Abstract
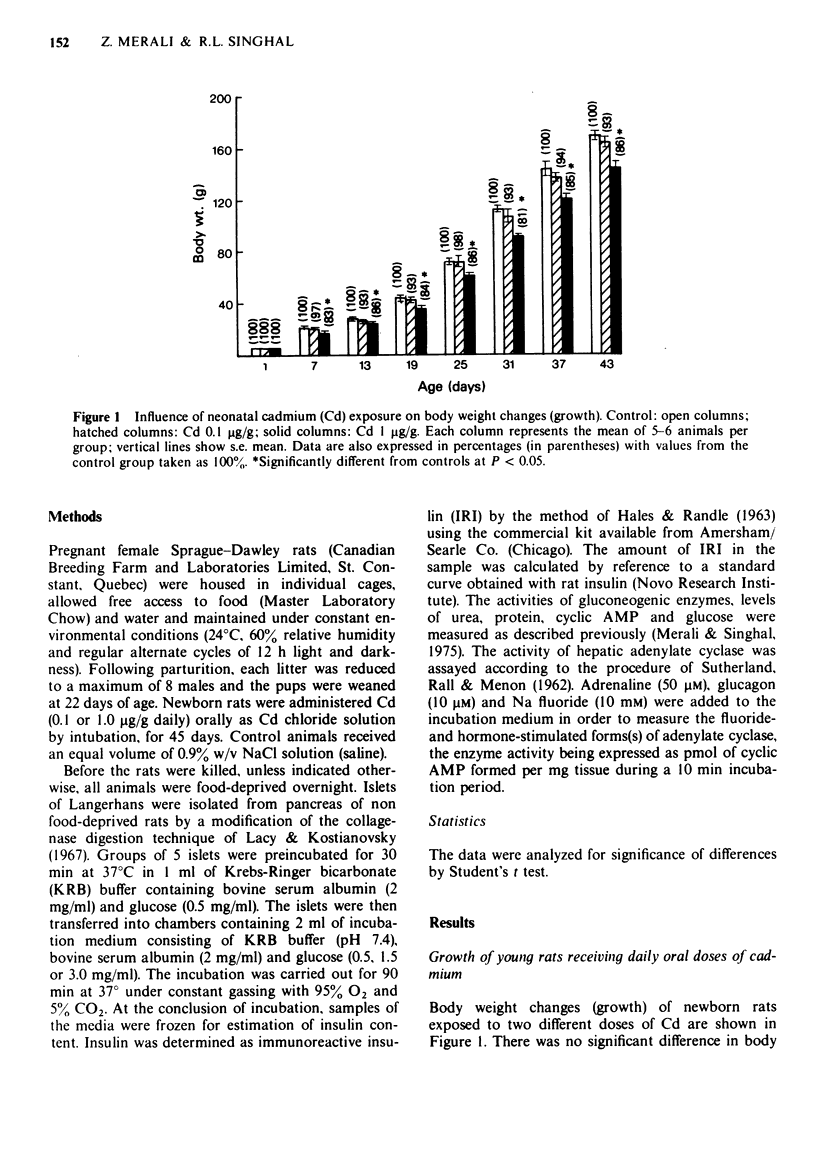
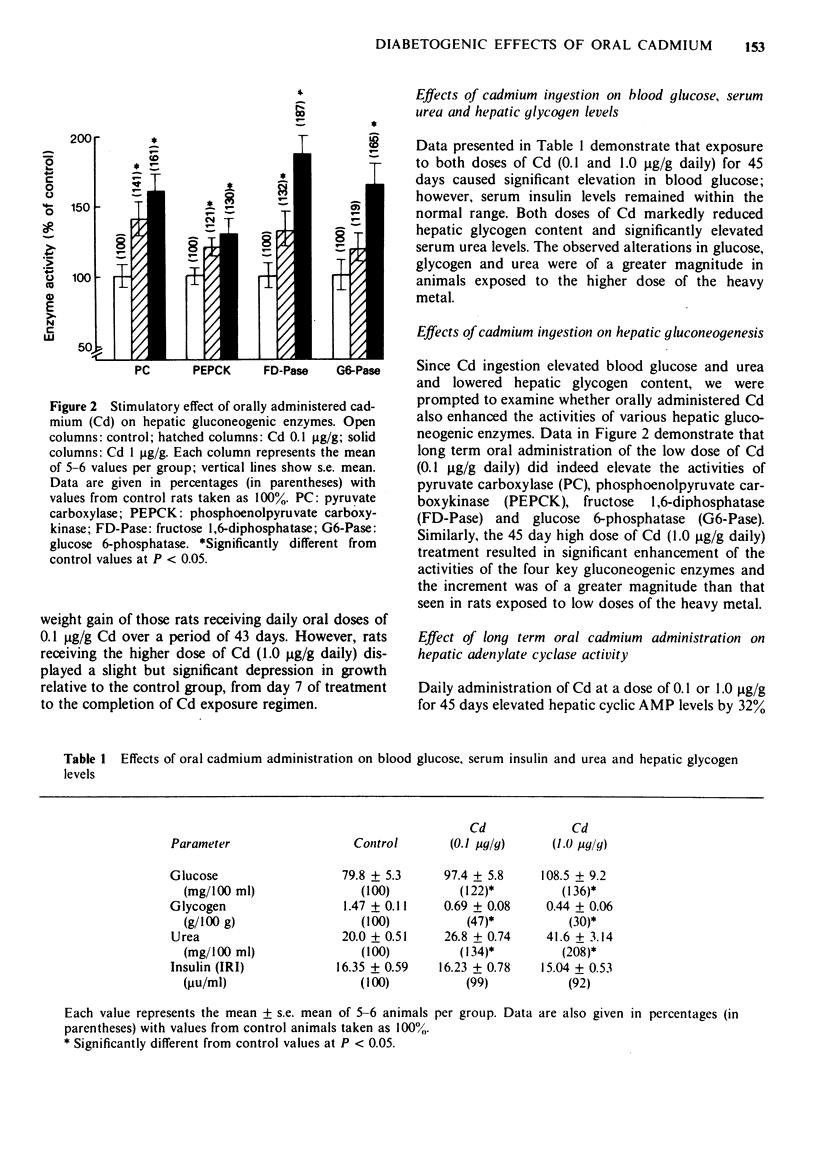
1 Chronic exposure of neonatal rats to oral cadmium (Cd) (0.1 and 1.0 micrograms/g daily for 45 days) disturbed glucose homeostasis, as reflected by hyperglycaemia, reduced liver glycogen and enhanced gluconeogenic potential of hepatic tissue. 2 This Cd-exposure regimen also increased hepatic cyclic adenosine 3',5'-monophosphate (cyclic AMP) which was accompanied by enhancement of basal, adrenaline and glucagon-stimulated form(s) of adenylate cyclase. 3 In order to assess the responsiveness of pancreatic beta cells to glucose, islets isolated from control as well as Cd-exposed animals were incubated in vitro and their rate of insulin secretion determined. In the presence of glucose 0.5 mg/ml, there was no significant difference in the rate of insulin release. However, at higher glucose concentrations (1.5 and 3.0 mg/ml), the islets from Cd-exposed rats released significantly less insulin than those of control animals. 4 The results are discussed in relation to the possible mechanism of the diabetogenic effect of Cd.
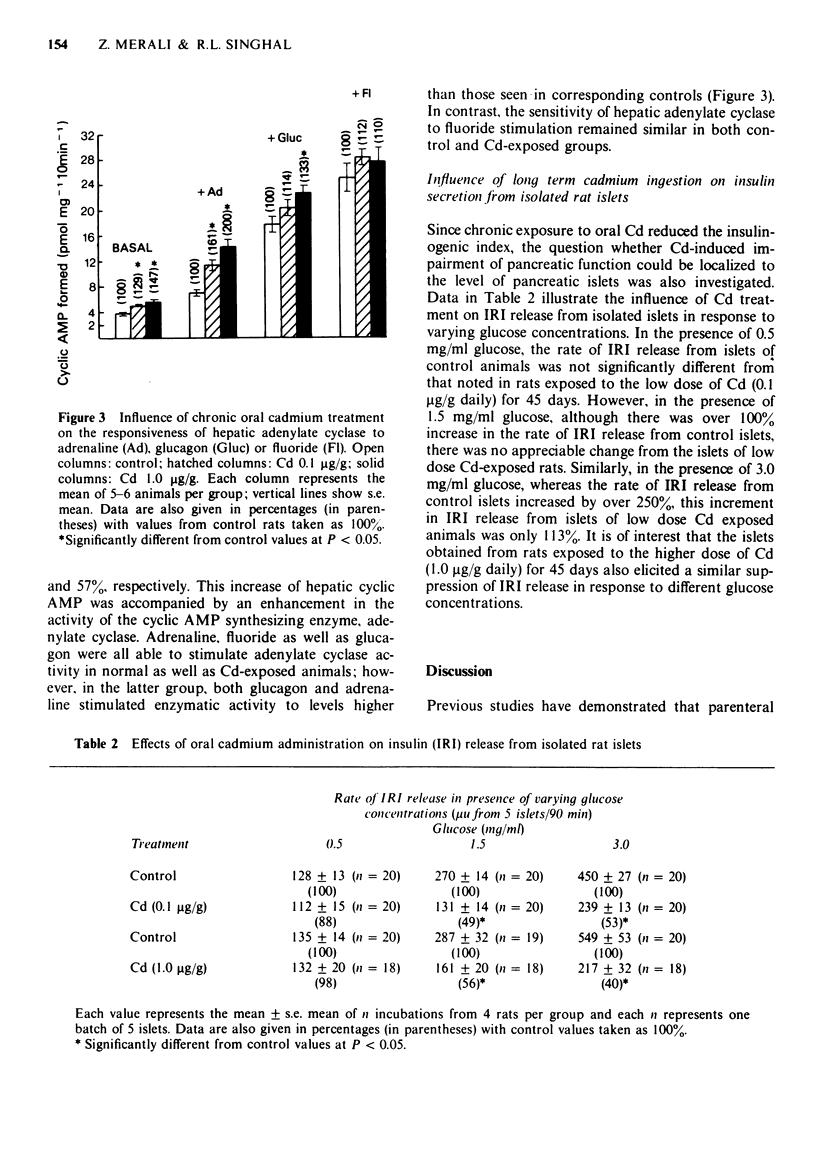
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBIERI L., COLOMBI R., STRANEO G. [Histological changes of the pancreatic islands in the rabbit after chronic cadmium poisoning]. Gen. 1961 Dec;44:1120–1133. [PubMed] [Google Scholar]
