Abstract
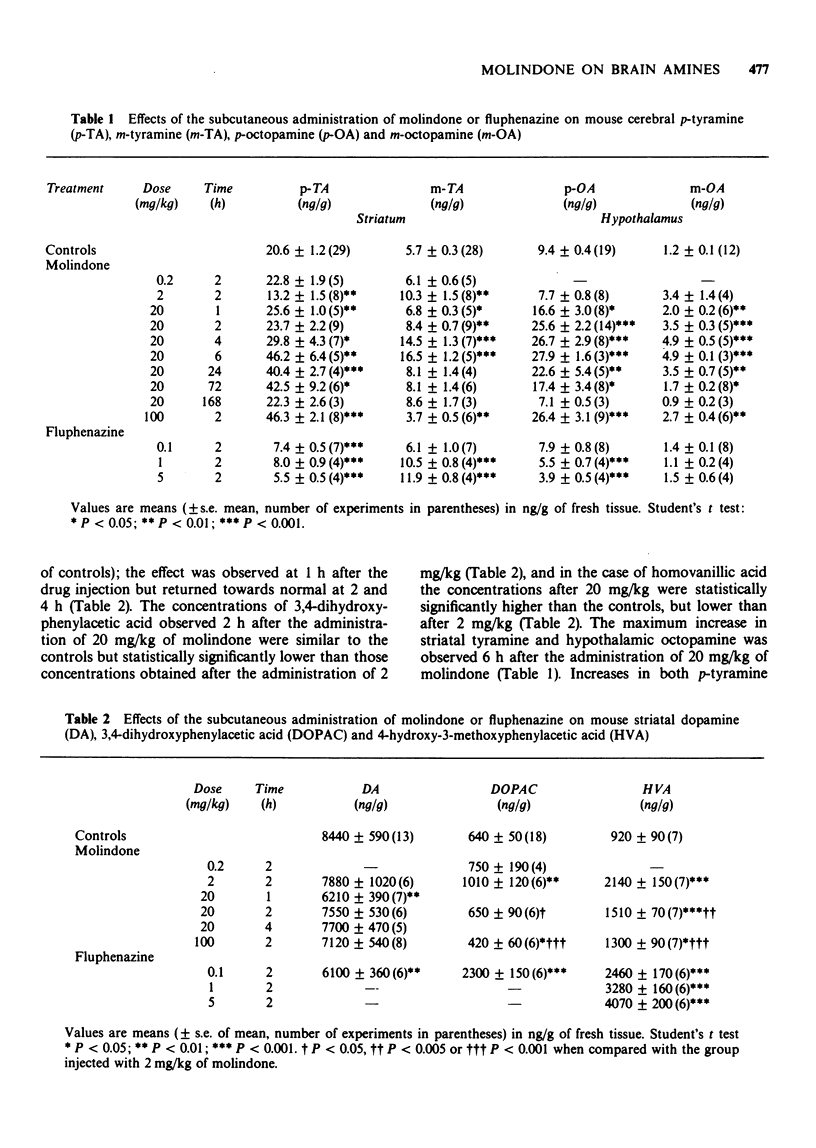
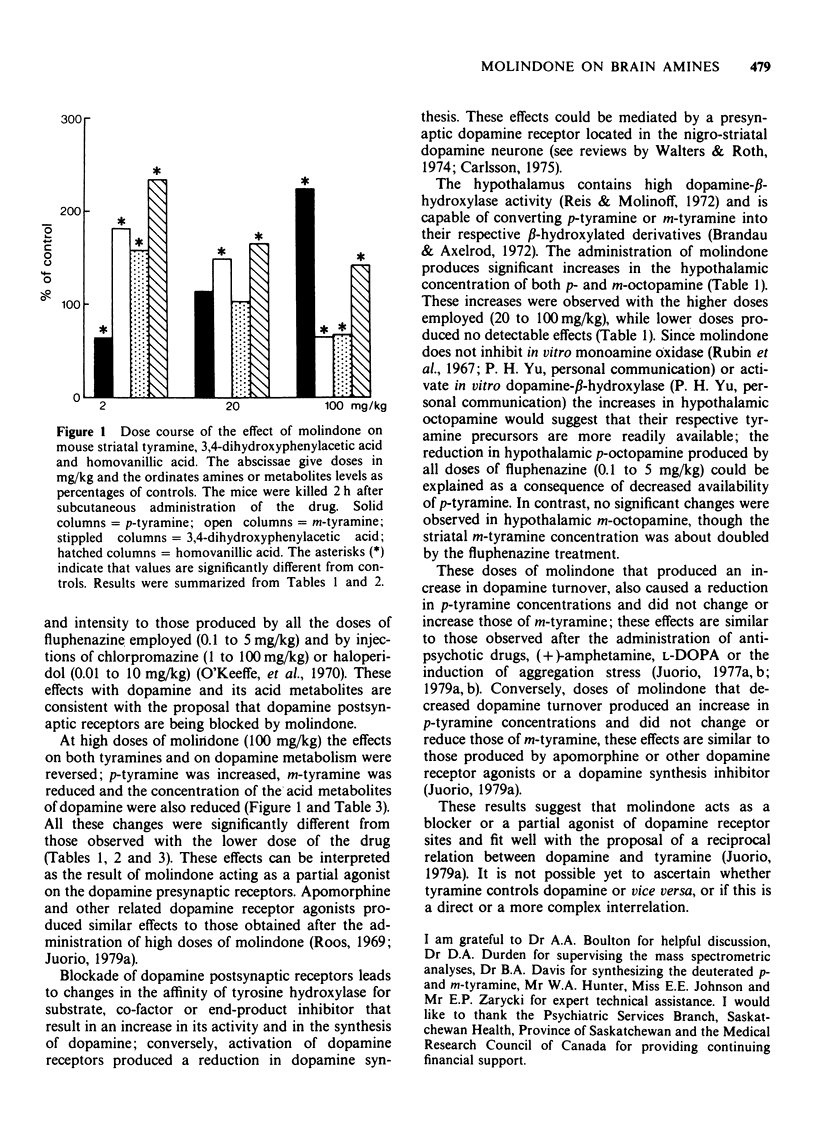
1 The concentrations of p- and m-tyramine, dopamine, 3,4-dihydroxyphenylacetic acid and homo-vanillic acid were measured in the mouse or rat striatum following the subcutaneous injection of molindone or fluphenazine. The mouse hypothalamic levels of the m- or p-isomers of octopamine were also analysed. 2 Endogenous concentrations of p- and m-tyramine in the mouse striatum and p- and m-octopamine in the mouse hypothalamus were 20.6, 5.7, 9.4 and 1.2 ng/g respectively. The rat striatum concentrations of p- and m-tyramine were 12.8 and 3.8 ng/g. 3 The administration of low doses of molindone (1 to 10 mg/kg) produced a reduction in striatal p-tyramine, an increase in m-tyramine and an increase in dopamine turnover. Similar effects were produced by all doses of fluphenazine (0.1 to 5 mg/kg) employed. These findings are consistent with those observed after blockade of dopamine postsynaptic receptors. 4 With high doses of molindone (100 mg/kg) the effects on both tyramines and on dopamine metabolism were reversed. These results can be interpreted as molindone acting as a partial agonist. 5 The concentrations of hypothalamic p- and m-octopamine were increased by the higher doses of molindone (20 to 100 mg/kg) employed while lower doses produced no significant effects. All doses of fluphenazine reduced hypothalamic p-octopamine. These changes seem to depend on differences in the availability of p-tyramine to be converted into p-octopamine. 6 These results suggest that molindone acts as a blocker or a partial agonist of dopamine receptor sites and fit well with the proposal of a reciprocal relation between dopamine and tyramine. It is not possible yet to ascertain whether tyramine controls dopamine or vice versa or if it is a direct or a more remote relation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDEN N. E., ROOS B. E., WERDINIUS B. On the occurrence of homovanillic acid in brain and cerebrospinal fluid and its determination by a fluorometric method. Life Sci. 1963 Jul;(7):448–458. doi: 10.1016/0024-3205(63)90132-2. [DOI] [PubMed] [Google Scholar]
- Boulton A. A., Juorio A. V. The tyramines: are they involved in the psychoses? Biol Psychiatry. 1979 Apr;14(2):413–419. [PubMed] [Google Scholar]
- Brandau K., Axelrod J. The biosynthesis of octopamine. Naunyn Schmiedebergs Arch Pharmacol. 1972;273(1):123–133. doi: 10.1007/BF00508085. [DOI] [PubMed] [Google Scholar]
