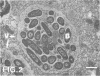
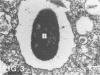
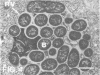
Abstract
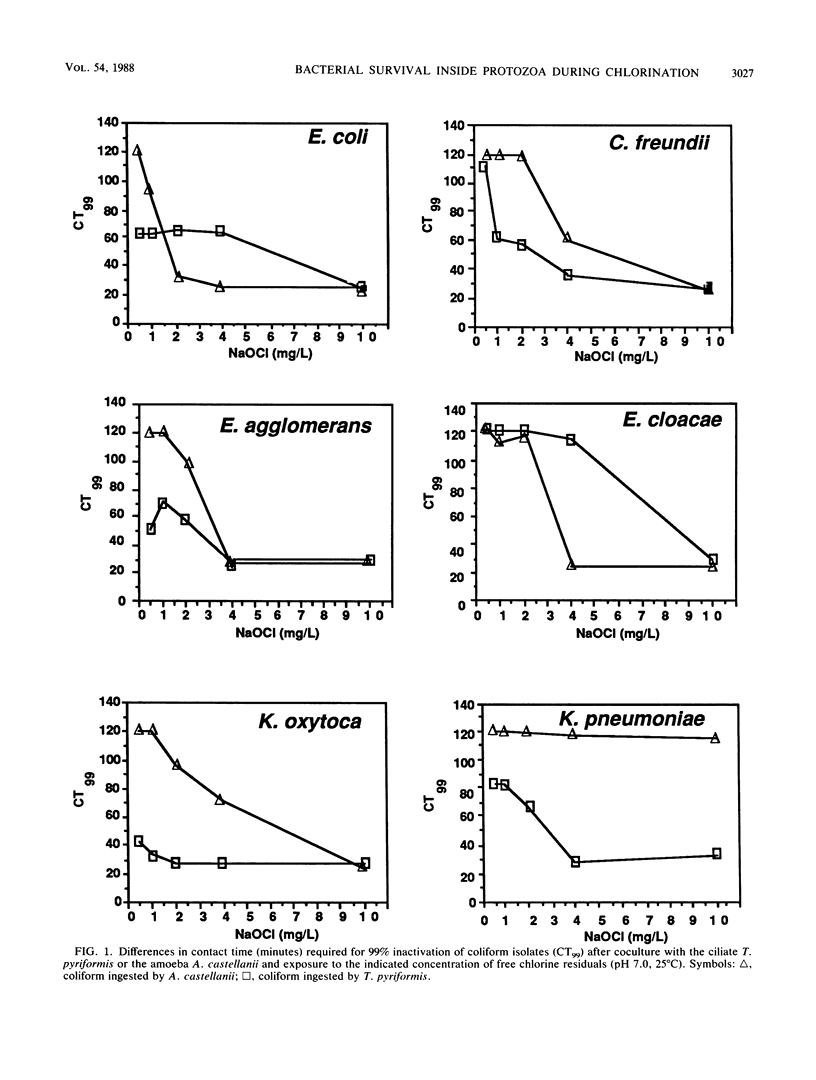
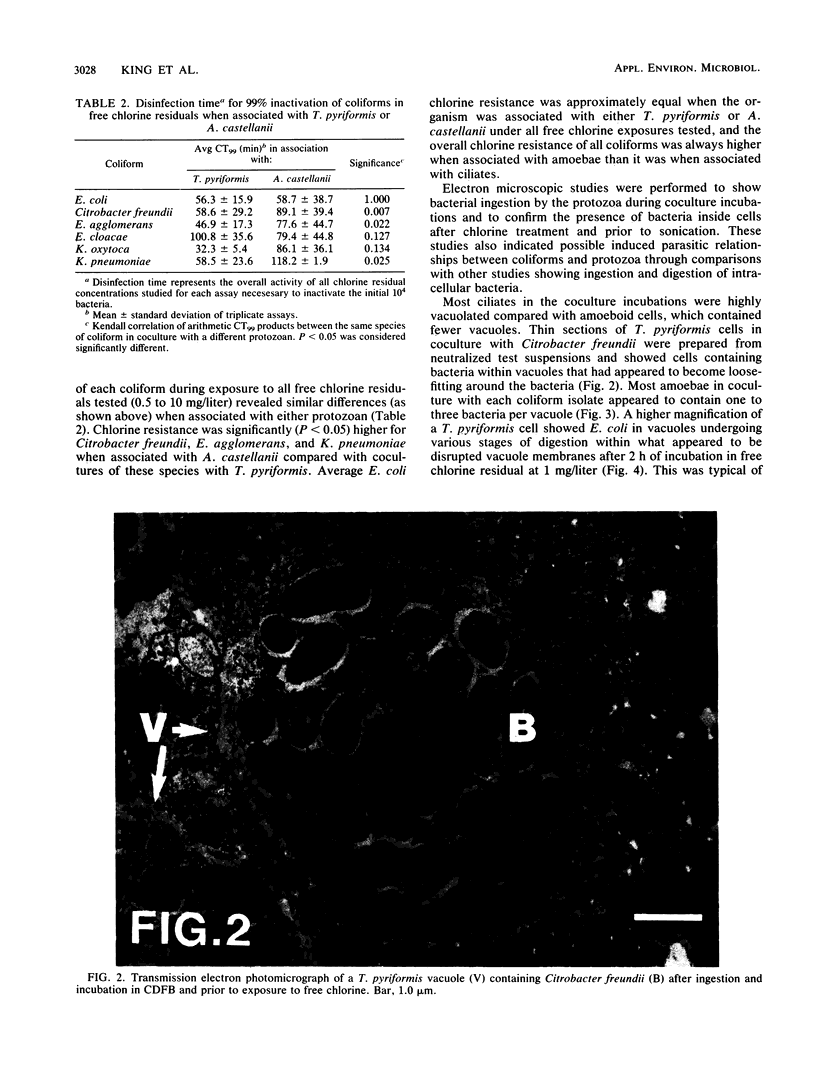
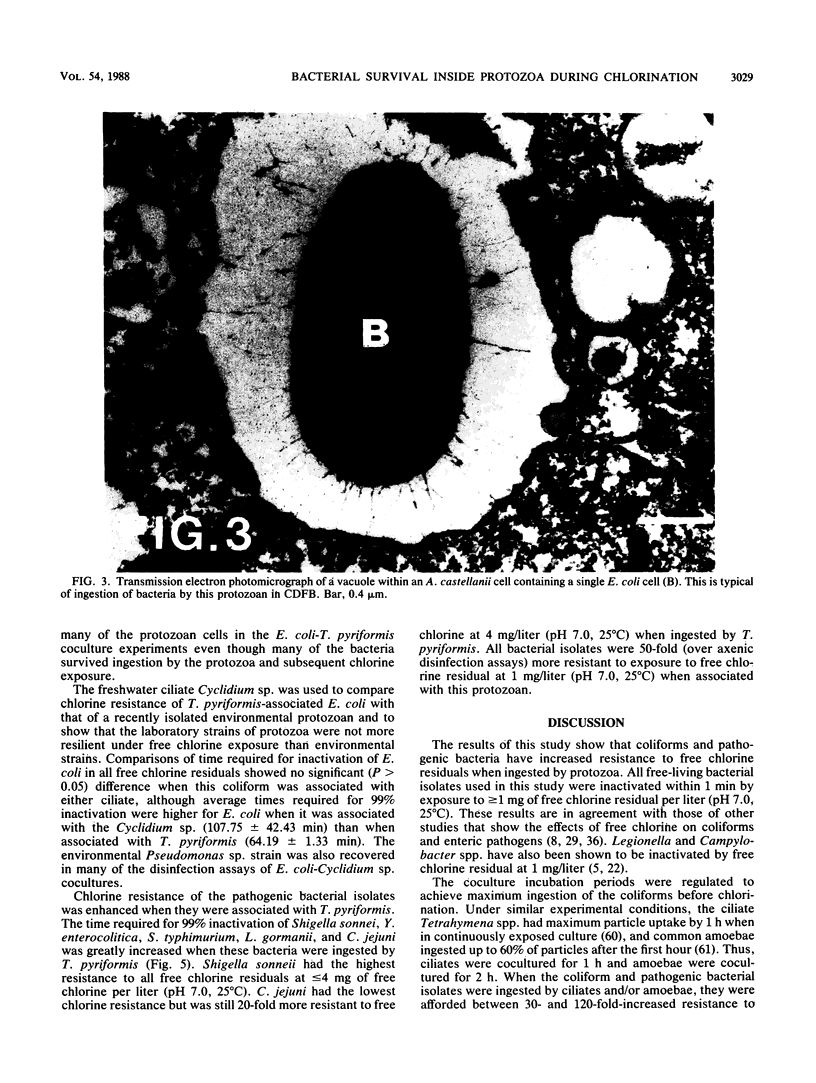
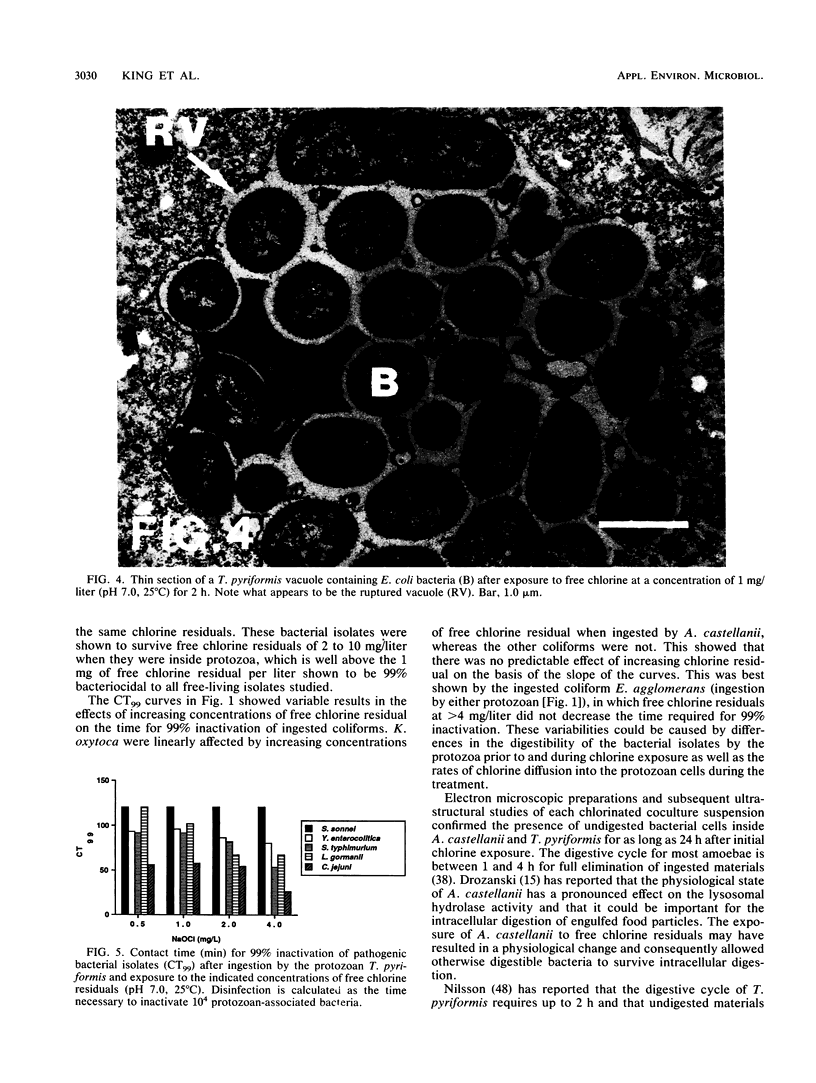
The susceptibility of coliform bacteria and bacterial pathogens to free chlorine residuals was determined before and after incubation with amoebae and ciliate protozoa. Viability of bacteria was quantified to determine their resistance to free chlorine residuals when ingested by laboratory strains of Acanthamoeba castellanii and Tetrahymena pyriformis. Cocultures of bacteria and protozoa were incubated to facilitate ingestion of the bacteria and then were chlorinated, neutralized, and sonicated to release intracellular bacteria. Qualitative susceptibility of protozoan strains to free chlorine was also assessed. Protozoa were shown to survive and grow after exposure to levels of free chlorine residuals that killed free-living bacteria. Ingested coliforms Escherichia coli, Citrobacter freundii, Enterobacter agglomerans, Enterobacter cloacae, Klebsiella pneumoniae, and Klebsiella oxytoca and bacterial pathogens Salmonella typhimurium, Yersinia enterocolitica, Shigella sonnei, Legionella gormanii, and Campylobacter jejuni had increased resistance to free chlorine residuals. Bacteria could be cultured from within treated protozoans well after the time required for 99% inactivation of free-living cells. All bacterial pathogens were greater than 50-fold more resistant to free chlorine when ingested by T. pyriformis. Escherichia coli ingested by a Cyclidium sp., a ciliate isolated from a drinking water reservoir, were also shown to be more resistant to free chlorine. The mechanism that increased resistance appeared to be survival within protozoan cells. This study indicates that bacteria can survive ingestion by protozoa. This bacterium-protozoan