Abstract
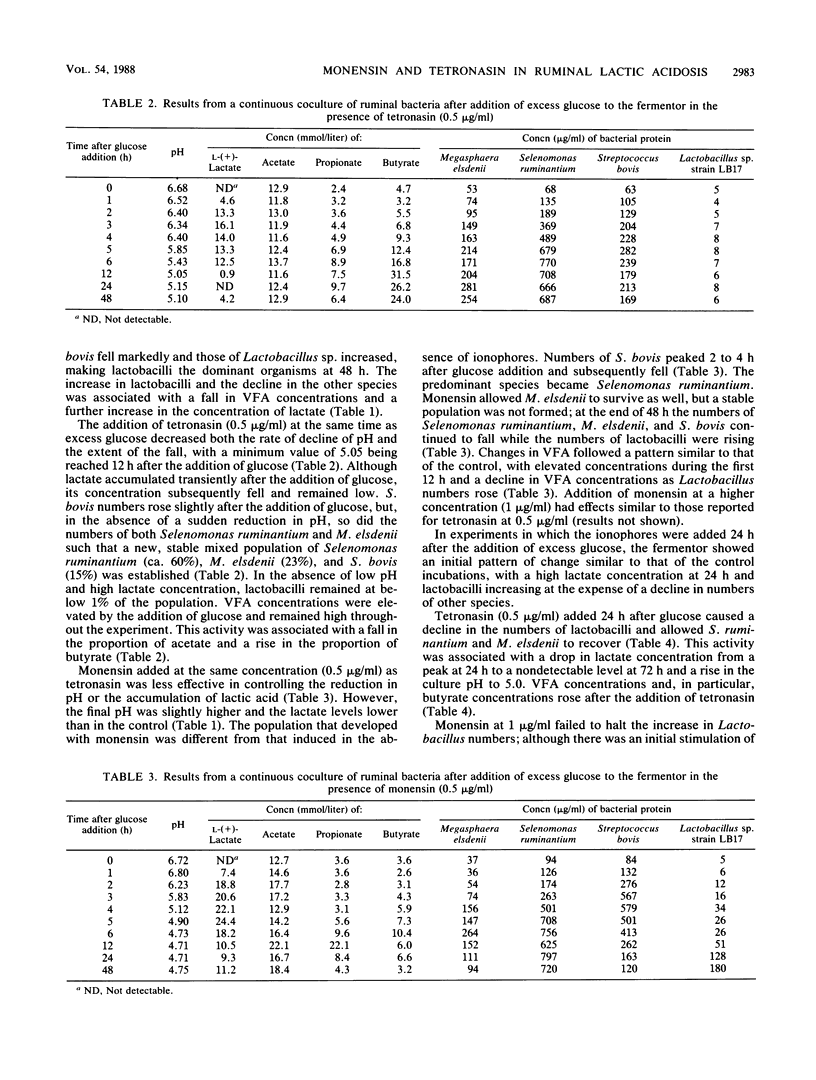
A continuous coculture of four ruminal bacteria, Megasphaera elsdenii, Selenomonas ruminantium, Streptococcus bovis, and Lactobacillus sp. strain LB17, was used to study the effects of the ionophores monensin and tetronasin on the changes in ruminal microbial ecology that occur during the onset of lactic acidosis. In control incubations, the system simulated the development of lactic acidosis in vivo, with an initial overgrowth of S. bovis when an excess of glucose was added to the fermentor. Lactobacillus sp. strain LB17 subsequently became dominant as pH fell and lactate concentration rose. Both ionophores were able to prevent the accumulation of lactic acid and maintain a healthy non-lactate-producing bacterial population when added at the same time as an excess of glucose. Tetronasin was more potent in this respect than monensin. When tetronasin was added to the culture 24 h after glucose, the proliferation of lactobacilli was reversed and a non-lactate-producing bacterial population developed, with an associated drop in lactate concentration in the fermentor. Rises in culture pH and volatile fatty acid concentrations accompanied these changes. Monensin was unable to suppress the growth of lactobacilli; therefore, in contrast to tetronasin, monensin added 24 h after the addition of glucose failed to reverse the acidosis. Numbers of lactobacilli and lactate concentrations remained high, whereas pH and volatile fatty acid concentrations were low.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brüggemann J., Giesecke D. Milchsäuregehalt im Pansen und mikrobieller Abbau in vitro. Zentralbl Veterinarmed A. 1968 Jul;15(5):470–476. [PubMed] [Google Scholar]
- Counotte G. H., Prins R. A., Janssen R. H., Debie M. J. Role of Megasphaera elsdenii in the Fermentation of dl-[2-C]lactate in the Rumen of Dairy Cattle. Appl Environ Microbiol. 1981 Oct;42(4):649–655. doi: 10.1128/aem.42.4.649-655.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis S. M., Nagaraja T. G., Bartley E. E. Effect of lasalocid or monensin on lactate production from in vitro rumen fermentation of various carbohydrates. J Dairy Sci. 1981 Dec;64(12):2350–2356. doi: 10.3168/jds.S0022-0302(81)82857-3. [DOI] [PubMed] [Google Scholar]
- Dennis S. M., Nagaraja T. G., Bartley E. E. Effects of lasalocid or monensin on lactate-producing or -using rumen bacteria. J Anim Sci. 1981 Feb;52(2):418–426. doi: 10.2527/jas1981.522418x. [DOI] [PubMed] [Google Scholar]
