Abstract
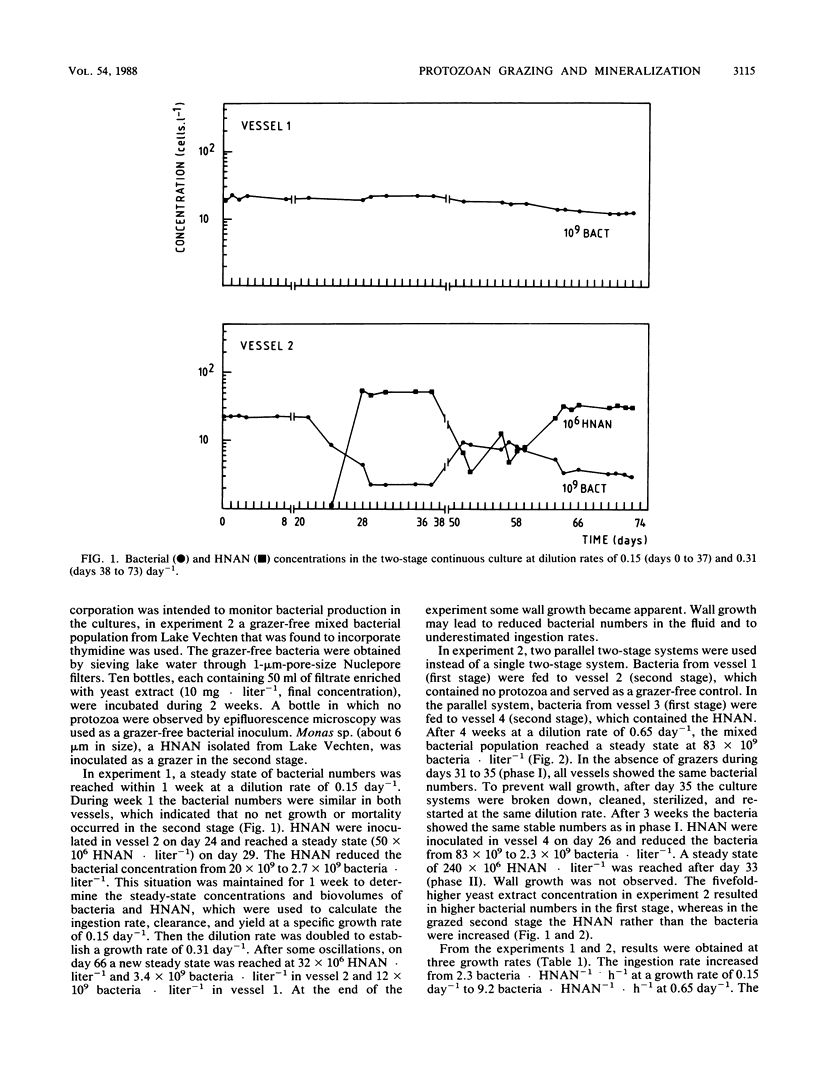
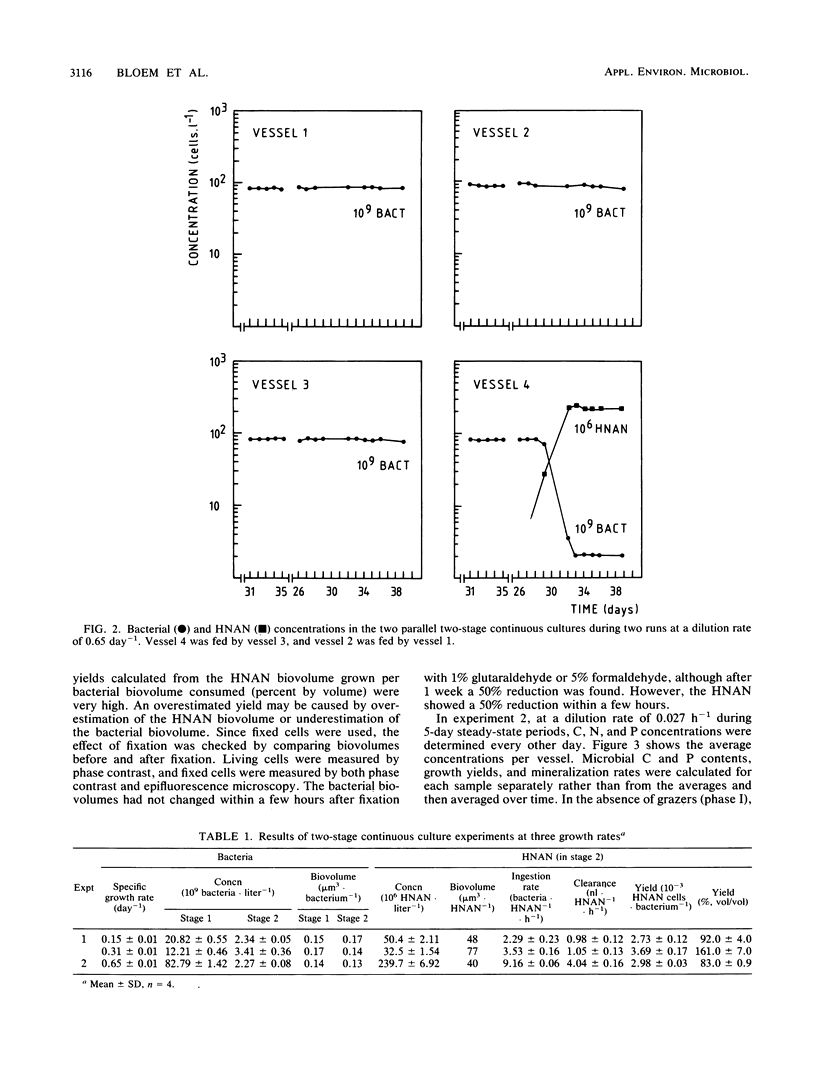
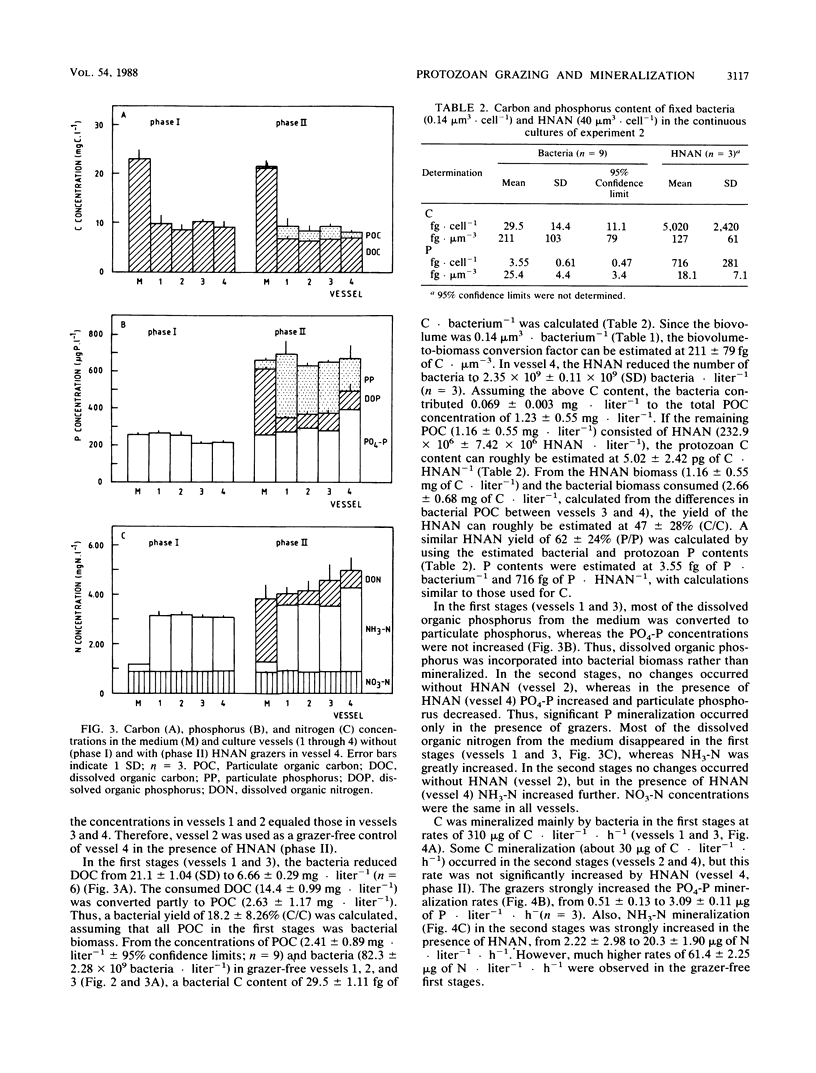
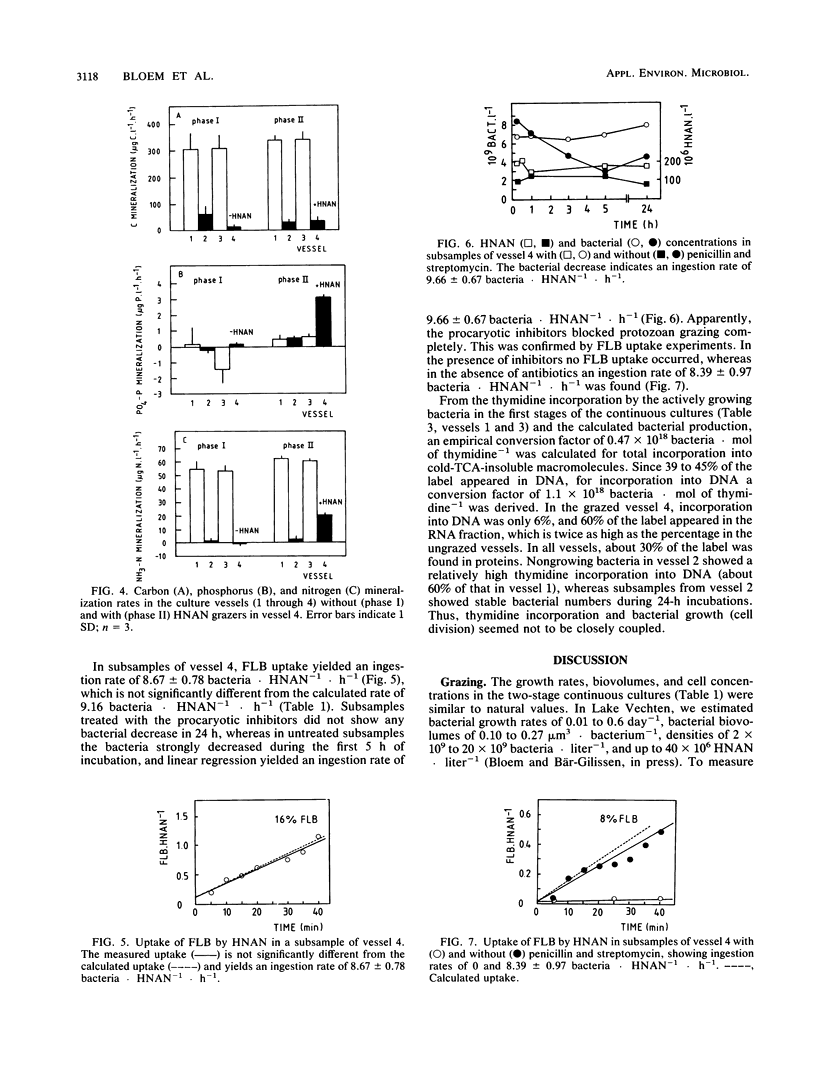
In two-stage continuous cultures, at bacterial concentrations, biovolumes, and growth rates similar to values found in Lake Vechten, ingestion rates of heterotrophic nanoflagellates (HNAN) increased from 2.3 bacteria HNAN−1 · h−1 at a growth rate of 0.15 day−1 to 9.2 bacteria · HNAN−1 · h−1 at a growth rate of 0.65 day−1. On a yeast extract medium with a C/N/P ratio of 100:15:1.2 (Redfield ratio), a mixed bacterial population showed a yield of 18% (C/C) and a specific carbon content of 211 fg of C · μm−3. The HNAN carbon content and yield were estimated at 127 fg of C · μm−3 and 47% (C/C). Although P was not growth limiting, HNAN accelerated the mineralization of PO4-P from dissolved organic matter by 600%. The major mechanism of P remineralization appeared to be direct consumption of bacteria by HNAN. N mineralization was performed mainly (70%) by bacteria but was increased 30% by HNAN. HNAN