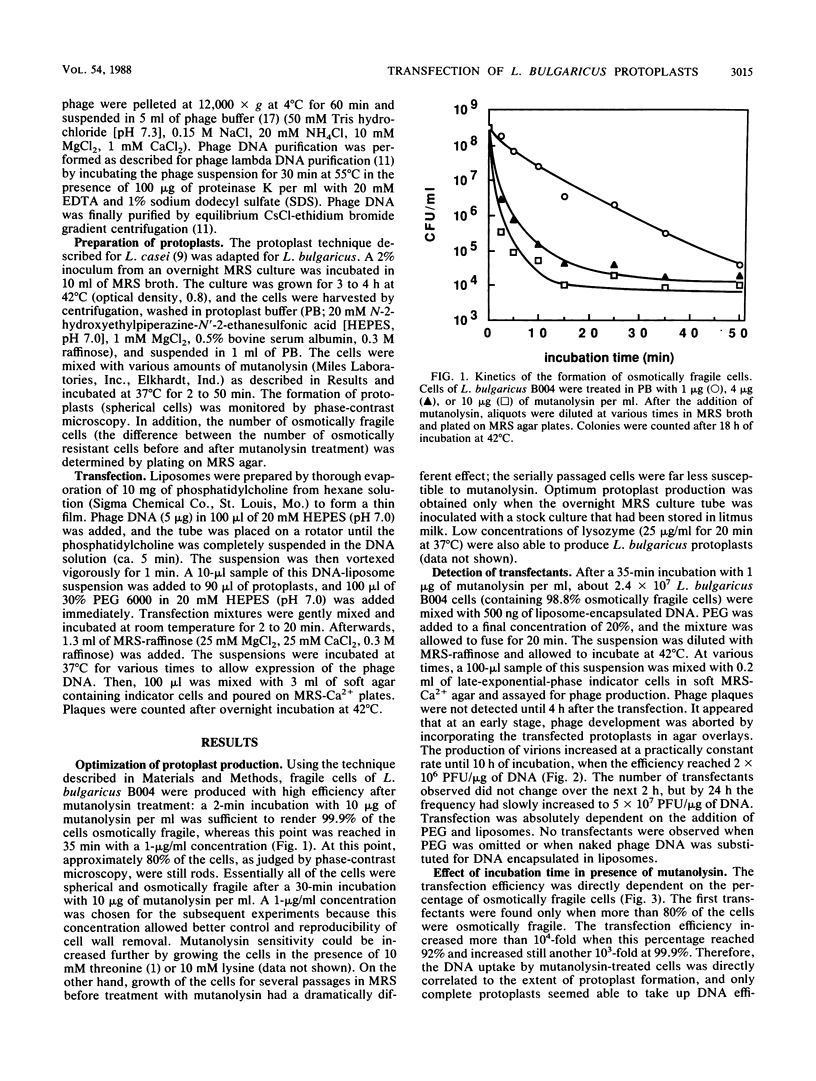
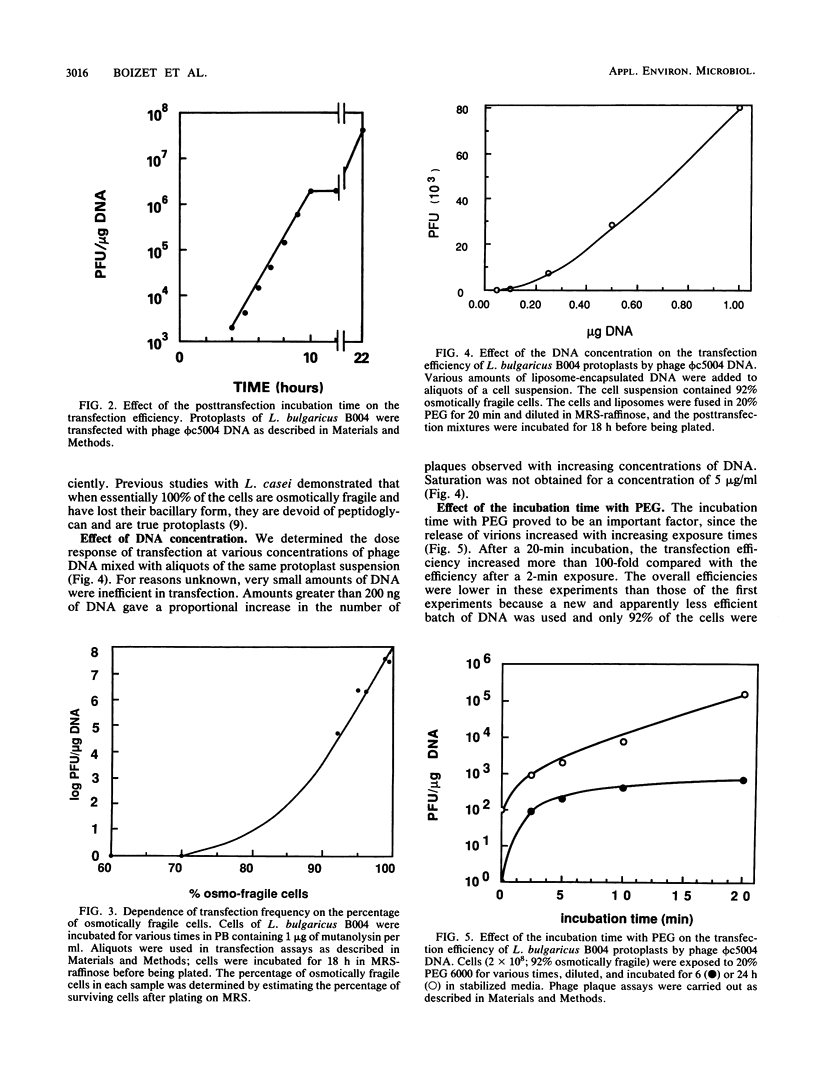
Abstract
A protoplast transfection system has been developed for Lactobacillus bulgaricus. The procedure involves a polyethylene glycol-mediated fusion of bacteriophage DNA encapsulated in liposomes into mutanolysin-treated cells. With L. bulgaricus B004 and DNA isolated from the phage phi c5004, transfection reached a maximum when at least 95% of the cells were osmotically fragile. The incorporation of phage DNA into liposomes was essential; no transfectants were detected in the absence of liposomes. The largest number of transfectants was observed after longer periods (20 min) of fusion of mutanolysin-treated cells and liposomes with polyethylene glycol. The maximum efficiency of 5 x 10(7) PFU/microgram of DNA was reached after a 24-h incubation in growth media prior to plating transfected cells in an agar overlay to detect the appearance of plaques. A minimum of 4 h of incubation in growth medium after fusion was required to detect the production and release of virions. The possibility that the high frequencies observed were due to bursting of transfected cells and subsequent infection of additional cells was found not to be a factor. The number of transfectants observed was directly proportional to the quantity of DNA added. These results define conditions appropriate for the introduction of DNA into L. bulgaricus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chassy B. M. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. doi: 10.1016/0006-291x(76)91188-8. [DOI] [PubMed] [Google Scholar]
- Gibson E. M., Chace N. M., London S. B., London J. Transfer of plasmid-mediated antibiotic resistance from streptococci to lactobacilli. J Bacteriol. 1979 Jan;137(1):614–619. doi: 10.1128/jb.137.1.614-619.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Wickner L. J., Chassy B. M. Production and Regeneration of Lactobacillus casei Protoplasts. Appl Environ Microbiol. 1984 Nov;48(5):994–1000. doi: 10.1128/aem.48.5.994-1000.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. H., Savage D. C. Genetic transformation of rifampicin resistance in Lactobacillus acidophilus. J Gen Microbiol. 1986 Aug;132(8):2107–2111. doi: 10.1099/00221287-132-8-2107. [DOI] [PubMed] [Google Scholar]