- Bunney B. S., Roth R. H., Aghajanian G. K. Effects of molindone on central dopaminergic neuronal activity and metabolism: similarity to other neuroleptics. Psychopharmacol Commun. 1975;1(4):349–358. [PubMed] [Google Scholar]
- Burt D. R., Enna S. J., Creese I., Snyder S. H. Dopamine receptor binding in the corpus striatum of mammalian brain. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4655–4659. doi: 10.1073/pnas.72.11.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSSON A., LINDQVIST M. EFFECT OF CHLORPROMAZINE OR HALOPERIDOL ON FORMATION OF 3METHOXYTYRAMINE AND NORMETANEPHRINE IN MOUSE BRAIN. Acta Pharmacol Toxicol (Copenh) 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- Clark M. L., Huber W. K., Sakata K., Fowles D. C., Serafetinides E. A. Molindone in chronic schizophrenia. Clin Pharmacol Ther. 1970 Sep-Oct;11(5):680–688. doi: 10.1002/cpt1970115680. [DOI] [PubMed] [Google Scholar]
- Danielson T. J., Boulton A. A., Robertson H. A. m-Octopamine, p-octopamine and phenylethanolamine in rat brain: a sensitive, specific assay and the effects of some drugs. J Neurochem. 1977 Dec;29(6):1131–1135. doi: 10.1111/j.1471-4159.1977.tb06519.x. [DOI] [PubMed] [Google Scholar]
- Gilder D. A., Fain W., Simpson L. L. A comparison of the abilities of chlorpromazine and molindone to interact adversely with guanethidine. J Pharmacol Exp Ther. 1976 Aug;198(2):255–263. [PubMed] [Google Scholar]
- Juorio A. V., Danielson T. J. Effect of haloperidol and d-amphetamine on cerebral tyramine and octopamine levels. Eur J Pharmacol. 1978 Jul 1;50(1):79–82. doi: 10.1016/0014-2999(78)90256-x. [DOI] [PubMed] [Google Scholar]
- Juorio A. V. Drug-induced changes in the formation, storage and metabolism of tyramine in the mouse. Br J Pharmacol. 1979 Jul;66(3):377–384. doi: 10.1111/j.1476-5381.1979.tb10841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juorio A. V. Effect of chlorpromazine and other anti-psychotic drugs on mouse striatal tyramines. Life Sci. 1977 May 15;20(10):1663–1667. doi: 10.1016/0024-3205(77)90340-x. [DOI] [PubMed] [Google Scholar]
- Juorio A. V. Effect of stress and L-DOPA administration on mouse striatal tyramine and homovanillic acid levels. Brain Res. 1979 Dec 21;179(1):186–189. doi: 10.1016/0006-8993(79)90505-5. [DOI] [PubMed] [Google Scholar]
- Juorio A. V. Effects of D-amphetamine and antipsychotic drug administration on striatal tyramine levels in the mouse. Brain Res. 1977 Apr 22;126(1):181–184. doi: 10.1016/0006-8993(77)90227-x. [DOI] [PubMed] [Google Scholar]
