim Biophys Acta. 1984 Sep 6;768(2):113–163. doi: 10.1016/0304-4173(84)90002-8. [DOI] [PubMed] [Google Scholar]
- Fathepure B. Z. Factors Affecting the Methanogenic Activity of Methanothrix soehngenii VNBF. Appl Environ Microbiol. 1987 Dec;53(12):2978–2982. doi: 10.1128/aem.53.12.2978-2982.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathepure B. Z., Nengu J. P., Boyd S. A. Anaerobic bacteria that dechlorinate perchloroethene. Appl Environ Microbiol. 1987 Nov;53(11):2671–2674. doi: 10.1128/aem.53.11.2671-2674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel M. M., Taddeo A. R., Fogel S. Biodegradation of chlorinated ethenes by a methane-utilizing mixed culture. Appl Environ Microbiol. 1986 Apr;51(4):720–724. doi: 10.1128/aem.51.4.720-724.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausinger R. P. Nickel utilization by microorganisms. Microbiol Rev. 1987 Mar;51(1):22–42. doi: 10.1128/mr.51.1.22-42.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstead R. L. Studies of the degradation of Mirex with an iron(II) porphyrin model system. J Agric Food Chem. 1976 May-Jun;24(3):620–624. doi: 10.1021/jf60205a024. [DOI] [PubMed] [Google Scholar]
- Jones W. J., Nagle D. P., Jr, Whitman W. B. Methanogens and the diversity of archaebacteria. Microbiol Rev. 1987 Mar;51(1):135–177. doi: 10.1128/mr.51.1.135-177.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifa S., Holmstead R. L., Casida J. E. Toxaphene degradation by iron(II) protoporphyrin systems. J Agric Food Chem. 1976 Mar-Apr;24(2):277–282. doi: 10.1021/jf60204a009. [DOI] [PubMed] [Google Scholar]
- Little C. D., Palumbo A. V., Herbes S. E., Lidstrom M. E., Tyndall R. L., Gilmer P. J. Trichloroethylene biodegradation by a methane-oxidizing bacterium. Appl Environ Microbiol. 1988 Apr;54(4):951–956. doi: 10.1128/aem.54.4.951-956.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974 May;27(5):985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. J., Montgomery S. O., Pritchard P. H. Trichloroethylene metabolism by microorganisms that degrade aromatic compounds. Appl Environ Microbiol. 1988 Feb;54(2):604–606. doi: 10.1128/aem.54.2.604-606.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers K. R., Baron S. F., Ferry J. G. Methanosarcina acetivorans sp. nov., an Acetotrophic Methane-Producing Bacterium Isolated from Marine Sediments. Appl Environ Microbiol. 1984 May;47(5):971–978. doi: 10.1128/aem.47.5.971-978.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel T. M., McCarty P. L. Biotransformation of tetrachloroethylene to trichloroethylene, dichloroethylene, vinyl chloride, and carbon dioxide under methanogenic conditions. Appl Environ Microbiol. 1985 May;49(5):1080–1083. doi: 10.1128/aem.49.5.1080-1083.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett L. P., Gibson D. T. Degradation of trichloroethylene by toluene dioxygenase in whole-cell studies with Pseudomonas putida F1. Appl Environ Microbiol. 1988 Jul;54(7):1703–1708. doi: 10.1128/aem.54.7.1703-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade R. S., Havlin R., Castro C. E. The oxidation of iron (II) porphyrins by organic molecules. J Am Chem Soc. 1969 Dec 17;91(26):7530–7530. doi: 10.1021/ja01054a063. [DOI] [PubMed] [Google Scholar]
- Wilson J. T., Wilson B. H. Biotransformation of trichloroethylene in soil. Appl Environ Microbiol. 1985 Jan;49(1):242–243. doi: 10.1128/aem.49.1.242-243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoro J. A., Hunter J. M., Eglinton G., Ware G. C. Degradation of p,p'-DDT in reducing environments. Nature. 1974 Jan 25;247(5438):235–237. doi: 10.1038/247235a0. [DOI] [PubMed] [Google Scholar]