Abstract
We have discovered that the mosquito Anopheles stephensi, a natural vector of human malaria, limits parasite development with inducible synthesis of nitric oxide (NO). Elevated expression of A. stephensi NO synthase (NOS), which is highly homologous to characterized NOS genes, was detected in the midgut and carcass soon after invasion of the midgut by Plasmodium. Early induction is likely primed by bacterial growth in the blood meal. Later increases in A. stephensi NOS expression and enzyme activity occurred at the beginning of sporozoite release. Circulating levels of nitrite/nitrate, end-products of NO synthesis, were significantly higher in Plasmodium-infected mosquitoes. Dietary provision of the NOS substrate l-arginine reduced Plasmodium infections in A. stephensi. In contrast, dietary provision of a NOS inhibitor significantly increased parasite numbers in infected mosquitoes, confirming that A. stephensi limits Plasmodium development with NO.
Global efforts to control malaria, caused by parasitic protozoa of the genus Plasmodium, have been hindered by insecticide-resistant mosquitoes, drug-resistant parasites, and socioeconomic obstacles. The drive to identify novel control strategies has, in part, focused on identifying mosquito gene products that impart refractory phenotypes, including those associated with the immune response to pathogens (1–3).
Plasmodium development in Anopheles begins with ingestion by the mosquito of blood containing gametocytes; parasite fertilization commences within minutes of ingestion. Mobile zygotes, or ookinetes, penetrate the midgut epithelium 24–36 hr later and rapidly transform into vegetative oocysts under the basal lamina in the hemolymph-filled, open circulatory system of the mosquito. After growth and development for 10–12 days, thousands of haploid sporozoites are released from each oocyst into the hemolymph. Hemolymph circulation and sporozoite movement carry a fraction of the parasites to the salivary glands, where they are released into the saliva during subsequent blood feeding.
We have discovered that A. stephensi, a major vector of human malaria in India and a competent laboratory host for the murine parasite P. berghei, limits parasite development with inducible synthesis of nitric oxide (NO). The free radical NO, produced during the oxidation of l-Arg to l-Citrulline (l-Cit) by NO synthase (NOS), has diverse roles in vertebrate physiology. Three genes encode distinct vertebrate NOS isoforms: two are constitutively expressed whereas the third is an inducible immune effector (4). Inducible NOS and NO are synthesized in response to bacterial, viral, and parasitic infections in vertebrates (5, 6). During Plasmodium infection in humans and mice, NO inactivates liver-invading sporozoites and blood-stage gametocytes (7, 8). Our discovery of inducible NOS activity in A. stephensi, therefore, demonstrates that mosquitoes and vertebrates share a conserved anti-Plasmodium defense.
MATERIALS AND METHODS
Mosquito Maintenance and Plasmodium Infections.
A. stephensi were produced at 27°C and 75% relative humidity under a 12-hr light/dark cycle. For P. berghei infections, mosquitoes were fed one time (day 0) on anesthetized Institute of Cancer Research mice and maintained on sugar cubes and water at 21°C and 75% relative humidity until use. For P. falciparum infections, mosquitoes were membrane-fed one time on human blood with strain NF54 and maintained on sugar and water at normal rearing conditions until use. Hereafter, these mosquitoes are referred to as “infected” or “P. sp.-infected.” Controls were fed one time on uninfected mice or human blood and maintained until use under the appropriate environmental conditions; these mosquitoes are referred to as “blood fed uninfected.” For studies of midgut bacteria, mosquitoes (non-blood fed, blood fed uninfected, and P. berghei-infected) were provided with sugar and either water- or gentamicin-soaked (50 μg/ml in water, Sigma) sterile cotton from 3 days before blood feeding until termination of the experiment. For NOS inhibition experiments, mosquitoes were provided with gentamicin-soaked (50 μg/ml in water) sterile cotton for 3 days before blood feeding. Immediately before membrane feeding, Nω-nitro-l-arginine methyl ester (1 mg/ml, l-NAME, Sigma) or the biologically inactive d-NAME (1 mg/ml, Sigma) was added to the P. falciparum blood culture. Blood fed mosquitoes were maintained on the same treatments, provided as water-soaked sterile cotton balls and changed daily, until dissection.
RNA Isolation and Analysis by Reverse Transcriptase–PCR (RT-PCR).
