Abstract
1 The effect of phenobarbitone on the single dose pharmacokinetics of the synthetic steroids, ethinyloestradiol (EE2) and norethisterone, has been studied in the rabbit and rat. 2 EE2 is subject to an extensive first pass effect (96%). The plasma clearance of EE2 approaches total hepatic blood flow. It is suggested that a secondary peak in EE2 plasma concentration time curves at 5 h is due to enterohepatic recycling. Phenobarbitone had no effect on plasma EE2 concentrations following intravenous administration and produced a variable decrease after oral administration. 3 In phenobarbitone-treated rabbits, following intravenous administration of norethisterone there was no significant change in the area under the curve (AUC) compared to controls. In contrast, following oral administration of norethisterone to treated rabbits, the AUC was 20% and the peak plasma concentration 17% of that in controls. 4 The data in rabbits are consistent with drugs which are highly extracted by the liver. 5 In rats, phenobarbitone had no effect on plasma norethisterone concentrations following intravenous or hepatic portal (bolus) administration, but caused a decrease in systemic availability after both infusion into the portal vein (over a period of 5 min) and oral administration. 6 It is concluded that the rate of delivery of norethisterone to the liver is important in determining whether or not enzyme induction will cause an increased first pass effect. 7 Phenobarbitone caused an increase in conjugation of norethisterone in the gastrointestinal tract of rats.
Full text
PDF









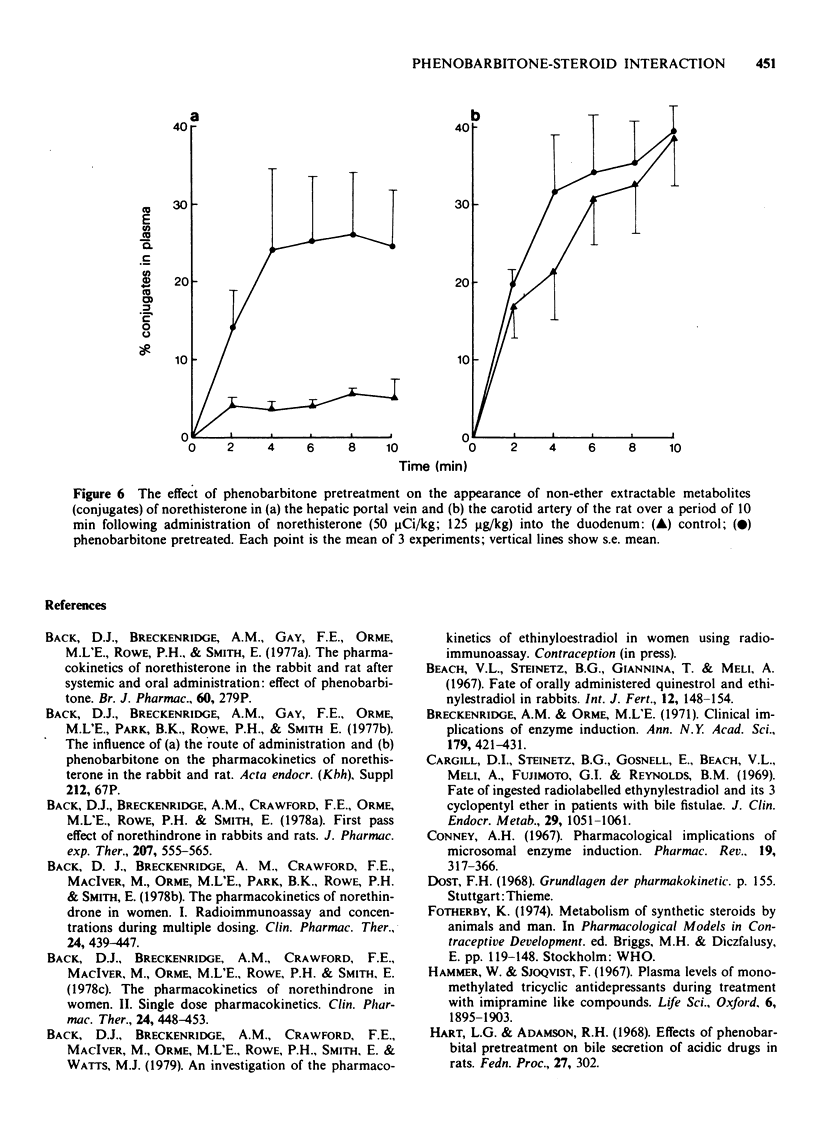

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back D. J., Breckenridge A. M., Crawford F. E., Mciver M., Orme M. L., Park B. K., Rowe P. H., Smith E. Kinetics of norethindrone in women. I. Radioimmunoassay and concentrations during multiple dosing. Clin Pharmacol Ther. 1978 Oct;24(4):439–447. doi: 10.1002/cpt1978244439. [DOI] [PubMed] [Google Scholar]
- Back D. J., Breckenridge A. M., Crawford F. E., Mciver M., Orme M. L., Rowe P. H., Smith E. Kinetics of norethindrone in women. II. Single-dose kinetics. Clin Pharmacol Ther. 1978 Oct;24(4):448–453. doi: 10.1002/cpt1978244448. [DOI] [PubMed] [Google Scholar]
- Back D. J., Breckenridge A. M., Crawford F. E., Orme M. L., Rowe P. H., Smith E. First-pass effect of norethindrone in rabbits and rats. J Pharmacol Exp Ther. 1978 Nov;207(2):555–565. [PubMed] [Google Scholar]
- Back D. J., Breckenridge A. M., Gay F. E., Orme ML'E, Rowe P. H., Smith E. The pharmacokinetics of norethisterone in the rabbit and rat after systemic and oral administration: effect of phenobarbitone [proceedings]. Br J Pharmacol. 1977 Jun;60(2):279P–279P. [PMC free article] [PubMed] [Google Scholar]
- Beach V. L., Steinetz B. G., Giannina T., Meli A. Fate of orally administered quinestrol and ethyinl estradiol in rabbits. Int J Fertil. 1967 Apr-Jun;12(2):148–154. [PubMed] [Google Scholar]
- Breckenridge A., Orme M. Clinical implications of enzyme induction. Ann N Y Acad Sci. 1971 Jul 6;179:421–431. doi: 10.1111/j.1749-6632.1971.tb46919.x. [DOI] [PubMed] [Google Scholar]
- Cargill D. I., Steinetz B. G., Gosnell E., Beach V. L., Meli A., Fujimoto G. I., Reynolds B. M. Fate of ingested radiolabeled ethynylestradiol and its 3-cyclopentyl ether in patients with bile fistulas. J Clin Endocrinol Metab. 1969 Aug;29(8):1051–1061. doi: 10.1210/jcem-29-8-1051. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Hammer W., Sjöqvist F. Plasma levels of monomethylated tricyclic antidepressants during treatment with imipramine-like compounds. Life Sci. 1967 Sep 1;6(17):1895–1903. doi: 10.1016/0024-3205(67)90218-4. [DOI] [PubMed] [Google Scholar]
