Abstract
1 Effects of nitroglycerine (NG) on the membrane and contractile properties of the smooth muscle cell of the isolated coronary artery of the pig were observed.
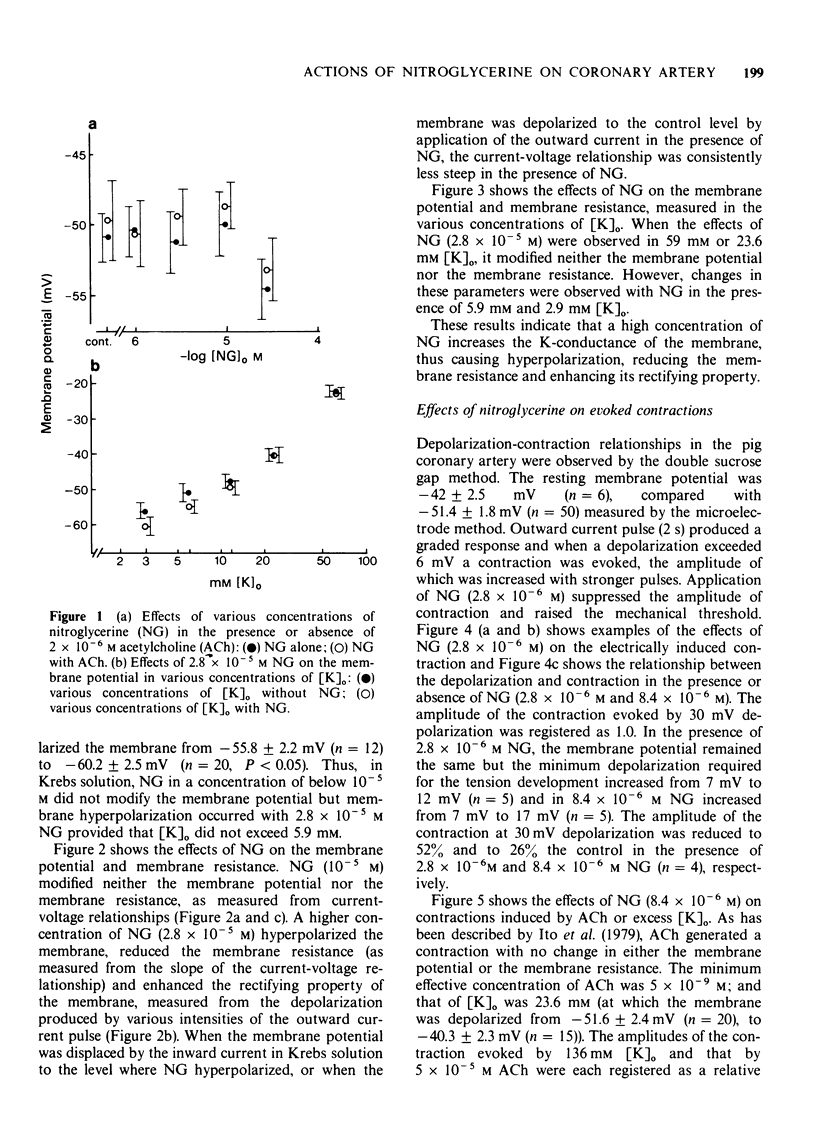
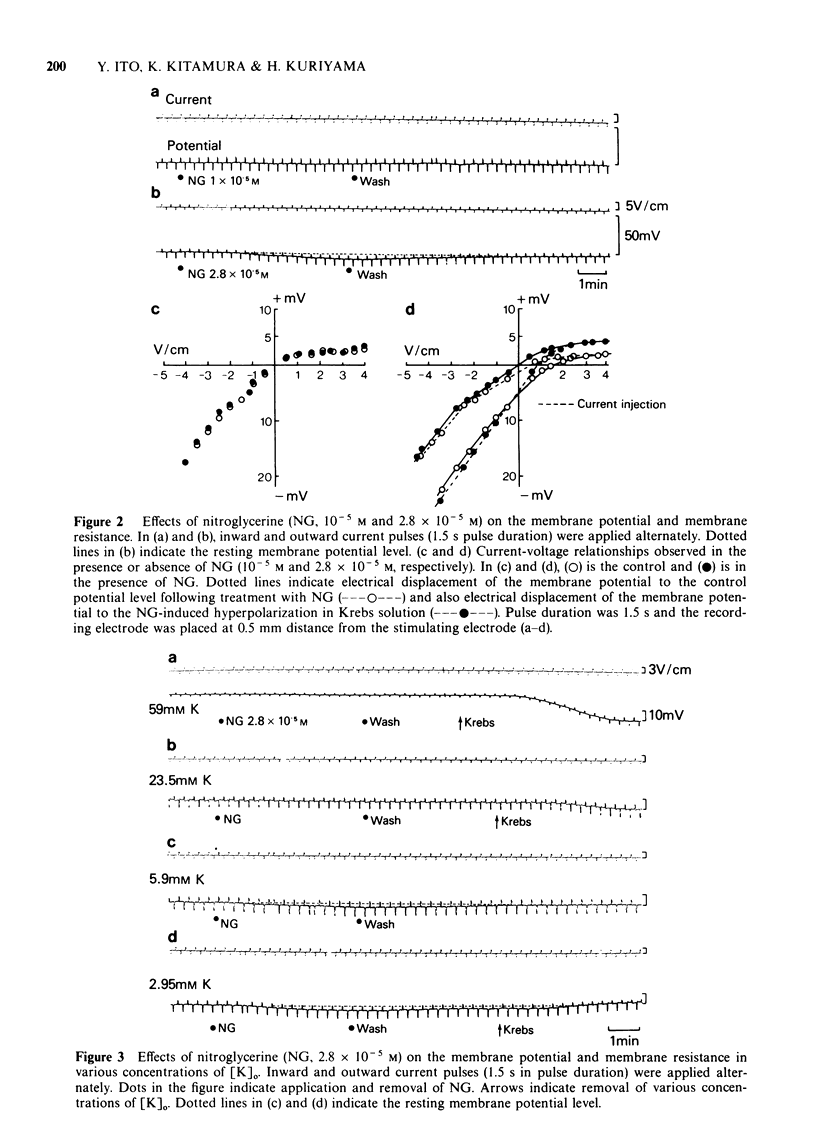
2 NG, up to a concentration of 10-5 M, modified neither the membrane potential nor the membrane resistance. Increased concentrations of NG (> 2.8 × 10-5 M) hyperpolarized the membrane, reduced the membrane resistance and enhanced the rectifying property of the membrane measured by depolarization pulses. These phenomena observed with a high concentration of NG are the result of an increase in the K-conductance of the membrane.
3 NG (2.8 × 10-5 M) did not modify the membrane potential displaced by various concentrations of excess [K]o. In low [K]o, NG (2.8 × 10-5 M) hyperpolarized the membrane to a greater extent than that observed in Krebs solution. The effects of NG (10-6 to 2.8 × 10-5 M) on the membrane potential were not modified by simultaneous application of 2 × 10-6 M acetylcholine (ACh).