did not enhance the decomposition of the relatively mineral-rich dissolved organic matter. An accelerated decomposition of organic carbon by protozoa may be restricted to mineral-poor substrates and may be explained mainly by protozoan nutrient regeneration. Growth and grazing in the cultures were compared with methods for in situ estimates. Thymidine incorporation by actively growing bacteria yielded an empirical conversion factor of 1.1 × 1018 bacteria per mol of thymidine incorporated into DNA. However, nongrowing bacteria also showed considerable incorporation. Protozoan grazing was found to be accurately measured by uptake of fluorescently labeled bacteria, whereas artificial fluorescent microspheres were not ingested, and selective prokaryotic inhibitors blocked not only bacterial growth but also protozoan grazing.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloem J., Bär-Gilissen M. J., Cappenberg T. E. Fixation, counting, and manipulation of heterotrophic nanoflagellates. Appl Environ Microbiol. 1986 Dec;52(6):1266–1272. doi: 10.1128/aem.52.6.1266-1272.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratbak G. Bacterial biovolume and biomass estimations. Appl Environ Microbiol. 1985 Jun;49(6):1488–1493. doi: 10.1128/aem.49.6.1488-1493.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratbak G., Dundas I. Bacterial dry matter content and biomass estimations. Appl Environ Microbiol. 1984 Oct;48(4):755–757. doi: 10.1128/aem.48.4.755-757.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curds C. R., Cockburn A. Continuous monoxenic culture of Tetrahymena pyriformis. J Gen Microbiol. 1971 Apr;66(1):95–108. doi: 10.1099/00221287-66-1-95. [DOI] [PubMed] [Google Scholar]
- Elisenreich S. J., Bannerman R. T., Armstrong D. E. A simplified phosphorus analysis technique. Environ Lett. 1975;9(1):43–53. doi: 10.1080/00139307509437455. [DOI] [PubMed] [Google Scholar]
- Lee S., Fuhrman J. A. Relationships between Biovolume and Biomass of Naturally Derived Marine Bacterioplankton. Appl Environ Microbiol. 1987 Jun;53(6):1298–1303. doi: 10.1128/aem.53.6.1298-1303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann B., Søndergaard M. Measurements of diel rates of bacterial secondary production in aquatic environments. Appl Environ Microbiol. 1984 Apr;47(4):632–638. doi: 10.1128/aem.47.4.632-638.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robarts R. D., Wicks R. J., Sephton L. M. Spatial and Temporal Variations in Bacterial Macromolecule Labeling with [methyl-H]Thymidine in a Hypertrophic Lake. Appl Environ Microbiol. 1986 Dec;52(6):1368–1373. doi: 10.1128/aem.52.6.1368-1373.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr B. F., Sherr E. B., Berman T. Grazing, growth, and ammonium excretion rates of a heterotrophic microflagellate fed with four species of bacteria. Appl Environ Microbiol. 1983 Apr;45(4):1196–1201. doi: 10.1128/aem.45.4.1196-1201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr B. F., Sherr E. B., Fallon R. D. Use of monodispersed, fluorescently labeled bacteria to estimate in situ protozoan bacterivory. Appl Environ Microbiol. 1987 May;53(5):958–965. doi: 10.1128/aem.53.5.958-965.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