Total RNA was isolated using TriZOL (Life Technologies,Gaithersburg, MD). Preliminary RT-PCR detected identical NOS expression in P. berghei- and P. falciparum-infected mosquitoes at 14–16 days post-blood meal (pBM) using degenerate primers designed against Drosophila nos (DNOS) (9). Temporal A. stephensi NOS (AsNOS) expression was studied by using semiquantitative RT-PCR (10, 11). Total RNA was isolated from 30–40 each non-blood fed, blood fed uninfected, and P. berghei-infected mosquitoes at 1, 2, 3, 6, 9, 14, and 18 days pBM or from 25–40 midguts and carcasses without midguts at 1, 2, and 3 days post-P. berghei infection. First-strand cDNA was synthesized by using 0.5 μg of oligo(dT) primer and Superscript II (Life Technologies). A 250-bp AsNOS fragment was amplified by 35 cycles of PCR (94°C for 40 sec, 53°C for 40 sec, and 72°C for 40 sec) with the primers 5′-ACATCAAGACGGAAATGGTTG 3′ and 5′-ACAGACGTAGATGTGGGCCTT 3′; for replicates of tissue-specific assays, a 180-bp AsNOS fragment was amplified using identical conditions and the nested primer pair 5′-ACTGCAAGATTCCCAAGGTAT 3′ and 5′-ATTCCGCCTCTTTGAGGGCAA 3′. Transcript abundance was normalized against a 300-bp β-actin fragment amplified by using the described PCR conditions for 15 cycles with primers designed against A. gambiae β-actin (12). For tissue-specific assays, AsNOS transcript abundance was normalized against a 460-bp ribosomal S7 protein gene fragment amplified using 25 cycles of PCR (94°C for 30 sec, 55°C for 30 sec, and 72°C for 40 sec) and published primers (13, 14). Amplimers were labeled by incorporation of [32P]dATP, separated in 1.5% agarose gels, transferred to nylon, and exposed to X-Omat film (Kodak). For signal quantitation, autoradiographs were scanned into rit120 software (Radiological Imaging Technology, Colorado Springs, CO) by using a Lumiscan 150 laser digitizer (Lumisys, Sunnyvale, CA). For midgut and carcass expression, data from three replicates were analyzed by one-tailed paired t test (α = 0.075).
AsNOS Cloning and Sequencing.
The genomic cognate of AsNOS (unpublished data) was constructed by using Genetics Computer Group software (15) assembly of sequence from four EMBL3 SP6/T7 clones (CLONTECH) isolated by high-stringency hybridization to [32P]dATP- or digoxigenin-labeled (Boehringer Mannheim) RT-PCR fragments. Coding sequence was confirmed with blast homology analysis (16) and 5′ and 3′ rapid amplification of cDNA ends (17).
Midgut Diaphorase Staining and Quantification of Midgut Bacteria.
Midgut AsNOS was measured quantitatively and examined in situ among non-blood fed, blood fed uninfected, and P. berghei- or P. falciparum-infected mosquitoes at 24 hr pBM by using diaphorase staining (18, 19, 20). Under these conditions, NOS is responsible for NADPH-dependent diaphorase reduction of indicator substrates (19, 21). For quantitative assays, 10 dissected midguts with blood removed were fixed in 4% paraformaldehyde, 1% glutaraldehyde in 0.1 M sodium phosphate (pH 7.0), triturated, and incubated in buffer with 1 mg/ml 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT, Sigma) and 1 mM NADPH for 1 hr at 37°C (20). Following color development, an equal volume of acid isopropanol (0.04 M HCl) was added to dissolve precipitated crystals, and the samples were read at 570 nm in a SpectraMax 250 microplate reader (Molecular Devices). Mean absorbances and standard errors were calculated from three experiments. For in situ staining, dissected midguts were fixed, washed, and incubated in buffer with 1 mM NADPH, 1 mg/ml nitroblue tetrazolium, and 0.01% Triton X-100 at 37°C for 10–15 min. The cofactor NADPH was omitted or replaced with NADH in controls. To determine whether diaphorase staining colocalized with parasites, midguts were treated with RNase A/T1 (205 units, Ambion, Austin, TX) and stained with propidium iodide (1 μg/ml, Molecular Probes). Interpretations of staining patterns were based on three preparations of 10 or more midguts per treatment group.
Tryptic soy agar plates with 5% sheep blood were used for quantification and gentamicin (50 μg/ml) sensitivity testing of midgut bacteria (22). Midguts from surface-sterilized mosquitoes were triturated in sterile Aedes saline (23). Following 24-hr incubation at 25°C, colony forming units were counted for triturate dilutions. Data from blood fed uninfected and infected midguts were combined for analysis because mean colony forming units were not significantly different from each other.
Plasmodium Infection: AsNOS Specific Activity, Hemolymph Nitrite/Nitrate Levels, and Effects of Mosquito Dietary Manipulation.