association provides bacteria with increased resistance to free chlorine residuals which can lead to persistence of bacteria in chlorine-treated water. We propose that resistance to digestion by predatory protozoa was an evolutionary precursor of pathogenicity in bacteria and that today it is a mechanism for survival of fastidious bacteria in dilute and inhospitable aquatic environments.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. L., Calomiris J. J., Seidler R. J. Selection of antibiotic-resistant standard plate count bacteria during water treatment. Appl Environ Microbiol. 1982 Aug;44(2):308–316. doi: 10.1128/aem.44.2.308-316.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaree J. M., Fields B. S., Feeley J. C., Gorman G. W., Martin W. T. Isolation of protozoa from water associated with a legionellosis outbreak and demonstration of intracellular multiplication of Legionella pneumophila. Appl Environ Microbiol. 1986 Feb;51(2):422–424. doi: 10.1128/aem.51.2.422-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Smith P. F., Wang W. L., Hoff J. C. Inactivation of Campylobacter jejuni by chlorine and monochloramine. Appl Environ Microbiol. 1986 Feb;51(2):307–311. doi: 10.1128/aem.51.2.307-311.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG S. L., BERG G., CLARKE N. A., KABLER P. W. Survival, and protection against chlorination, of human enteric pathogens in free-living nematodes isolated from water supplies. Am J Trop Med Hyg. 1960 Mar;9:136–142. doi: 10.4269/ajtmh.1960.9.136. [DOI] [PubMed] [Google Scholar]
- Craun G. F. Waterborne giardiasis in the United States: a review. Am J Public Health. 1979 Aug;69(8):817–819. doi: 10.2105/ajph.69.8.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonckheere J., van de Voorde H. Differences in destruction of cysts of pathogenic and nonpathogenic Naegleria and Acanthamoeba by chlorine. Appl Environ Microbiol. 1976 Feb;31(2):294–297. doi: 10.1128/aem.31.2.294-297.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozanski W., Chmielewski T. Electron microscopic studies of Acanthamoeba castellanii infected with obligate intracellular bacterial parasite. Acta Microbiol Pol. 1979;28(2):123–133. [PubMed] [Google Scholar]