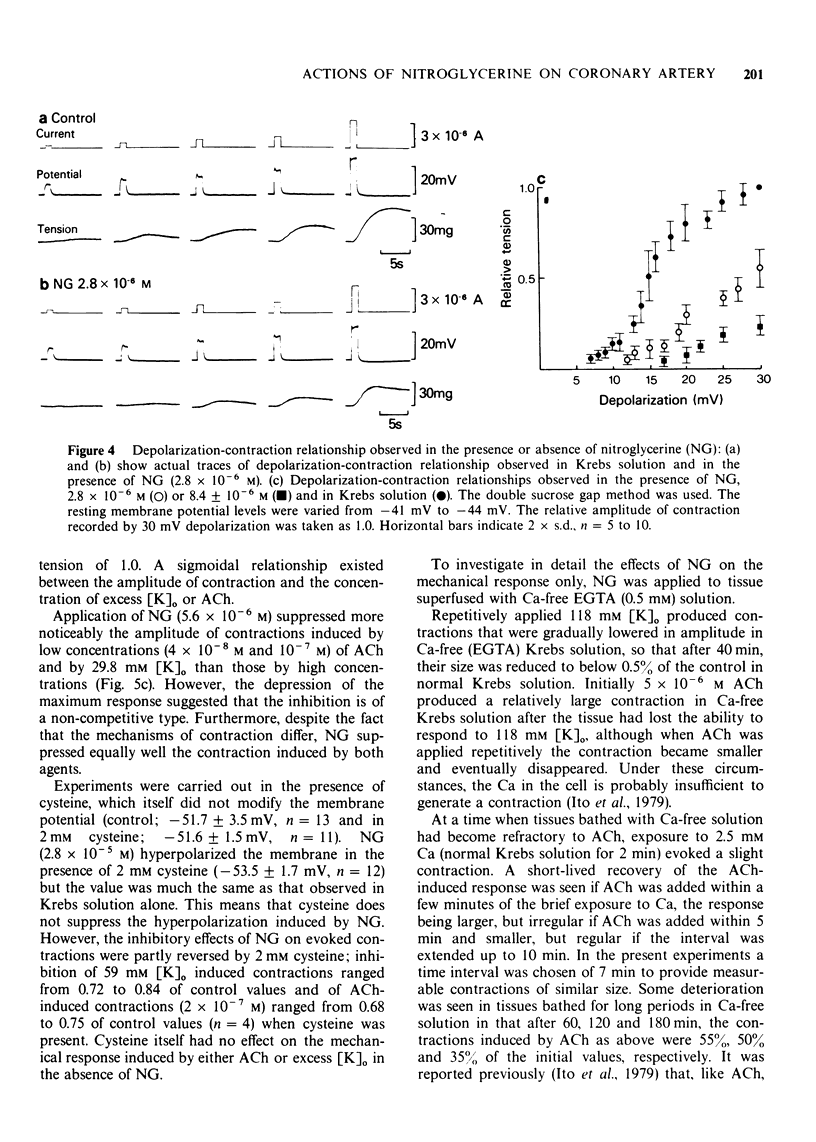
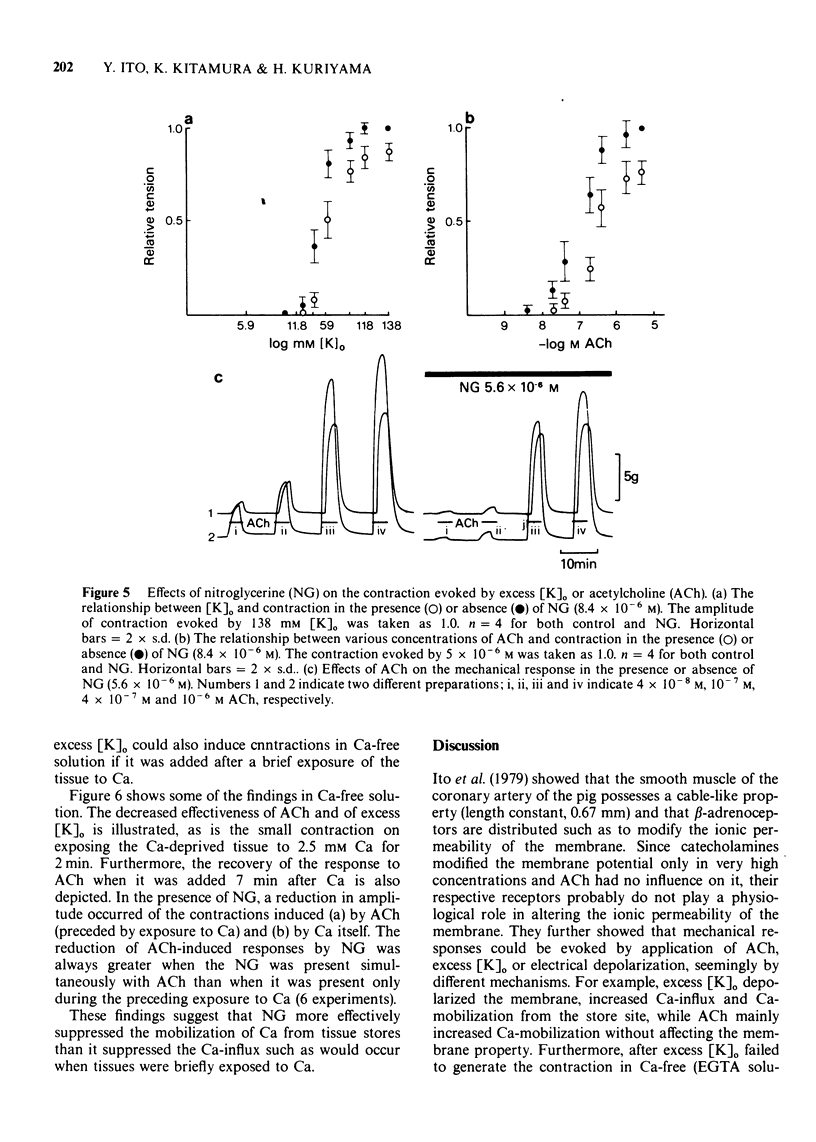
4 NG (2.8 × 10-6 M) consistently raised the mechanical threshold required for tension development and suppressed the amplitude of the contraction evoked by excess [K]o, ACh or electrical depolarization of the membrane. The dose-response curve shifted to the right in the presence of NG noncompetitively in all the conditions employed to develop the tension.
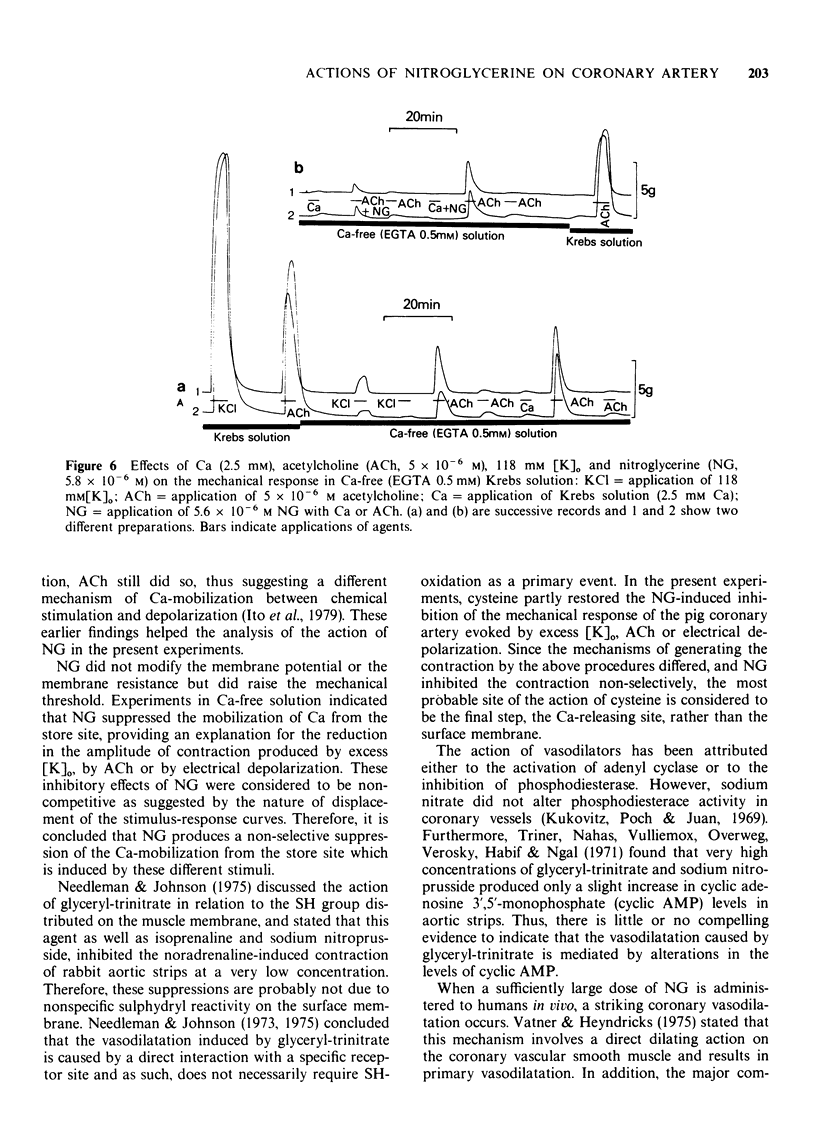
5 When the tissue was immersed in Ca-free (EGTA) solution, ACh (5 × 10-6 M) evoked a contraction even after the tissue had lost the ability to contract to repetitive applications of 118 mM [K]o in Ca-free (EGTA) solution. However, the tissue finally failed to contract to repetitively applied ACh. At this stage, 2.5 mM [Ca]o evoked a small contraction, after which the response was briefly restored to 5 × 10-6 M ACh. This transient response to ACh was reduced by NG (5.6 × 10-6 M) when NG was added either simultaneously with ACh or with the previous Ca application. However, the inhibition was greater in the former than the latter case.