Specific activities of AsNOS in lysates of 40 each non-blood fed, blood fed uninfected, or P. berghei-infected mosquitoes were measured as the rate of conversion of [3H]Arg to [3H]Cit per mg of homogenate at 6 and 9 days pBM using the NOSdetect kit (Stratagene). Assays followed blood digestion (4–5 days at 21°C, unpublished observation) to avoid interference by host blood proteins. Activity was measured with or without the NOS inhibitor l-NAME (1 mM). Data were analyzed using contingency tables (α = 0.05). Hemolymph nitrite/nitrate (NO2−/NO3−) concentration was measured using a modified cadmium reduction/Griess reagent microassay (24) after collection (25) from groups of 100–200 blood fed uninfected and P. berghei-infected mosquitoes at 7, 9–10, and 14–15 days pBM. Midgut blood contamination of hemolymph precluded the use of earlier time points. Data for each time point were analyzed by one-tailed paired t test (α = 0.075).
The impact of NO on Plasmodium in A. stephensi was assessed with dietary supplements. Three days before blood feeding, two replicates of four groups of mosquitoes were provided with sugar cubes and water alone or sugar cubes and the NOS substrate l-Arg (0.2% or 0.002%) in water or l-Cit (0.2%) in water. On day 0, all of the groups were fed on a single P. berghei-infected hamster. At day 10, mosquitoes were dissected to count midgut oocysts. In other experiments, two replicates of three groups of mosquitoes were provided with sugar cubes and gentamicin (50 μg/ml) in water for three days before blood feeding. On day 0, l-NAME (1 mg/ml) or d-NAME (1 mg/ml) was added to aliquots of a single P. falciparum culture immediately before mosquito feeding; a third aliquot was left as an untreated control. Blood fed mosquitoes were maintained on sugar and l-NAME- or d-NAME-treated or untreated water until dissection at 7 days pBM to count midgut oocysts. Data from treatment replicates were combined because infection means and prevalences were not significantly different from each other. Effects on infection were compared using contingency table analyses (α = 0.05) for amino acid supplements and Tukey’s test (α = 0.05) for l-NAME/D-NAME experiments.
RESULTS
Isolation and Sequence Analysis of AsNOS.
A span of ≈30 kb of AsNOS genomic sequence (unpublished data) encodes a large ORF of 1,247 aa, including all of the characteristic NOS cofactor-binding domains (Fig. 1). The sequence surrounding the initiation methionine conforms strictly to the Kozak consensus for translational start sites (26). The deduced AsNOS protein (1,247 aa) is intermediate in size to DNOS (1,350 aa, Fig. 1) and Rhodnius NOS (1,174 aa) (9, 27). Amino acid homology to DNOS (69–81% identity) and Rhodnius NOS (60–73% identity) varies across the coding sequence with the highest homology around the putative encoded heme and calmodulin cofactor-binding domains. The deduced amino acid sequences of DNOS, Rhodnius NOS, and AsNOS show the highest level of homology to vertebrate neuronal NOS, followed by decreasing homologies to vertebrate endothelial and inducible NOS genes. The homology of three distinct insect genes to a neuronal isoform should be regarded more as a reflection of gene ancestry than functional activity. Genomic structure conservation suggests that the three human NOS genes arose from a single precursor gene by duplication events (28); our analysis (unpublished results) strongly suggests that AsNOS and the human NOS genes share a common ancestor.
Figure 1.
Comparison of AsNOS (GenBank accession no. AF053344) and DNOS (9) deduced amino acid sequences. The first AsNOS amino acid is the putative initiation methionine; alignment with DNOS begins at D81. Presumed cofactor-binding domains for heme, calmodulin (CaM), FMN, FAD pyrophosphate (FAD PPi), FAD isoalloxazine (FAD Iso), NADPH ribose, NADPH adenine, and NADPH are double-underlined (9). Abbreviations for the amino acid residues are as follows: A, Ala; C, Cys; D, Asp; E, Glu; F, Phe; G, Gly; H, His; I, Ile; K, Lys; L, Leu; M, Met; N, Asn; P, Pro; Q, Gln; R, Arg; S, Ser; T, Thr; V, Val; W, Trp; and Y, Tyr.
Temporal and Tissue-Specific Expression of AsNOS.
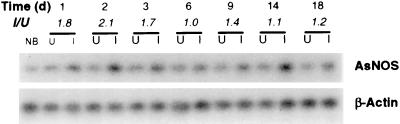
To determine the temporal pattern of AsNOS expression after ingestion of Plasmodium, we analyzed total RNA from uninfected and Plasmodium-infected A. stephensi using RT-PCR with AsNOS-specific primers and A. gambiae-derived β-actin primers (Fig. 2). After normalization to β-actin, it was apparent that, although AsNOS expression could be induced by blood feeding alone, a stronger induction occurred in infected mosquitoes at 1–3 days pBM (Fig. 2), coincident with P. berghei invasion and early oocyst development. At 6 days pBM, during oocyst growth in infected mosquitoes, no induction was observed. By 9 days pBM, however, at the initiation of sporozoite release, AsNOS expression was again elevated in infected mosquitoes. By 14 days pBM, after sporozoite penetration of the salivary glands, induction of AsNOS expression was nearly undetectable in infected mosquitoes.