- BONNELL J. A., KAZANTZIS G., KING E. A follow-up study of men exposed to cadmium oxide fume. Br J Ind Med. 1959 Apr;16(2):135–147. doi: 10.1136/oem.16.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitensky M. W., Gorman R. E., Neufeld A. H. Selective effects of insulin on hepatic epinephrine responsive adenyl cyclase activity. Endocrinology. 1972 May;90(5):1331–1335. doi: 10.1210/endo-90-5-1331. [DOI] [PubMed] [Google Scholar]
- Ghafghazi T., Mennear J. H. Effects of acute and subacute cadmium administration on carbohydrate metabolism in mice. Toxicol Appl Pharmacol. 1973 Oct;26(2):231–240. doi: 10.1016/0041-008x(73)90256-1. [DOI] [PubMed] [Google Scholar]
- Ghagghazi T., Mennear J. H. The inhibitory effect of cadmium on the secretory activity of the isolated perfused rat pancreas. Toxicol Appl Pharmacol. 1975 Jan;31(1):134–142. doi: 10.1016/0041-008x(75)90061-7. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havu N. Sulfhydryl inhibitors and pancreatic islet tissue. Acta Endocrinol Suppl (Copenh) 1969;139:1–231. [PubMed] [Google Scholar]
- Ithakissios D. S., Ghafghazi T., Mennear J. H., Kessler W. V. Effect of multiple doses of cadmium on glucose metabolism and insulin secretion in the rat. Toxicol Appl Pharmacol. 1975 Jan;31(1):143–149. doi: 10.1016/0041-008x(75)90062-9. [DOI] [PubMed] [Google Scholar]
- KAZANTZIS G., FLYNN F. V., SPOWAGE J. S., TROTT D. G. Renal tubular malfunction and pulmonary emphysema in cadmium pigment workers. Q J Med. 1963 Apr;32:165–192. [PubMed] [Google Scholar]