- Drozański W. Activity and distribution of bacteriolytic N-acetyl-muramidase during growth of Acanthamoeba castellanii in axenic culture. Acta Microbiol Pol. 1978;27(3):243–256. [PubMed] [Google Scholar]
- Elliott A. M., Clemmons G. L. An ultrastructural study of ingestion and digestion in Tetrahymena pyriformis. J Protozool. 1966 May;13(2):311–323. doi: 10.1111/j.1550-7408.1966.tb01912.x. [DOI] [PubMed] [Google Scholar]
- Feely D. E., Chase D. G., Hardin E. L., Erlandsen S. L. Ultrastructural evidence for the presence of bacteria, viral-like particles, and mycoplasma-like organisms associated with Giardia spp. J Protozool. 1988 Feb;35(1):151–158. doi: 10.1111/j.1550-7408.1988.tb04095.x. [DOI] [PubMed] [Google Scholar]
- Fields B. S., Barbaree J. M., Shotts E. B., Jr, Feeley J. C., Morrill W. E., Sanden G. N., Dykstra M. J. Comparison of guinea pig and protozoan models for determining virulence of Legionella species. Infect Immun. 1986 Sep;53(3):553–559. doi: 10.1128/iai.53.3.553-559.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. S., Shotts E. B., Jr, Feeley J. C., Gorman G. W., Martin W. T. Proliferation of Legionella pneumophila as an intracellular parasite of the ciliated protozoan Tetrahymena pyriformis. Appl Environ Microbiol. 1984 Mar;47(3):467–471. doi: 10.1128/aem.47.3.467-471.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher-Hoch S. P., Bartlett C. L., Tobin J. O., Gillett M. B., Nelson A. M., Pritchard J. E., Smith M. G., Swann R. A., Talbot J. M., Thomas J. A. Investigation and control of an outbreaks of legionnaires' disease in a district general hospital. Lancet. 1981 Apr 25;1(8226):932–936. doi: 10.1016/s0140-6736(81)91626-3. [DOI] [PubMed] [Google Scholar]