- McCarthy D. M., Lin J. H., Rinckel L. A., Savage D. C. Genetic transformation in Lactobacillus sp. strain 100-33 of the capacity to colonize the nonsecreting gastric epithelium in mice. Appl Environ Microbiol. 1988 Feb;54(2):416–422. doi: 10.1128/aem.54.2.416-422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli L., Cocconcelli P. S., Bottazzi V., Damiani G., Ferretti L., Sgaramella V. Lactobacillus protoplast transformation. Plasmid. 1987 Jan;17(1):73–75. doi: 10.1016/0147-619x(87)90013-8. [DOI] [PubMed] [Google Scholar]
- Muriana P. M., Klaenhammer T. R. Conjugal Transfer of Plasmid-Encoded Determinants for Bacteriocin Production and Immunity in Lactobacillus acidophilus 88. Appl Environ Microbiol. 1987 Mar;53(3):553–560. doi: 10.1128/aem.53.3.553-560.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Kadota M., Sakurai T. Prophage Curing in Lactobacillus casei by Isolation of a Thermoinducible Mutant. Appl Environ Microbiol. 1982 Jun;43(6):1284–1287. doi: 10.1128/aem.43.6.1284-1287.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrago A. W., Chassy B. M., Dobrogosz W. J. Conjugal plasmid transfer (pAM beta 1) in Lactobacillus plantarum. Appl Environ Microbiol. 1986 Sep;52(3):574–576. doi: 10.1128/aem.52.3.574-576.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W. Conjugal transfer of plasmid pAM beta 1 in Lactobacillus reuteri and between lactobacilli and Enterococcus faecalis. Appl Environ Microbiol. 1987 Nov;53(11):2693–2695. doi: 10.1128/aem.53.11.2693-2695.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovo M., Morelli L., Bottazzi V., Gasson M. J. Conjugal Transfer of Broad-Host-Range Plasmid pAMbeta1 into Enteric Species of Lactic Acid Bacteria. Appl Environ Microbiol. 1983 Sep;46(3):753–755. doi: 10.1128/aem.46.3.753-755.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]


