Abstract
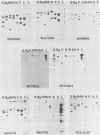
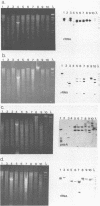
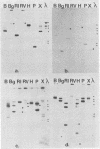
The relatedness of several marine Synechococcus spp. was estimated by DNA hybridization. Strains isolated from various geographical locations and representing a diversity of DNA base compositions and phycobiliprotein profiles were compared by restriction fragment length polymorphisms for a number of genes. DNAs from two marine red algae and a cryptomonad alga (which exhibit a phycobiliprotein composition similar to that of the marine Synechococcus spp.) and Synechococcus strain PCC6301 (Anacystis nidulans) were also included in the comparison. Strains WH8008, WH8018, and WH7805 were shown to be very similar to one another, as were strains WH7802 and WH7803. Strains WH8110 and WH5701 were clearly unrelated to any of the other strains, and no marine Synechococcus isolate showed any similarity to the freshwater Synechococcus strain PCC6301 or the eucaryotic algae. The method is relatively straightforward and sensitive and uses a variety of basic molecular biology techniques. Its utility in ascertaining the genetic relatedness and diversity of marine Synechococcus spp. and possible extension to field studies are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberte R. S., Wood A. M., Kursar T. A., Guillard R. R. Novel Phycoerythrins in Marine Synechococcus spp. : Characterization and Evolutionary and Ecological Implications. Plant Physiol. 1984 Jul;75(3):732–739. doi: 10.1104/pp.75.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen L., Doolittle W. F., Fox G. E. Cyanobacterial evolution: results of 16S ribosomal ribonucleic acid sequence analyses. Can J Biochem. 1979 Jun;57(6):879–888. doi: 10.1139/o79-108. [DOI] [PubMed] [Google Scholar]
- Cannon F. C., Riedel G. E., Ausubel F. M. Overlapping sequences of Klebsiella pneumoniae nifDNA cloned and characterized. Mol Gen Genet. 1979 Jul 2;174(1):59–66. doi: 10.1007/BF00433306. [DOI] [PubMed] [Google Scholar]
- Douglas S. E., Doolittle W. F. Complete nucleotide sequence of the 23S rRNA gene of the Cyanobacterium, Anacystis nidulans. Nucleic Acids Res. 1984 Apr 11;12(7):3373–3386. doi: 10.1093/nar/12.7.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hori H., Osawa S. Evolutionary change in 5S rRNA secondary structure and a phylogenic tree of 352 5S rRNA species. Biosystems. 1986;19(3):163–172. doi: 10.1016/0303-2647(86)90037-7. [DOI] [PubMed] [Google Scholar]
- Kursar T. A., Swift H., Alberte R. S. Morphology of a novel cyanobacterium and characterization of light-harvesting complexes from it: Implications for phycobiliprotein evolution. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6888–6892. doi: 10.1073/pnas.78.11.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G. R., Carr N. G. Resistance of DNA from filamentous and unicellular cyanobacteria to restriction endonuclease cleavage. Biochim Biophys Acta. 1984 Feb 24;781(1-2):45–55. doi: 10.1016/0167-4781(84)90122-2. [DOI] [PubMed] [Google Scholar]
- Li W. K., Rao D. V., Harrison W. G., Smith J. C., Cullen J. J., Irwin B., Platt T. Autotrophic picoplankton in the tropical ocean. Science. 1983 Jan 21;219(4582):292–295. doi: 10.1126/science.219.4582.292. [DOI] [PubMed] [Google Scholar]
- Maly P., Brimacombe R. Refined secondary structure models for the 16S and 23S ribosomal RNA of Escherichia coli. Nucleic Acids Res. 1983 Nov 11;11(21):7263–7286. doi: 10.1093/nar/11.21.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevarech M., Rice D., Haselkorn R. Nucleotide sequence of a cyanobacterial nifH gene coding for nitrogenase reductase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6476–6480. doi: 10.1073/pnas.77.11.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan B., Schultes N., Chen L., Bogorad L. Nucleotide sequence of a multiple-copy gene for the B protein of photosystem II of a cyanobacterium. Proc Natl Acad Sci U S A. 1984 May;81(9):2693–2697. doi: 10.1073/pnas.81.9.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J., Borman P. A rapid biochemical method for purifying high molecular weight bacterial chromosomal DNA for restriction enzyme analysis. Nucleic Acids Res. 1987 Apr 24;15(8):3631–3631. doi: 10.1093/nar/15.8.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott G. H., Carr N. G. Homology between nucleic acids of blue-green algae and chloroplasts of Euglena gracilis. Science. 1972 Mar 17;175(4027):1259–1261. doi: 10.1126/science.175.4027.1259. [DOI] [PubMed] [Google Scholar]
- Reichelt B. Y., Delaney S. F. The nucleotide sequence for the large subunit of ribulose 1,5-bisphosphate carboxylase from a unicellular cyanobacterium, Synechococcus PCC6301. DNA. 1983;2(2):121–129. doi: 10.1089/dna.1983.2.121. [DOI] [PubMed] [Google Scholar]
- Rice D., Mazur B. J., Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982 Nov 10;257(21):13157–13163. [PubMed] [Google Scholar]
- Rigaud G., Grange T., Pictet R. The use of NaOH as transfer solution of DNA onto nylon membrane decreases the hybridization efficiency. Nucleic Acids Res. 1987 Jan 26;15(2):857–857. doi: 10.1093/nar/15.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippka R., Cohen-Bazire G. The cyanobacteriales: a legitimate order based on the type strain Cyanobacterium stanieri? Ann Microbiol (Paris) 1983 Jul-Aug;134B(1):21–36. doi: 10.1016/s0769-2609(83)80094-5. [DOI] [PubMed] [Google Scholar]
- Schwartz R. M., Dayhoff M. O. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. Science. 1978 Jan 27;199(4327):395–403. doi: 10.1126/science.202030. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Elwood H. J., Gunderson J. H. Evolutionary diversity of eukaryotic small-subunit rRNA genes. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1383–1387. doi: 10.1073/pnas.83.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterbury J. B., Willey J. M., Franks D. G., Valois F. W., Watson S. W. A cyanobacterium capable of swimming motility. Science. 1985 Oct 4;230(4721):74–76. doi: 10.1126/science.230.4721.74. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman M., Gregory R. P., Carr N. G. Novel Role for Phycoerythrin in a Marine Cyanobacterium, Synechococcus Strain DC2. Science. 1985 Nov 15;230(4727):818–820. doi: 10.1126/science.230.4727.818. [DOI] [PubMed] [Google Scholar]
- de Lorimier R., Bryant D. A., Porter R. D., Liu W. Y., Jay E., Stevens S. E., Jr Genes for the alpha and beta subunits of phycocyanin. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7946–7950. doi: 10.1073/pnas.81.24.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]