Abstract
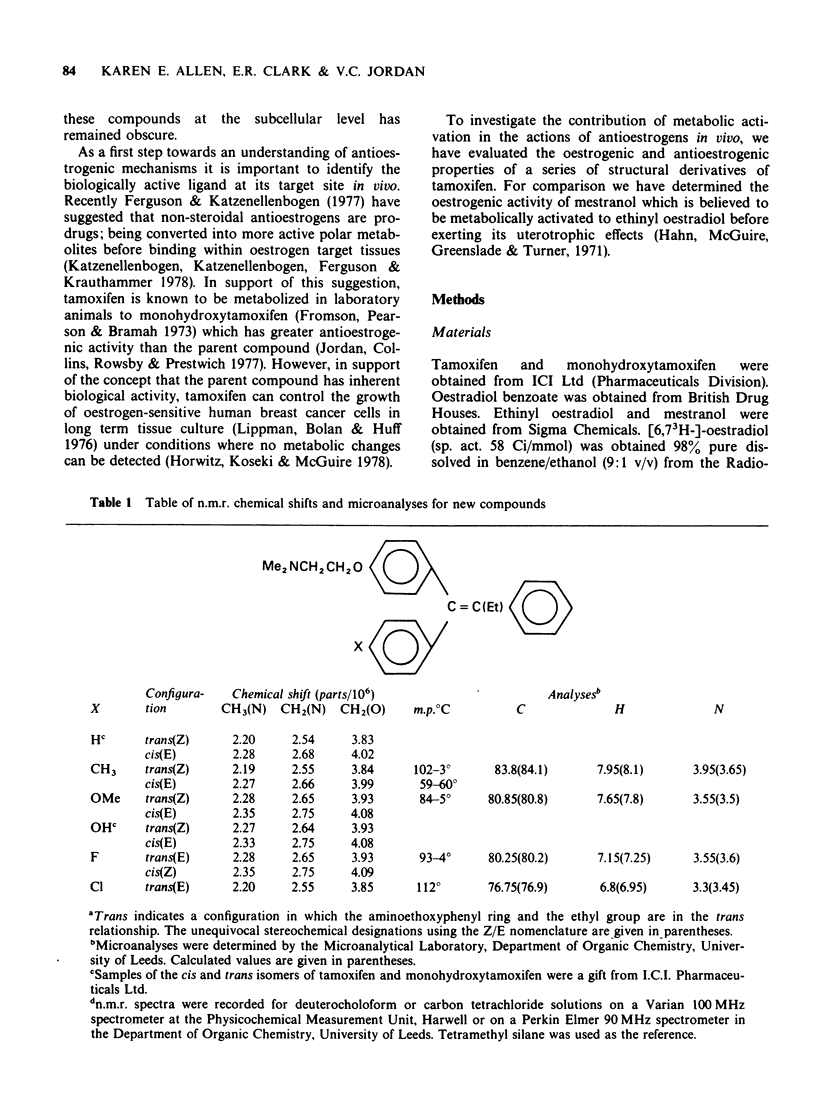
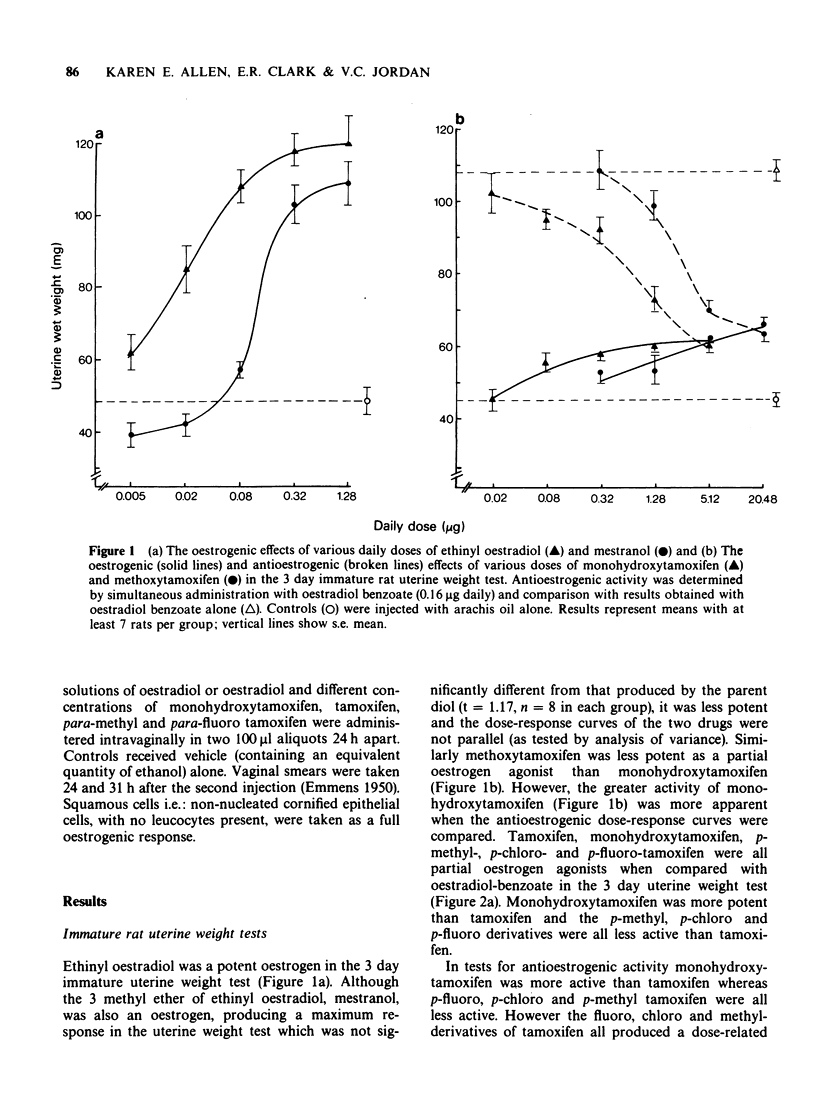
1 The oestrogenic and antioestrogenic activities of tamoxifen and monohydroxytamoxifen have been compared with those of para-methoxy, -methyl, -fluoro, and -chloro tamoxifen in the 3 day immature rat uterine weight test.
2 The oestrogenic activity of mestranol, a steroid with low oestrogen receptor binding affinity which is believed to be demethylated to ethinyl oestradiol before exerting its effects, was