Abstract
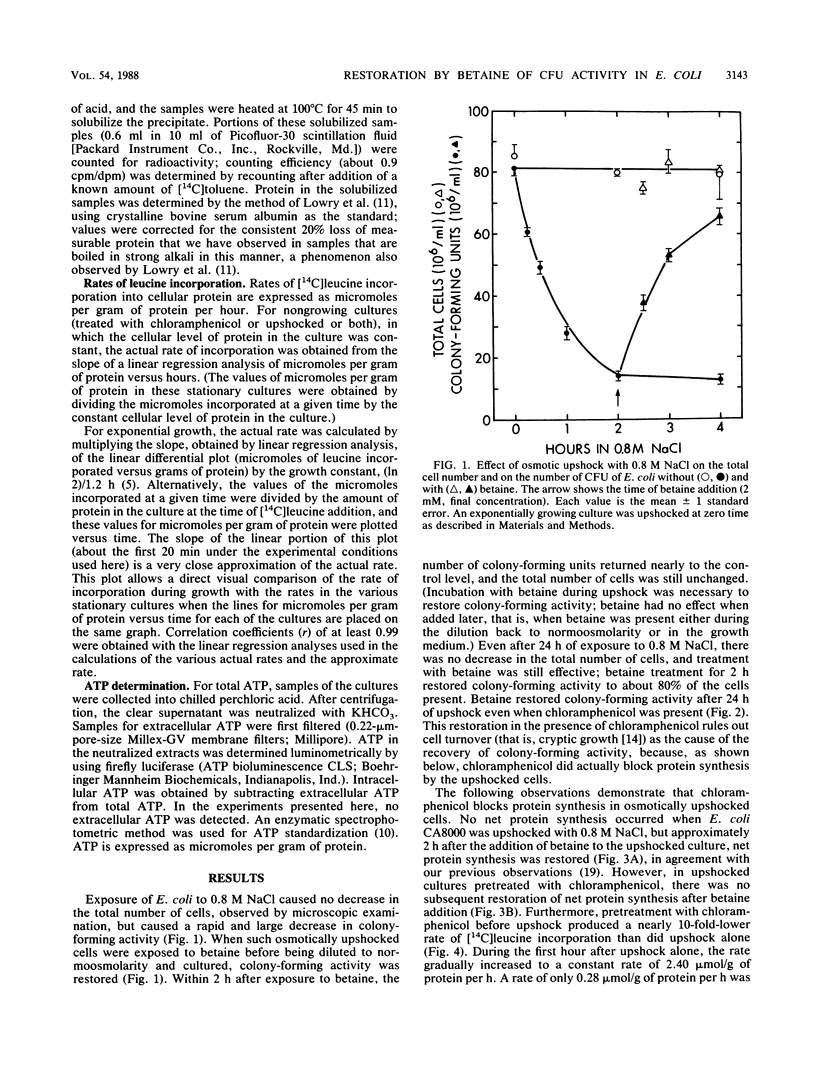
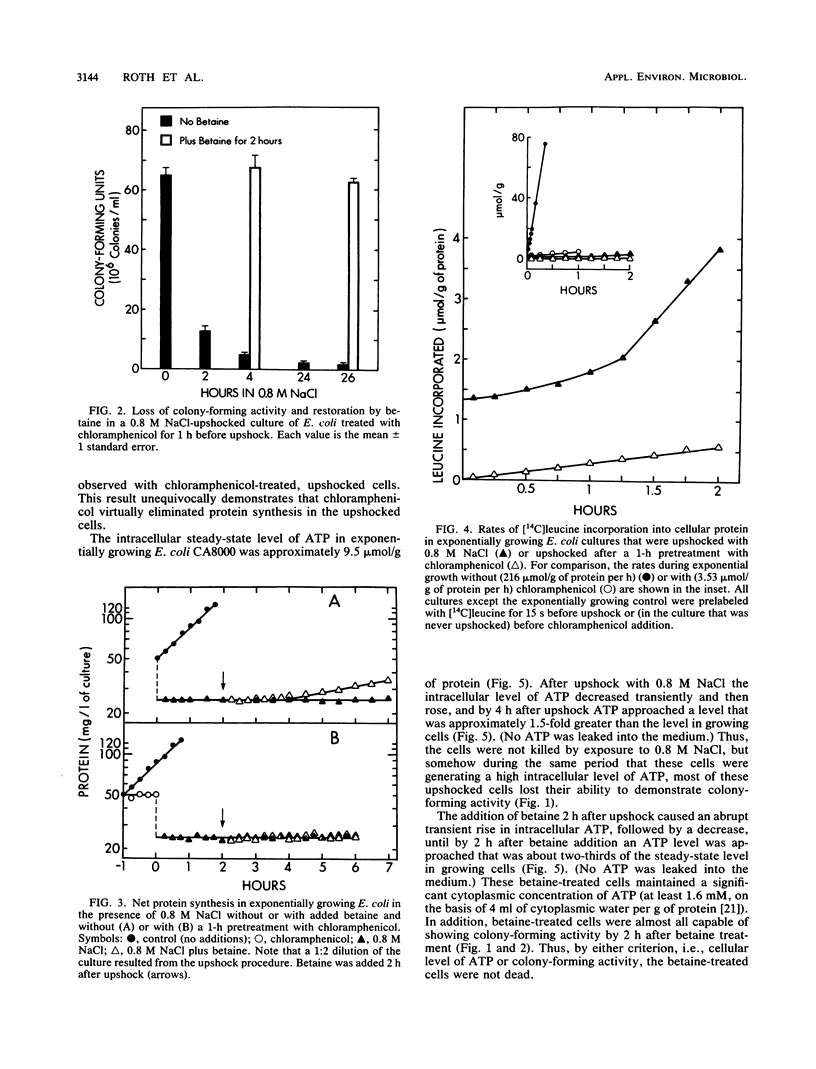
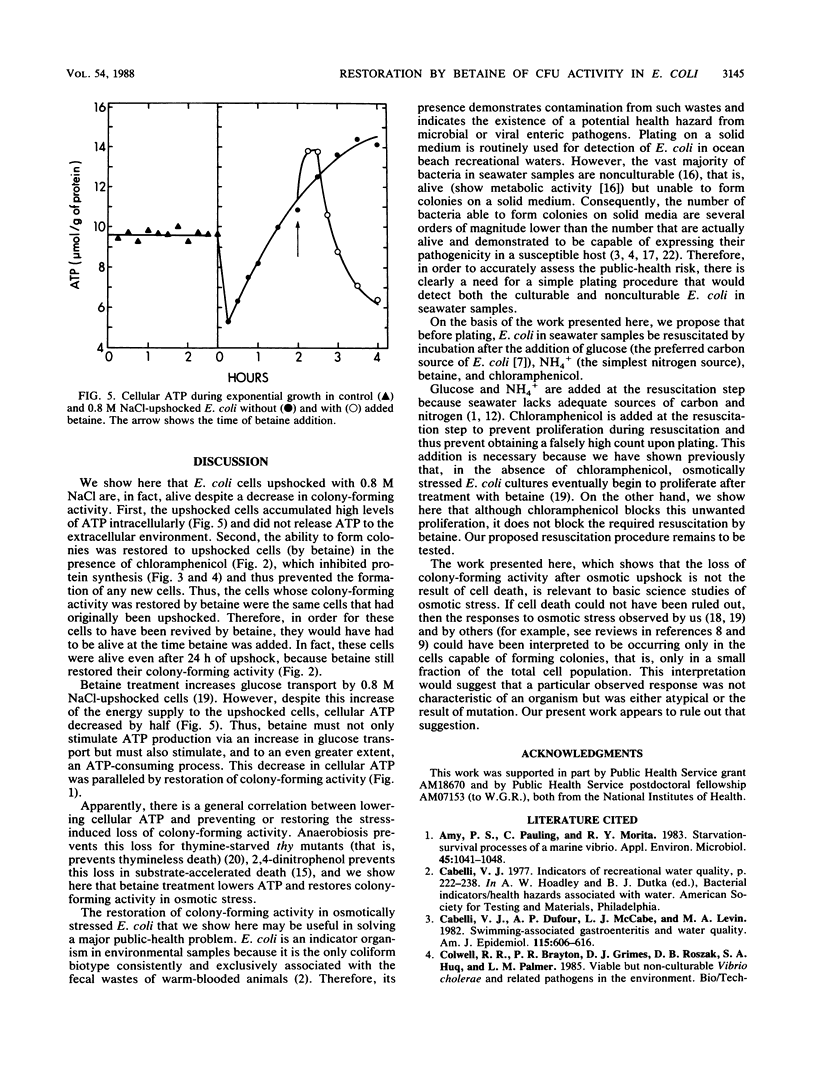
Exposure of Escherichia coli to 0.8 M NaCl caused a rapid and large decrease in colony-forming activity. When such osmotically upshocked cells were exposed to betaine, colony-forming activity was restored. Betaine was able to restore colony-forming activity even when chloramphenicol inhibited protein synthesis. Thus, restoration was not the result of cell turnover. The cells were not killed by exposure to 0.8 M NaCl, because during exposure they accumulated ATP intracellularly. Betaine treatment caused this cellular ATP to decrease to a lower level. This work may provide the foundation for a simple plating procedure to quantitatively detect nonculturable E. coli in ocean beach recreational waters.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy P. S., Pauling C., Morita R. Y. Starvation-survival processes of a marine Vibrio. Appl Environ Microbiol. 1983 Mar;45(3):1041–1048. doi: 10.1128/aem.45.3.1041-1048.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelli V. J., Dufour A. P., McCabe L. J., Levin M. A. Swimming-associated gastroenteritis and water quality. Am J Epidemiol. 1982 Apr;115(4):606–616. doi: 10.1093/oxfordjournals.aje.a113342. [DOI] [PubMed] [Google Scholar]