- LAVERTY R., SHARMAN D. F. MODIFICATION BY DRUGS OF THE METABOLISM OF 3,4-DIHYDROXYPHENYLETHYLAMINE, NORADRENALINE AND 5-HYDROXYTRYPTAMINE IN THE BRAIN. Br J Pharmacol Chemother. 1965 Jun;24:759–772. doi: 10.1111/j.1476-5381.1965.tb01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAVERTY R., SHARMAN D. F. THE ESTIMATION OF SMALL QUANTITIES OF 3,4-DIHYDROXYPHENYLETHYLAMINE IN TISSUES. Br J Pharmacol Chemother. 1965 Apr;24:538–548. doi: 10.1111/j.1476-5381.1965.tb01744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinoff P. B., Landsberg L., Axelrod J. An enzymatic assay for octopamine and other beta-hydroxylated phenylethylamines. J Pharmacol Exp Ther. 1969 Dec;170(2):253–261. [PubMed] [Google Scholar]
- Murphy G. F., Robinson D., Sharman D. F. The effect of tropolone on the formation of 3,4-dihydroxyphenylacetic acid and 4-hydroxy-3-methoxyphenylacetic acid in the brain of the mouse. Br J Pharmacol. 1969 May;36(1):107–115. doi: 10.1111/j.1476-5381.1969.tb08308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keeffe R., Sharman D. F., Vogt M. Effect of drugs used in psychoses on cerebral dopamine metabolism. Br J Pharmacol. 1970 Feb;38(2):287–304. doi: 10.1111/j.1476-5381.1970.tb08517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips S. R., Davis B. A., Durden D. A., Boulton A. A. Identification and distribution of m-tyramine in the rat. Can J Biochem. 1975 Jan;53(1):65–69. doi: 10.1139/o75-010. [DOI] [PubMed] [Google Scholar]
- Philips S. R., Durden D. A., Boulton A. A. Identification and distribution of p-tyramine in the rat. Can J Biochem. 1974 May;52(5):366–373. doi: 10.1139/o74-055. [DOI] [PubMed] [Google Scholar]
- Reis D. J., Molinoff P. B. Brain dopamine- -hydroxylase: regional distribution and effects of lesions and 6-hydroxy-dopamine on activity. J Neurochem. 1972 Jan;19(1):195–204. doi: 10.1111/j.1471-4159.1972.tb01269.x. [DOI] [PubMed] [Google Scholar]
- Roos B. E. Decrease in homovanillic acid as evidence for dopamine receptor stimulation by apomorphine in the neostriatum of the rat. J Pharm Pharmacol. 1969 Apr;21(4):263–264. doi: 10.1111/j.2042-7158.1969.tb08243.x. [DOI] [PubMed] [Google Scholar]
- Rubin A. A., Yen H. C., Pfeffer M. Psychopharmacological profile of molindone. Nature. 1967 Nov 11;216(5115):578–579. doi: 10.1038/216578a0. [DOI] [PubMed] [Google Scholar]
- Saavedra J. M. Enzymatic-isotopic method for octopamine at the picogram level. Anal Biochem. 1974 Jun;59(2):628–633. doi: 10.1016/0003-2697(74)90316-9. [DOI] [PubMed] [Google Scholar]
- Seeman P., Chau-Wong M., Tedesco J., Wong K. Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4376–4380. doi: 10.1073/pnas.72.11.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]