less potent than ethinyl oestradiol when assayed in the 3 day immature rat uterine weight test. Similarly, para-methoxytamoxifen was less active than monohydroxytamoxifen in oestrogenic and antioestrogenic tests.
3 The introduction of a para-methoxy group into tamoxifen did not affect oestrogenic or antioestrogenic activity.
4 All the derivatives of tamoxifen were partial oestrogen agonists when compared with oestradiol benzoate in the 3 d immature rat uterine weight test. All test compounds inhibited the uterotrophic activity of oestradiol benzoate (0.16 μg daily) in a dose-related manner. The order of potency was: monohydroxytamoxifen > tamoxifen ≡ methoxytamoxifen > p-fluoro ≡ p-chloro ≡ p-methyltamoxifen.
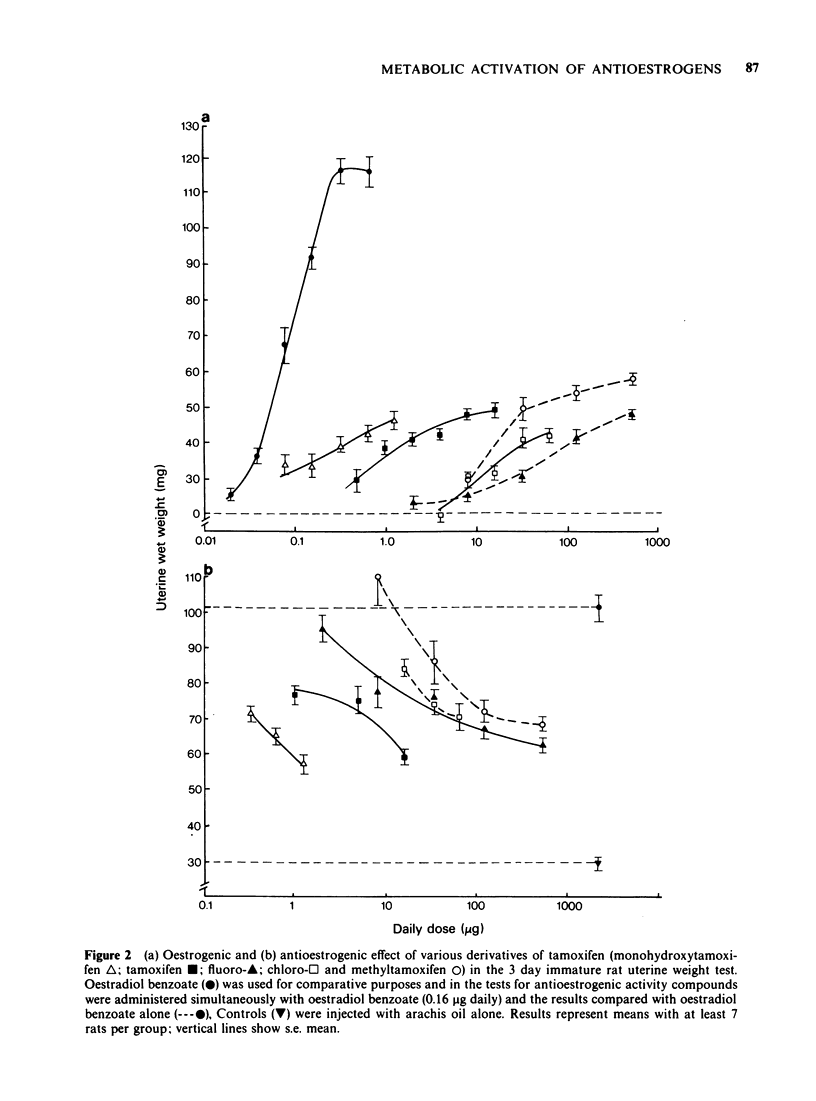
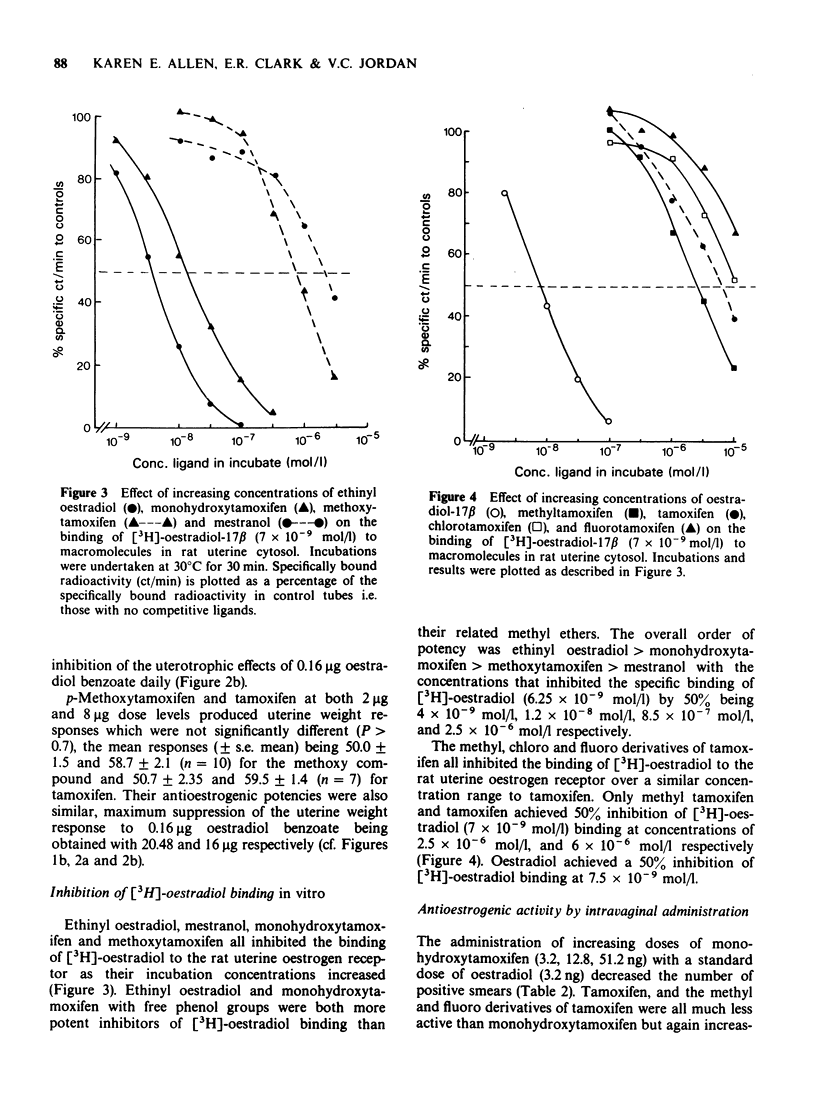
5 Tamoxifen was approximately equiactive with its p-methyl, p-fluoro and p-chloro derivatives in the ability to inhibit [3H]-oestradiol binding to rat uterine oestrogen receptors in vitro.