- Dietzler D. N., Leckie M. P., Bergstein P. E., Sughrue M. J. Evidence for the coordinate control of glycogen synthesis, glucose utilization, and glycolysis in Escherichia coli. I. Quantitative covariance of the rate of glucose utilization and the cellular level of fructose 1,6-diphosphate during exponential growth and nutrient limitation. J Biol Chem. 1975 Sep 25;250(18):7188–7193. [PubMed] [Google Scholar]
- Dietzler D. N., Leckie M. P., Lais C. J. Rates of glycogen synthesis and the cellular levels of ATP and FDP during exponential growth and the nitrogen-limited stationary phase of Escherichia coli W4597 (K). Arch Biochem Biophys. 1973 Jun;156(2):684–693. doi: 10.1016/0003-9861(73)90321-4. [DOI] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Novitsky J. A., Morita R. Y. Survival of a psychrophilic marine Vibrio under long-term nutrient starvation. Appl Environ Microbiol. 1977 Mar;33(3):635–641. doi: 10.1128/aem.33.3.635-641.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. ACCELERATED DEATH OF AEROBACTER AEROGENES STARVED IN THE PRESENCE OF GROWTH-LIMITING SUBSTRATES. J Gen Microbiol. 1964 Mar;34:459–473. doi: 10.1099/00221287-34-3-459. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- Perroud B., Le Rudulier D. Glycine betaine transport in Escherichia coli: osmotic modulation. J Bacteriol. 1985 Jan;161(1):393–401. doi: 10.1128/jb.161.1.393-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Grimes D. J., Colwell R. R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984 Mar;30(3):334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- Roth W. G., Leckie M. P., Dietzler D. N. Osmotic stress drastically inhibits active transport of carbohydrates by Escherichia coli. Biochem Biophys Res Commun. 1985 Jan 16;126(1):434–441. doi: 10.1016/0006-291x(85)90624-2. [DOI] [PubMed] [Google Scholar]
- Roth W. G., Porter S. E., Leckie M. P., Porter B. E., Dietzler D. N. Restoration of cell volume and the reversal of carbohydrate transport and growth inhibition of osmotically upshocked Escherichia coli. Biochem Biophys Res Commun. 1985 Jan 16;126(1):442–449. doi: 10.1016/0006-291x(85)90625-4. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]