- HOBSON P. N. CONTINUOUS CULTURE OF RUMEN BACTERIA: APPARATUS. J Gen Microbiol. 1965 Feb;38:161–166. doi: 10.1099/00221287-38-2-161. [DOI] [PubMed] [Google Scholar]
- HOBSON P. N. CONTINUOUS CULTURE OF SOME ANEROBIC AND FACULTATIVELY ANAEROBIC RUMEN BACTERIA. J Gen Microbiol. 1965 Feb;38:167–180. doi: 10.1099/00221287-38-2-167. [DOI] [PubMed] [Google Scholar]
- Hobson P. N., Summers R. The continuous culture of anaerobic bacteria. J Gen Microbiol. 1967 Apr;47(1):53–65. doi: 10.1099/00221287-47-1-53. [DOI] [PubMed] [Google Scholar]
- Mann S. O. Some effects on the rumen micro-organisms of overfeeding a high barley ration. J Appl Bacteriol. 1970 Jun;33(2):403–409. doi: 10.1111/j.1365-2672.1970.tb02213.x. [DOI] [PubMed] [Google Scholar]
- Nagaraja T. G., Avery T. B., Bartley E. E., Roof S. K., Dayton A. D. Effect of lasalocid, monensin or thiopeptin on lactic acidosis in cattle. J Anim Sci. 1982 Mar;54(3):649–658. doi: 10.2527/jas1982.543649x. [DOI] [PubMed] [Google Scholar]
- Newbold C. J., Wallace R. J., Watt N. D., Richardson A. J. Effect of the novel ionophore tetronasin (ICI 139603) on ruminal microorganisms. Appl Environ Microbiol. 1988 Feb;54(2):544–547. doi: 10.1128/aem.54.2.544-547.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Substrate preferences in rumen bacteria: evidence of catabolite regulatory mechanisms. Appl Environ Microbiol. 1978 Aug;36(2):319–329. doi: 10.1128/aem.36.2.319-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Cotta M. A., Dombrowski D. B. Rumen Bacterial Competition in Continuous Culture: Streptococcus bovis Versus Megasphaera elsdenii. Appl Environ Microbiol. 1981 Jun;41(6):1394–1399. doi: 10.1128/aem.41.6.1394-1399.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Dombrowski D. B. Effect of pH on the efficiency of growth by pure cultures of rumen bacteria in continuous culture. Appl Environ Microbiol. 1980 Mar;39(3):604–610. doi: 10.1128/aem.39.3.604-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satter L. D., Esdale W. J. In vitro lactate metabolism by ruminal ingesta. Appl Microbiol. 1968 May;16(5):680–688. doi: 10.1128/am.16.5.680-688.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slyter L. L. Influence of acidosis on rumen function. J Anim Sci. 1976 Oct;43(4):910–929. doi: 10.2527/jas1976.434910x. [DOI] [PubMed] [Google Scholar]
- Wallace R. J. Control of lactate production by Selenomonas ruminantium: homotropic activation of lactate dehydrogenase by pyruvate. J Gen Microbiol. 1978 Jul;107(1):45–52. doi: 10.1099/00221287-107-1-45. [DOI] [PubMed] [Google Scholar]