6 Tamoxifen was approximately equiactive with its p-methyl and p-fluoro derivatives in the ability to inhibit vaginal cornification of ovariectomized rats upon intravaginal administration with oestradiol (3.2 ng total dose).
7 Since tamoxifen in vivo was more active as a partial oestrogen agonist and antagonist than the para substituted fluoro, chloro and methyl derivatives that cannot undergo metabolic hydroxylation to monohydroxytamoxifen, whereas the antioestrogenic activity of the compounds upon local application in the vaginal cornification test was equivalent as was their ability to inhibit [3H]-oestradiol-17β binding to the oestrogen receptor in vitro, it is suggested that at low doses; i.e. over the range of the partial agonist dose-response curve, the biological activity of tamoxifen is the net result of the activities of the parent compound and its metabolites.
8 The results demonstrate that metabolic activation of non-steroidal antioestrogens is only an advantage and not a requirement for antioestrogenic activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam H. K., Douglas E. J., Kemp J. V. The metabolism of tamoxifen in human. Biochem Pharmacol. 1979;28(1):145–147. doi: 10.1016/0006-2952(79)90283-1. [DOI] [PubMed] [Google Scholar]
- Bishop P. M. Clomiphene. Br Med Bull. 1970 Jan;26(1):22–25. doi: 10.1093/oxfordjournals.bmb.a070737. [DOI] [PubMed] [Google Scholar]
- Clark E. R., McCracken A. M. Investigation of aqueous Tween 80 as a vehicle for intravaginal administration. J Endocrinol. 1971 Dec;51(4):797–798. doi: 10.1677/joe.0.0510797. [DOI] [PubMed] [Google Scholar]
- Collins D. J., Hobbs J. J., Emmens C. W. Antiestrogenic and antifertility compounds. 4. 1,1,2-Triarylalkan-1-ols and 1,1,2-Triarylalk-1-enes containing basic ether groups. J Med Chem. 1971 Oct;14(10):952–957. doi: 10.1021/jm00292a016. [DOI] [PubMed] [Google Scholar]
- Dorfman R. I., Kincl F. A. Uterotrophic activity of various phenolic steroids. Acta Endocrinol (Copenh) 1966 Aug;52(4):619–626. doi: 10.1530/acta.0.0520619. [DOI] [PubMed] [Google Scholar]
- EMMENS C. W. The intravaginal assay of naturally occurring oestrogens. J Endocrinol. 1950 Jan;6(3):302–307. doi: 10.1677/joe.0.0060302. [DOI] [PubMed] [Google Scholar]
- Eisenfeld A. Oral contraceptives: ethinyl estradiol binds with higher affinity than mestranol to macromolecules from the sites of anti-fertility action. Endocrinology. 1974 Mar;94(3):803–807. doi: 10.1210/endo-94-3-803. [DOI] [PubMed] [Google Scholar]
- Ferguson E. R., Katzenellenbogen B. S. A comparative study of antiestrogen action: temporal patterns of antagonism of estrogen stimulated uterine growth and effects on estrogen receptor levels. Endocrinology. 1977 May;100(5):1242–1251. doi: 10.1210/endo-100-5-1242. [DOI] [PubMed] [Google Scholar]
- Fromson J. M., Pearson S., Bramah S. The metabolism of tamoxifen (I.C.I. 46,474). I. In laboratory animals. Xenobiotica. 1973 Nov;3(11):693–709. doi: 10.3109/00498257309151594. [DOI] [PubMed] [Google Scholar]
- HOLTKAMP D. E., GRESLIN J. G., ROOT C. A., LERNER L. J. Gonadotrophin inhibiting and anti-fecundity effects of chloramiphene. Proc Soc Exp Biol Med. 1960 Oct;105:197–201. doi: 10.3181/00379727-105-26054. [DOI] [PubMed] [Google Scholar]