Figure 2.
AsNOS expression in uninfected and Plasmodium-infected A. stephensi. Total RNA from non-blood fed (NB), blood fed uninfected (U), and P. berghei-infected (I) A. stephensi was assayed by using semi-quantitative RT-PCR for AsNOS at 1–18 days pBM. AsNOS transcript abundance, reflected by hybridization signal intensity, was normalized for each time point against β-actin to correct for sample-to-sample differences in PCR template. The ratio of normalized infected/uninfected (I/U) indicates the fold-induction of AsNOS expression in infected mosquitoes.
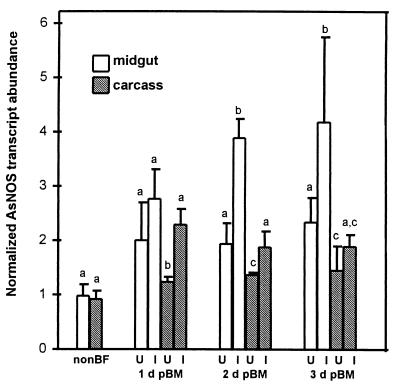
Because early induction of AsNOS suggested an effect on the midgut phase of parasite development, we examined the first few days after blood feeding in greater detail, beginning with the impact of blood alone on gene expression. AsNOS expression was significantly higher in both midguts (1–3 days pBM) and carcasses (1–2 days pBM) of blood fed uninfected mosquitoes than in the corresponding tissues of non-blood fed mosquitoes (comparison not shown, α = 0.075) with uninfected midgut expression significantly higher than uninfected carcass expression at each time point (Fig. 3). Adult A. stephensi possess at least five different species of Gram-negative midgut bacteria, which are acquired during larval development, are maintained transtadially, and show blood meal-induced growth for 3–5 days (22). We also observed blood meal-associated bacterial growth in the midgut (Table 1) and determined that although gentamicin (50 μg/ml) was bactericidal to these organisms in vitro, it was bacteriostatic in the midgut environment (Table 1). Induction of AsNOS after an uninfected blood meal was lower in mosquitoes treated with gentamicin (50 μg/ml) compared with untreated mosquitoes (unpublished data), suggesting that midgut bacterial growth coincident with blood feeding induces AsNOS expression.
Figure 3.
AsNOS expression in uninfected and Plasmodium-infected A. stephensi. Total RNA from midguts and carcasses of non-blood fed (nonBF), blood fed uninfected (U), and P. berghei-infected (I) A. stephensi was assayed by using semi-quantitative RT-PCR for AsNOS at 1, 2, and 3 days post-blood meal (d pBM). Transcript abundance was normalized against a ribosomal S7 protein gene (13, 14) to correct for sample-to-sample differences in PCR template. Data from three treatment replicates at each time point were analyzed by paired t test (α = 0.075); significant differences within groups are indicated by different letters.
Table 1.
Blood meal and antibiotic effects on midgut bacteria (×105 CFU per gut ± SEM)
| Days post-blood meal | 1 | 3 | |
|---|---|---|---|
| nonBF | BF and Pb | BF and Pb | |
| Gentamicin (−) | 1.0 ± 0.4a | 1.9 ± 0.3a | 6.2 ± 0.9b |
| Gentamicin (+) | ND | 1.4 ± 0.1a | 1.4 ± 0.2a |
Data were analyzed by Tukey’s test (α = 0.05). Significant differences are indicated by different letters. CFU, colony-forming unit; nonBF, non-blood fed, BF & Pb, blood fed uninfected and P. berghei-infected (see Materials and Methods); ND, not done.
The addition of parasites to the blood meal enhanced AsNOS expression in the midgut above uninfected levels at 1, 2, and 3 days pBM (Fig. 3); only the increase at 1 day pBM was not statistically significant. Although carcass expression from infected mosquitoes also was consistently greater than from uninfected mosquitoes, carcass expression was equal to or less than midgut expression at all time points (Fig. 3).