6 Cysteine (1 to 2 mM), without modifying the membrane potential or membrane resistance, partly restored the contraction evoked by excess [K]o or ACh which had been reduced by NG.
7 The mechanism of action of NG on the smooth muscle cell of the coronary artery of the pig is postulated to be due to a nonselective suppression of the Ca-mobilization from the store site with no noticeable change in the membrane properties.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Correlation between membrane potential, spike discharge and tension in smooth muscle. J Physiol. 1955 Apr 28;128(1):200–221. doi: 10.1113/jphysiol.1955.sp005299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S., Takeda K. Effect of vasodilators upon the isolated taenia coli of the guinea pig. J Pharmacol Exp Ther. 1967 Jun;156(3):557–564. [PubMed] [Google Scholar]
- Ito Y., Suzuki H., Kuriyama H. On the roles of calcium ion during potassium induced contracture in the smooth muscle cells of the rabbit main pulmonary artery. Jpn J Physiol. 1977;27(6):755–770. doi: 10.2170/jjphysiol.27.755. [DOI] [PubMed] [Google Scholar]
- Needleman P., Johnson E. M., Jr Mechanism of tolerance development to organic nitrates. J Pharmacol Exp Ther. 1973 Mar;184(3):709–715. [PubMed] [Google Scholar]
- Schnaar R. L., Sparks H. V. Response of large and small coronary arteries to nitroglycerin, NaNO 2 , and adenosine. Am J Physiol. 1972 Jul;223(1):223–228. doi: 10.1152/ajplegacy.1972.223.1.223. [DOI] [PubMed] [Google Scholar]
- Triner L., Nahas G. G., Vulliemoz Y., Overweg N. T., Verosky M., Habif D. V., Ngai S. H. Cyclic AMP and smooth muscle function. Ann N Y Acad Sci. 1971 Dec 30;185:458–476. doi: 10.1111/j.1749-6632.1971.tb45273.x. [DOI] [PubMed] [Google Scholar]
- Winbury M. M., Howe B. B., Hefner M. A. Effect of nitrates and other coronary dilators on large and small coronary vessels: an hypothesis for the mechanism of action of nitrates. J Pharmacol Exp Ther. 1969 Jul;168(1):70–95. [PubMed] [Google Scholar]