- Kello D., Kostial K. Influence of age and milk diet on cadmium absorption from the gut. Toxicol Appl Pharmacol. 1977 May;40(2):277–282. doi: 10.1016/0041-008x(77)90098-9. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Menahan L. A., Wieland O. Glucagon-like action of N6,2'-O-dibutyryl cyclic 3',5'-AMP on perfused rat liver. Biochem Biophys Res Commun. 1967 Dec 29;29(6):880–885. doi: 10.1016/0006-291x(67)90303-8. [DOI] [PubMed] [Google Scholar]
- Merali Z., Kacew S., Singhal R. L. Response of hepatic carbohydrate and cyclic AMP metabolism to cadmium treatment in rats. Can J Physiol Pharmacol. 1975 Feb;53(1):174–184. doi: 10.1139/y75-024. [DOI] [PubMed] [Google Scholar]
- Merali Z., Singhal R. L. Prevention by zinc of cadmium-induced alterations in pancreatic and hepatic functions. Br J Pharmacol. 1976 Aug;57(4):573–579. doi: 10.1111/j.1476-5381.1976.tb10387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z., Singhal R. L. Protective effect of selenium on certain hepatotoxic and pancreotoxic manifestations of subacute cadmium administration. J Pharmacol Exp Ther. 1975 Oct;195(1):58–66. [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]
- Sasser L. B., Jarboe G. E. Intestinal absorption and retention of cadmium in neonatal rat. Toxicol Appl Pharmacol. 1977 Aug;41(2):423–431. doi: 10.1016/0041-008x(77)90043-6. [DOI] [PubMed] [Google Scholar]
- Wicks W. D. Induction of hepatic enzymes by adenosine 3',5'-monophosphate in organ culture. J Biol Chem. 1969 Jul 25;244(14):3941–3950. [PubMed] [Google Scholar]