- Görtz H. D. Infections of Paramecium bursaria with bacteria and yeasts. J Cell Sci. 1982 Dec;58:445–453. doi: 10.1242/jcs.58.1.445. [DOI] [PubMed] [Google Scholar]
- Humphreys W. J., Spurlock B. O., Johnson J. S. Transmission electron microscopy of tissue prepared for scanning electron microscopy by ethanol-cryofracturing. Stain Technol. 1975 Mar;50(2):119–125. doi: 10.3109/10520297509117045. [DOI] [PubMed] [Google Scholar]
- Jacquemin J. L., Simitzis-Le Flohic A. M., Chauveau N. Recherche des amibes libres dans l'eau. Etude du réseau d'alimentation en eaux de la ville de Poitiers. Bull Soc Pathol Exot Filiales. 1981 Sep-Oct;74(5):521–524. [PubMed] [Google Scholar]
- Kuchta J. M., States S. J., McGlaughlin J. E., Overmeyer J. H., Wadowsky R. M., McNamara A. M., Wolford R. S., Yee R. B. Enhanced chlorine resistance of tap water-adapted Legionella pneumophila as compared with agar medium-passaged strains. Appl Environ Microbiol. 1985 Jul;50(1):21–26. doi: 10.1128/aem.50.1.21-26.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeChevallier M. W., Cawthon C. D., Lee R. G. Factors promoting survival of bacteria in chlorinated water supplies. Appl Environ Microbiol. 1988 Mar;54(3):649–654. doi: 10.1128/aem.54.3.649-654.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeters G. A., Camper A. K. Enumeration of indicator bacteria exposed to chlorine. Adv Appl Microbiol. 1983;29:177–193. doi: 10.1016/s0065-2164(08)70357-5. [DOI] [PubMed] [Google Scholar]
- McFeters G. A., Kippin J. S., LeChevallier M. W. Injured coliforms in drinking water. Appl Environ Microbiol. 1986 Jan;51(1):1–5. doi: 10.1128/aem.51.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama Y., King W. M., Carpenter D. O. Edrophonium-induced inward membrane current in single neurons physically isolated from Aplysia californica. Brain Res. 1988 Jan 12;438(1-2):95–100. doi: 10.1016/0006-8993(88)91327-3. [DOI] [PubMed] [Google Scholar]