The difference between apparent midgut AsNOS induction by bacteria and by Plasmodium also was reflected in diaphorase staining of non-blood fed, blood fed uninfected, and infected midguts at 24 hr pBM. We observed that the purplish-blue diformazan precipitated more rapidly in infected midguts (P. berghei and P. falciparum) than in blood fed or non-blood fed midguts and did not occur without NADPH or when NADH was substituted in the reaction mixture (data not shown). Soluble NADPH-dependent diaphorase reactions at 24 hr pBM showed a significant increase from non-blood fed (0.095 ± 0.012 OD units) to blood fed uninfected (0.205 ± 0.027 OD units) to P. berghei-infected midguts (0.293 ± 0.035 OD units), confirming that increased synthesis of AsNOS was correlated directly with induction of expression. Staining of uninfected blood fed midguts was uniform (Fig. 4A). In contrast, both P. berghei- (Fig. 4B) and P. falciparum-infected midguts (not shown) at 24 hr pBM also showed darkly stained individual cells concentrated in the posterior midgut, where Plasmodium development typically occurs (29). Double-staining of midguts with diaphorase and propidium iodide demonstrated that, in some cases, diaphorase-positive cells were colocalized with immature oocysts of both species (data not shown).
Figure 4.
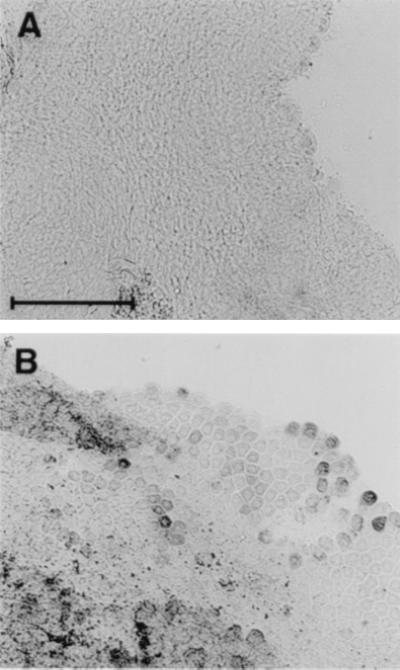
Representative diaphorase staining of midguts from blood fed uninfected and Plasmodium-infected A. stephensi. (A) Uninfected 24 hr pBM. (Bar = 300 μm.) (B) P. berghei-infected 24 hr pBM (same magnification as A).
Effect of Infection on AsNOS Activity and Hemolymph NO2−/NO3− Levels.
In control reactions without l-NAME, 3 of 4 groups of blood fed mosquitoes had lower specific activities than non-blood fed mosquitoes (Table 2). The presence of fully matured ova, which contain as much as 120 μg of protein (30), in blood fed mosquitoes may have resulted in an underestimation of AsNOS activity. The difference in AsNOS specific activity between infected and uninfected mosquitoes was much greater at 9 days than at 6 days (P < 0.01, Table 2) in control reactions. Relatively static enzyme activity at 6 days likely reflects the absence of RT-PCR detectable induction of AsNOS in infected mosquitoes at this time (Fig. 2). At 9 days pBM, however, when AsNOS expression was induced in infected mosquitoes (Fig. 2), specific activity was 5.3-fold higher in infected mosquitoes. The effect of l-NAME on AsNOS activity in infected mosquitoes showed similar temporal differences. AsNOS activity in infected mosquitoes was relatively unchanged by l-NAME at 6 days, whereas activity in infected mosquitoes was significantly inhibited by l-NAME three days later (P < 0.005, Table 2).
Table 2.
AsNOS specific activity in uninfected and infected mosquitoes
| Sample | × 104 CPM/mg/min
|
|
|---|---|---|
| Control | l-name | |
| non-blood fed | 38.4 | 13.7 |
| 6 days infected | 14.4 | 16.3 |
| 6 days uninfected | 9.9 | 10.0 |
| 9 days infected | 66.0 | 20.0 |
| 9 days uninfected | 12.4 | 8.4 |
Data analysis by contingency tables (1 df, χ0.05 = 3.84) indicated significant differences at 6 and 9 days in parasite induction of AsNOS in control reactions (χcalc = 6.78) and l-name inhibition in infected mosquitoes (χcalc = 9.40).
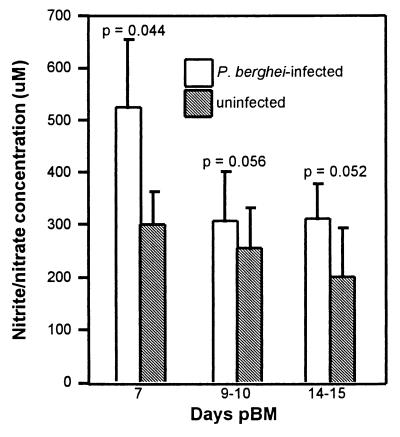
Hemolymph levels of NO2−/NO3−, the stable reaction products of NO, were significantly higher in Plasmodium-infected mosquitoes than in uninfected mosquitoes at all time points, with the greatest relative difference at 7 days pBM (Fig. 5). At 7 days pBM, higher NO2−/NO3− levels in infected mosquitoes likely reflect sustained induction from 1–3 days pBM (Fig. 2). In the mouse, NO production may last for 4–5 days after synthesis of inducible NOS (31). Levels at 9–10 days pBM may be correlated with induction (Fig. 2) and a higher specific activity in infected mosquitoes at this time (Table 2). AsNOS did not appear to be induced in infected mosquitoes at 14 days pBM (Fig. 2); elevated NO2−/NO3− at this time may be the result of sustained NO production of AsNOS induced at 9 days.