- Helton E. D., Goldzieher J. W. The pharmacokinetics of ethynyl estrogens. A review. Contraception. 1977 Mar;15(3):255–284. doi: 10.1016/0010-7824(77)90112-3. [DOI] [PubMed] [Google Scholar]
- Hempel E., Böhm W., Carol W., Klinger G. Medikamentöse Enzyminduktion und hormonale Kontrazeption. Zentralbl Gynakol. 1973 Oct 12;95(41):1451–1457. [PubMed] [Google Scholar]
- Janz D., Schmidt D. Letter: Anti-epileptic drugs and failure of oral contraceptives. Lancet. 1974 Jun 1;1(7866):1113–1113. doi: 10.1016/s0140-6736(74)90593-5. [DOI] [PubMed] [Google Scholar]
- Kappus H., Bolt H. M., Remmer H. Demethylation of mestranol to ethinyloestradiol in vitro and in vivo. Acta Endocrinol (Copenh) 1972 Oct;71(2):374–384. doi: 10.1530/acta.0.0710374. [DOI] [PubMed] [Google Scholar]
- Kenyon I. E. Unplanned pregnancy in an epileptic. Br Med J. 1972 Mar 11;1(5801):686–687. doi: 10.1136/bmj.1.5801.686-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen C. D. Biliary flow after microsomal enzyme induction. J Pharmacol Exp Ther. 1969 Aug;168(2):218–223. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin W., Welch R. M., Conney A. H. Decreased uterotropic potency of oral contraceptives in rats pretreated with phenobarbital. Endocrinology. 1968 Jul;83(1):149–156. doi: 10.1210/endo-83-1-149. [DOI] [PubMed] [Google Scholar]
- Neutze J. M., Wyler F., Rudolph A. M. Use of radioactive microspheres to assess distribution of cardiac output in rabbits. Am J Physiol. 1968 Aug;215(2):486–495. doi: 10.1152/ajplegacy.1968.215.2.486. [DOI] [PubMed] [Google Scholar]
- Nieschlag E., Mauss J., Coert A., Kićović P. Plasma androgen levels in men after oral administration of testosterone or testosterone undecanoate. Acta Endocrinol (Copenh) 1975 Jun;79(2):366–374. doi: 10.1530/acta.0.0790366. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Riegelman S., Rowland M., Epstein W. L. Griseofulvin-phenobarbital interaction in man. JAMA. 1970 Jul 20;213(3):426–431. [PubMed] [Google Scholar]
- Shand D. G., Rangno R. E. The disposition of propranolol. I. Elimination during oral absorption in man. Pharmacology. 1972;7(3):159–168. doi: 10.1159/000136285. [DOI] [PubMed] [Google Scholar]
- Steinetz B. G., Meli A., Giannina T., Beach V. L. Studies on biliary metabolites of orally administered ethynylestradiol (EE) and its 3-cyclopentyl ether (EECPE-quinestrol). Proc Soc Exp Biol Med. 1967 Apr;124(4):1283–1289. doi: 10.3181/00379727-124-31989. [DOI] [PubMed] [Google Scholar]
- WILLIAMS C. H., Jr, KAMIN H. Microsomal triphosphopyridine nucleotide-cytochrome c reductase of liver. J Biol Chem. 1962 Feb;237:587–595. [PubMed] [Google Scholar]
- Welch R. M., Levin W., Conney A. H. Stimulatory effect of phenobarbital on the metabolism in vivo of estradiol-17-beta and estrone in the rat. J Pharmacol Exp Ther. 1968 Mar;160(1):171–178. [PubMed] [Google Scholar]
- Wilkinson G. R., Shand D. G. Commentary: a physiological approach to hepatic drug clearance. Clin Pharmacol Ther. 1975 Oct;18(4):377–390. doi: 10.1002/cpt1975184377. [DOI] [PubMed] [Google Scholar]
- Yates M. S., Hiley C. R., Roberts P. J., Back D. J., Crawford F. E. Differential effects of hepatic microsomal enzyme inducing agents on liver blood flow. Biochem Pharmacol. 1978;27(22):2617–2621. doi: 10.1016/0006-2952(78)90336-2. [DOI] [PubMed] [Google Scholar]