- Preer J. R., Jr, Preer L. B., Jurand A. Kappa and other endosymbionts in Paramecium aurelia. Bacteriol Rev. 1974 Jun;38(2):113–163. doi: 10.1128/br.38.2.113-163.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway H. F., Olson B. H. Chlorine resistance patterns of bacteria from two drinking water distribution systems. Appl Environ Microbiol. 1982 Oct;44(4):972–987. doi: 10.1128/aem.44.4.972-987.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein T. L., Blum J. J. Lysosomal physiology in Tetrahymena. 3. Pharmacological studies on acid hydrolase release and the ingestion and egestion of dimethylbenzanthracene particles. J Cell Biol. 1974 Sep;62(3):844–859. doi: 10.1083/jcb.62.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALT G. W. Feeding activity by Amoeba proteus. Exp Cell Res. 1961 Sep;24:618–620. doi: 10.1016/0014-4827(61)90469-4. [DOI] [PubMed] [Google Scholar]
- Sherr B. F., Sherr E. B., Rassoulzadegan F. Rates of digestion of bacteria by marine phagotrophic protozoa: temperature dependence. Appl Environ Microbiol. 1988 May;54(5):1091–1095. doi: 10.1128/aem.54.5.1091-1095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- States S. J., Conley L. F., Kuchta J. M., Oleck B. M., Lipovich M. J., Wolford R. S., Wadowsky R. M., McNamara A. M., Sykora J. L., Keleti G. Survival and multiplication of Legionella pneumophila in municipal drinking water systems. Appl Environ Microbiol. 1987 May;53(5):979–986. doi: 10.1128/aem.53.5.979-986.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout J. E., Yu V. L., Best M. G. Ecology of Legionella pneumophila within water distribution systems. Appl Environ Microbiol. 1985 Jan;49(1):221–228. doi: 10.1128/aem.49.1.221-228.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonckheere J. F. Pathogenic and nonpathogenic Acanthamoeba spp. in thermally polluted discharges and surface waters. J Protozool. 1981 Feb;28(1):56–59. doi: 10.1111/j.1550-7408.1981.tb02804.x. [DOI] [PubMed] [Google Scholar]