Figure 5.
Hemolymph nitrite/nitrate of blood fed uninfected and P. berghei-infected A. stephensi was determined at 7, 9–10, and 14–15 days pBM by using a cadmium reduction/Griess reagent microassay (24). Means were analyzed by using a paired t test (α = 0.075); P values are reported above the bars.
Dietary Manipulation of Mosquitoes and Its Impact on Plasmodium Infection.
Despite the correlation of parasite development with AsNOS expression and NO production, laboratory reared A. stephensi still support parasite infections. Because l-Arg is at low levels in mammalian blood and is essential for both ovarian and Plasmodium development (32–34), NO production may be limited by Plasmodium infection. Based on this hypothesis and a study of dietary l-Arg effects on Cryptosporidium infections in mice (35), we provided A. stephensi with either l-Arg or l-Cit supplements and examined the effect on P. berghei development. No differences in survivorship were observed across the treatments; individual infections ranged from 1–885 oocysts/gut. At 0.2% l-Arg, the percentage of infected mosquitoes was reduced by 28%; there was also a significant reduction at a 1,000-fold dilution of the supplement (Table 3). No reduction in infections was observed with l-Cit (Table 3), indicating that parasite killing was l-Arg-dependent. Although the percentage of mosquitoes infected was correlated with treatment (Table 3), the mean number of oocysts/mosquito did not vary among treatments (data not shown, ANOVA α = 0.05), suggesting that parasites were destroyed before oocyst formation.
Table 3.
Effects of amino acid supplements on oocyst infection
| Diet | Percentage infected (n) |
|---|---|
| Water only | 85 (151)a |
| 0.2% l-Arg | 57 (171)b |
| 0.002% l-Arg | 70 (75)b |
| 0.2% l-Cit | 81 (94)a |
Data were analyzed by contingency tables (α = 0.05) with significant differences indicated by different letters. n, number of mosquitoes dissected.
The enhanced early expression of AsNOS in infected mosquitoes (Figs. 2 and 3) suggested that the l-Arg effect was mediated by AsNOS. To support this conclusion, we examined the effect of l-NAME, which appeared to interfere with induced AsNOS (Table 2), on Plasmodium development. For these experiments, mosquitoes were maintained on gentamicin (50 μg/ml) before blood feeding to inhibit bacterial growth that could have confounded the l-NAME experiments. No differences in survivorship were observed across the treatments. Provision of l-NAME in the blood meal and water significantly increased P. falciparum oocyst infection in A. stephensi; the d-NAME and water-fed control groups had nearly identical mean oocyst infections (Table 4).
Table 4.
Effects of l- and d-name on oocyst infection
| Treatment
|
|||
|---|---|---|---|
| Water | l-NAME | d-NAME | |
| Oocysts/gut (±SEM) | 72.9 ± 4.6a | 129.2 ± 6.4b | 76.7 ± 4.2a |
| Total dissected | 128 | 133 | 130 |
Data were analyzed by Tukey’s test (α = 0.05); significant differences are indicated by different letters. NAME, Nω-nitro-arginine methyl ester.
DISCUSSION
We have identified an invertebrate NOS gene that shares significant sequence homology with other insect and vertebrate NOS genes and is pathogen-inducible. Our results showed that AsNOS is induced after Plasmodium infection, which suggests that mosquitoes and vertebrates share a conserved response against this multi-host pathogen. We propose that this response, like the vertebrate response, is a defense reaction that limits Plasmodium development and support this assertion with data that show not only temporal and tissue-specific association of AsNOS expression and activity with Plasmodium development but also that experimental manipulation of this response alters pathogen infection.
AsNOS induction by Plasmodium infection at 1 day pBM is likely a response to parasite invasion of the midgut, which is maximal at this time for P. berghei (29). The opposing effects of l-Arg and l-NAME suggest that parasites are targeted before oocyst development, a hypothesis consistent with the demonstrated susceptibility of gametocytes to NO damage (8). The induction observed in response to normal bacterial growth could prime an amplified midgut response to parasite infection: gentamicin-treated mosquitoes had slightly higher mean oocyst infections than did untreated mosquitoes (data not shown), an observation also made by others (36). Diaphorase staining confirmed that early parasite-induced AsNOS expression was correlated with a quantitative increase in AsNOS activity but also demonstrated that this response was qualitatively different from the response to bacterial growth. Because ookinete-associated pathology does not appear to be limited to the area of invasion (37), the pattern of diaphorase activity seen in infected mosquitoes may result from ookinete movement as well as parasite invasion.