- Hahn D. W., McGuire J. L., Greenslade F. C., Turner G. D., DaVanzo J. P. Molecular parameters involved in the estrogenicity of mestranol and ethynylestradiol. Proc Soc Exp Biol Med. 1971 Sep;137(4):1180–1185. doi: 10.3181/00379727-137-35751. [DOI] [PubMed] [Google Scholar]
- Harper M. J., Walpole A. L. A new derivative of triphenylethylene: effect on implantation and mode of action in rats. J Reprod Fertil. 1967 Feb;13(1):101–119. doi: 10.1530/jrf.0.0130101. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Koseki Y., McGuire W. L. Estrogen control of progesterone receptor in human breast cancer: role of estradiol and antiestrogen. Endocrinology. 1978 Nov;103(5):1742–1751. doi: 10.1210/endo-103-5-1742. [DOI] [PubMed] [Google Scholar]
- Jordan V. C., Allen K. E. Evaluation of the antitumour activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma model. Eur J Cancer. 1980 Feb;16(2):239–251. doi: 10.1016/0014-2964(80)90156-5. [DOI] [PubMed] [Google Scholar]
- Jordan V. C. Antiestrogenic and antitumor properties of tamoxifen in laboratory animals. Cancer Treat Rep. 1976 Oct;60(10):1409–1419. [PubMed] [Google Scholar]
- Jordan V. C., Collins M. M., Rowsby L., Prestwich G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977 Nov;75(2):305–316. doi: 10.1677/joe.0.0750305. [DOI] [PubMed] [Google Scholar]
- KINCL F. A., BIRCH A. J., DORFMAN R. I. PITUITARY GONADOTROPIC INHIBITORY ACTIVITY OF VARIOUS STEROIDS IN OVARIECTOMIZED-INTACT FEMALE RATS IN PARABIOSIS. Proc Soc Exp Biol Med. 1964 Nov;117:549–552. doi: 10.3181/00379727-117-29634. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Katzenellenbogen J. A., Ferguson E. R., Krauthammer N. Anti-estrogen interaction with uterine estrogen receptors. Studies with a radiolabeled anti-estrogen (CI-628). J Biol Chem. 1978 Feb 10;253(3):697–707. [PubMed] [Google Scholar]
- Korenman S. G. Comparative binding affinity of estrogens and its relation to estrogenic potency. Steroids. 1969 Feb;13(2):163–177. doi: 10.1016/0039-128x(69)90004-x. [DOI] [PubMed] [Google Scholar]
- LERNER L. J., HOLTHAUS F. J., Jr, THOMPSON C. R. A non-steroidal estrogen antiagonist 1-(p-2-diethylaminoethoxyphenyl)-1-phenyl-2-p-methoxyphenyl ethanol. Endocrinology. 1958 Sep;63(3):295–318. doi: 10.1210/endo-63-3-295. [DOI] [PubMed] [Google Scholar]
- Lednicer D., Lyster S. C., Duncan G. W. Mammalian antifertility agents. IV. Basic 3,4-dihydronaphthalenes and 1,2,3,4-tetrahydro-1-naphthols. J Med Chem. 1967 Jan;10(1):78–84. doi: 10.1021/jm00313a016. [DOI] [PubMed] [Google Scholar]
- Lippman M., Bolan G., Huff K. Interactions of antiestrogens with human breast cancer in long-term tissue culture. Cancer Treat Rep. 1976 Oct;60(10):1421–1429. [PubMed] [Google Scholar]
- Mouridsen H., Palshof T., Patterson J., Battersby L. Tamoxifen in advanced breast cancer. Cancer Treat Rev. 1978 Sep;5(3):131–141. doi: 10.1016/s0305-7372(78)80017-6. [DOI] [PubMed] [Google Scholar]
- Nicholson R. I., Golder M. P. The effect of synthetic anti-oestrogens on the growth and biochemistry of rat mammary tumours. Eur J Cancer. 1975 Aug;11(8):571–579. doi: 10.1016/0014-2964(75)90129-2. [DOI] [PubMed] [Google Scholar]
- Williams K. I. The metabolism of radioactive 17 alpha-ethynylestradiol 3-methyl ether (Mestranol) by women. Steroids. 1969 Apr;13(4):539–544. doi: 10.1016/0039-128x(69)90064-6. [DOI] [PubMed] [Google Scholar]