Our observations of parallel induction of AsNOS in the midgut and carcass during the first three days after blood feeding confirms observations of others that the midgut appears to function as a first line of defense against bacterial growth and ingested parasites, whereas carcass induction may be a signal-mediated secondary response (1, 3) that acts against organisms that breach the midgut barrier. Because lipopolysaccharide is a potent inducer of NOS expression (38), lipopolysaccharide synthesized during bacterial growth could enhance AsNOS expression after blood feeding, whereas parasite invasion and P. berghei metabolites further stimulate expression in the highly reactive midgut and carcass. Plasmodium antigens are potent inducers of mouse macrophage NO production (39). Fat body may be the source of carcass expression. However, nearly every vertebrate cell type can produce inducible NOS (40), suggesting that a variety of tissues be examined for expression.
The biological significance of enhanced gene expression and enzyme activity at 9 days pBM is unknown, but correlation with the onset of sporozoite release suggests that mosquito NO may interfere with this phase of parasite development as well. Sporozoite inactivation by vertebrate NO has been demonstrated during hepatocyte invasion (7), and preliminary in vitro experiments from our laboratory indicate that salivary gland sporozoites are inactivated within hours of exposure to NO levels similar to those detected in hemolymph. Interestingly, hemolymph NO2−/NO3− levels were much higher than levels in naive (10–50 μM) or Plasmodium-infected (up to 100–200 μM) mice and humans (24, 41), implying that Plasmodium is exposed to a uniquely hostile environment in the mosquito host.
Acknowledgments
We thank Y. Liu, M. Dowler, and J. Glass for technical support; R. Wirtz, J. Ribeiro, and D. Wink for helpful discussions. This work was supported by National Institutes of Health, United Nations Development Program/World Bank/World Health Organization Special Program for Research and Training in Tropical Diseases, Uniformed Services University of the Health Sciences, and U.S. Department of Defense.
ABBREVIATIONS
- l-NAME
Nω-nitro-l-arginine methyl ester
- NOS
NO synthase
- DNOS
Drosophila NOS
- RT-PCR
reverse transcriptase-PCR
- pBM
post-blood meal
- AsNOS
A. stephensi NOS
- l-Cit
l-citrulline
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF053344).
References
- 1.Dimopoulos G, Richman A, della Torre A, Kafatos F C, Louis C. Proc Natl Acad Sci USA. 1996;93:13066–13071. doi: 10.1073/pnas.93.23.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richman A M, Dimopoulos G, Seeley D, Kafatos F C. EMBO J. 1997;16:6114–6119. doi: 10.1093/emboj/16.20.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimopoulos G, Richman A M, Muller H-M, Kafatos F C. Proc Natl Acad Sci USA. 1997;94:11508–11513. doi: 10.1073/pnas.94.21.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stuehr D J. In: Methods in Enzymology. Packer L, editor. 268A. San Diego: Academic; 1996. pp. 324–333. [DOI] [PubMed] [Google Scholar]
- 5.Nathan C F, Hibbs J B., Jr Curr Opin Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 6.Karupiah G, Xie Q-W, Buller M L, Nathan C F, Duarte C, MacMicking J D. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 7.Mellouk S, Hoffman S L, Liu Z-Z, de la Vega P, Billiar T R, Nussler A K. Infect Immun. 1994;62:4043–4046. doi: 10.1128/iai.62.9.4043-4046.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naotunne T de S, Karunaweera N D, Mendis K N, Carter R. Immunology. 1993;78:555–562. [PMC free article] [PubMed] [Google Scholar]
- 9.Regulski M, Tully T. Proc Natl Acad Sci USA. 1995;92:9072–9076. doi: 10.1073/pnas.92.20.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohler K M, Butler L D. Mol Immunol. 1991;28:437–447. doi: 10.1016/0161-5890(91)90157-f. [DOI] [PubMed] [Google Scholar]
- 11.Kell W M, LeClaire R D, Schmidt R, Lansford M. Methods Mol Cell Biol. 1995;5:105–111. [Google Scholar]
- 12.Cui L, Webb B A. J Gen Virol. 1997;78:1807–1817. doi: 10.1099/0022-1317-78-7-1807. [DOI] [PubMed] [Google Scholar]
- 13.Salazar C E, Mills-Hamm D, Kumar V, Collins F H. Nucleic Acids Res. 1993;21:4147. doi: 10.1093/nar/21.17.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barillas-Mury C, Charlesworth A, Gross I, Richman A M, Hoffmann J A, Kafatos F C. EMBO J. 1996;15:4691–4701. [PMC free article] [PubMed] [Google Scholar]
- 15.Devereux J, Haeberli P, Smithies O. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 17.Frohman M A. In: PCR Protocols: A Guide to Methods and Applications. Innis M A, Gelfand D H, Sninsky J J, White T J, editors. San Diego: Academic; 1990. pp. 28–38. [Google Scholar]
- 18.Mosmann T. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro J M C, Nussenzveig R H. FEBS Lett. 1993;330:165–168. doi: 10.1016/0014-5793(93)80265-v. [DOI] [PubMed] [Google Scholar]
- 20.Nussenzveig R H, Bentley D L, Ribeiro J M C. J Exp Biol. 1995;198:1093–1098. doi: 10.1242/jeb.198.5.1093. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto T, Nakane M, Pollock J S, Kuk J E, Forstermann U. Neurosci Lett. 1993;155:61–64. doi: 10.1016/0304-3940(93)90673-9. [DOI] [PubMed] [Google Scholar]
- 22.Pumpuni C B, Demaio J, Kent M, Davis J R, Beier J C. Am J Trop Med Hyg. 1996;54:214–218. doi: 10.4269/ajtmh.1996.54.214. [DOI] [PubMed] [Google Scholar]
- 23.Hagedorn H H, Turner S, Hagedorn E A, Pontecorvo D, Greenbaum P, Pfeiffer D, Wheelock G, Flanagan T R. J Insect Physiol. 1977;23:203–206. doi: 10.1016/0022-1910(77)90030-0. [DOI] [PubMed] [Google Scholar]
- 24.Vodovotz Y. BioTechniques. 1996;20:390–394. doi: 10.2144/19962003390. [DOI] [PubMed] [Google Scholar]
- 25.Munkirs D D, Christensen B M, Tracy J W. J Invertebr Pathol. 1990;56:267–270. doi: 10.1016/0022-2011(90)90110-r. [DOI] [PubMed] [Google Scholar]
- 26.Kozak M. Nucleic Acids Res. 1984;12:857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuda M, Hirai M, Miura K, Matsumura H, Ando K, Chinzei Y. Eur J Biochem. 1996;242:807–812. doi: 10.1111/j.1432-1033.1996.0807r.x. [DOI] [PubMed] [Google Scholar]
- 28.Hall A V, Antoniou H, Wang Y, Cheung A H, Arbus A M, Olson S L, Lu W C, Kau C, Marsden P A. J Biol Chem. 1994;269:33082–33090. [PubMed] [Google Scholar]
- 29.Ramaswamy M S, Kulasekera R, Wanniarachchi I C, Srikrishnaraj K A, Ramaswamy R. Med Vet Entomol. 1997;11:290–296. doi: 10.1111/j.1365-2915.1997.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 30.Briegel H. J Med Entomol. 1990;27:839–850. doi: 10.1093/jmedent/27.5.839. [DOI] [PubMed] [Google Scholar]
- 31.Vodovotz Y, Kwon N, Popischil M, Manning J, Paik J, Nathan C. J Immunol. 1994;152:4110–4118. [PubMed] [Google Scholar]
- 32.Ball G H, Chao J. Exp Parasitol. 1976;39:115–118. doi: 10.1016/0014-4894(76)90018-7. [DOI] [PubMed] [Google Scholar]
- 33.France K R, Judson C L. J Insect Physiol. 1979;25:841–846. [Google Scholar]
- 34.Briegel H. J Insect Physiol. 1986;32:455–462. [Google Scholar]
- 35.Leitch G J, He Q. Infect Immun. 1994;62:5173–5176. doi: 10.1128/iai.62.11.5173-5176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beier M S, Pumpuni C B, Beier J C, Davis J R. J Med Entomol. 1994;31:561–565. doi: 10.1093/jmedent/31.4.561. [DOI] [PubMed] [Google Scholar]
- 37.Torii M, Nakamura K, Sieber K P, Miller L H, Aikawa M. J Protozool. 1992;39:449–454. doi: 10.1111/j.1550-7408.1992.tb04830.x. [DOI] [PubMed] [Google Scholar]
- 38.Wu C-C, C. Thiemermann C. In: Methods in Enzymology. Packer L, editor. 268A. San Diego: Academic; 1996. pp. 408–420. [DOI] [PubMed] [Google Scholar]
- 39.Rockett K A, Kwiatkowski D, Bate C A W, Awburn M M, Rockett E J, Clark I A. J Infect. 1996;32:187–196. doi: 10.1016/s0163-4453(96)80018-1. [DOI] [PubMed] [Google Scholar]
- 40.Nathan C. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 41.Hegesh E, Shiloah J. Clin Chim Acta. 1982;125:107–115. doi: 10.1016/0009-8981(82)90187-5. [DOI] [PubMed] [Google Scholar]