Abstract
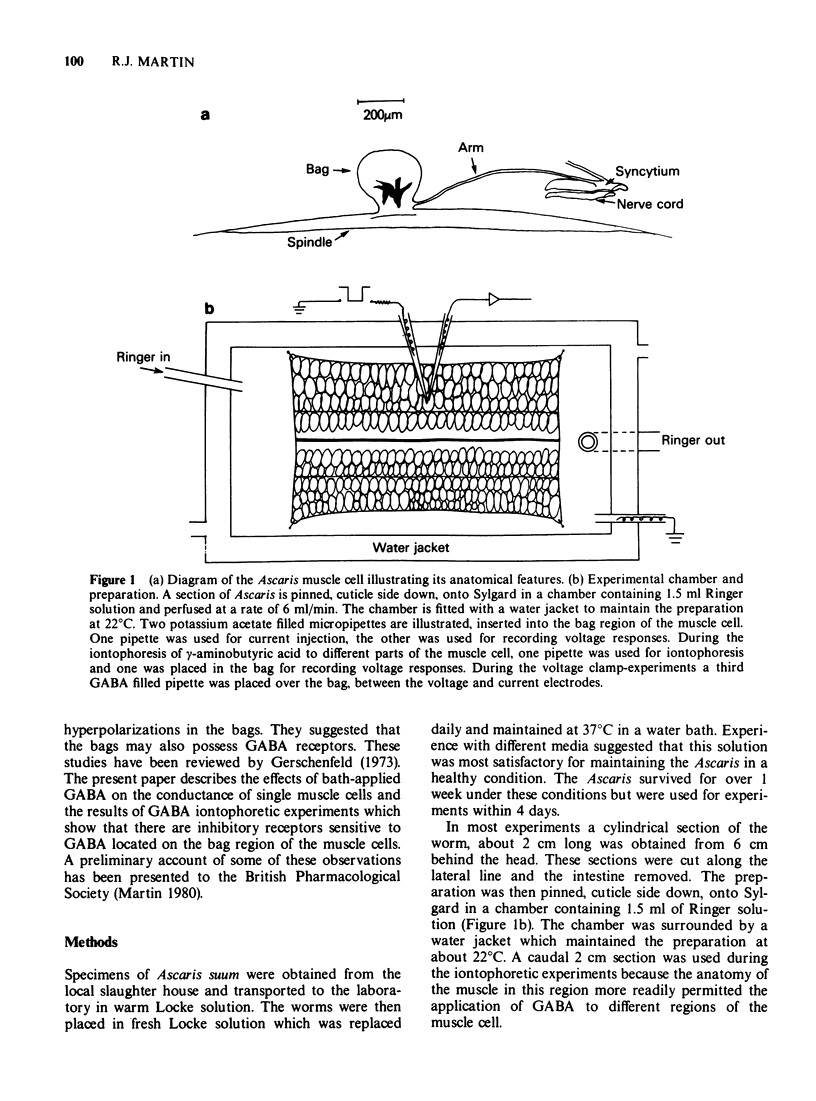
1 Twin intracellular recordings were made from the bag region of Ascaris muscle in order to make conductance measurements. The preparation was bathed in a cool (22°C) Ringer solution to abolish the large spontaneous depolarizing potentials and to improve stability for recording.
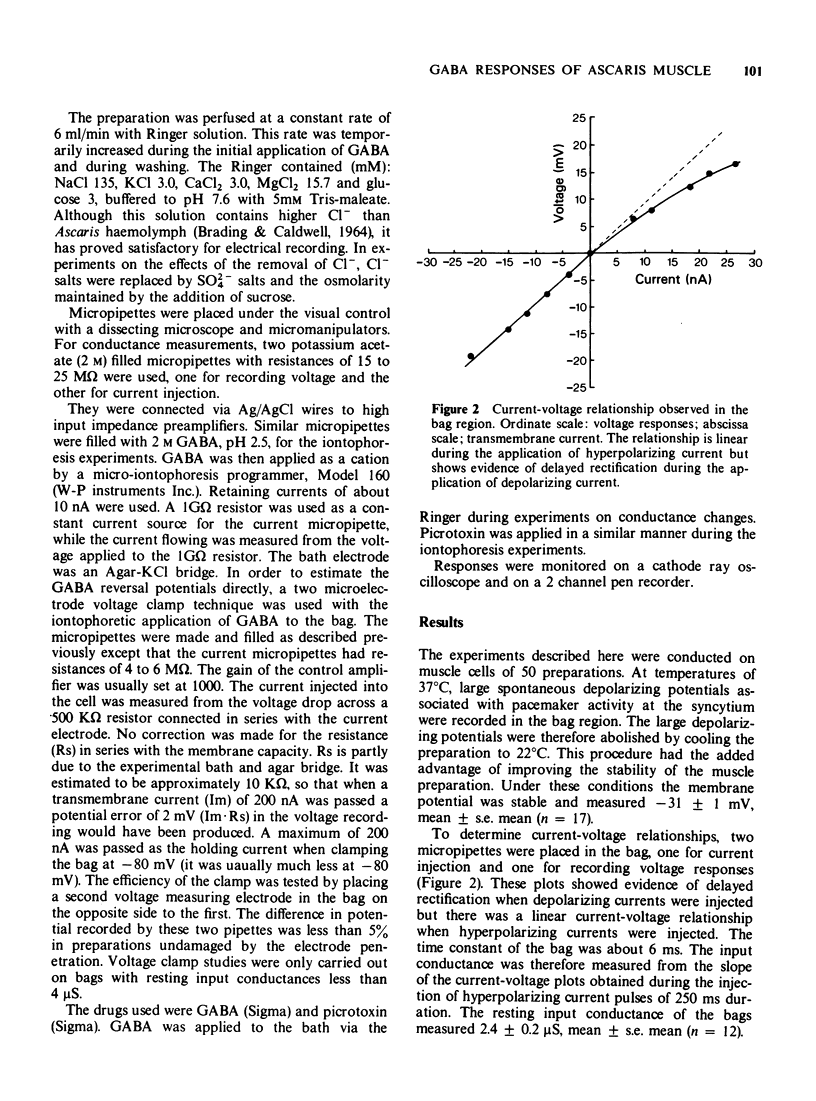
2 The resting membrane potential was -31 ± 1 mV, mean ± s.e. mean (n = 17). The current—voltage plots were linear in the hyperpolarizing direction but showed evidence of delayed rectification during the application of depolarizing currents. The input conductance of the bag was measured from the slope of these plots during the application of hyperpolarizing current. The resting conductance of the bags was 2.4 ± 0.2 μS, mean ± s.e. mean (n = 12).
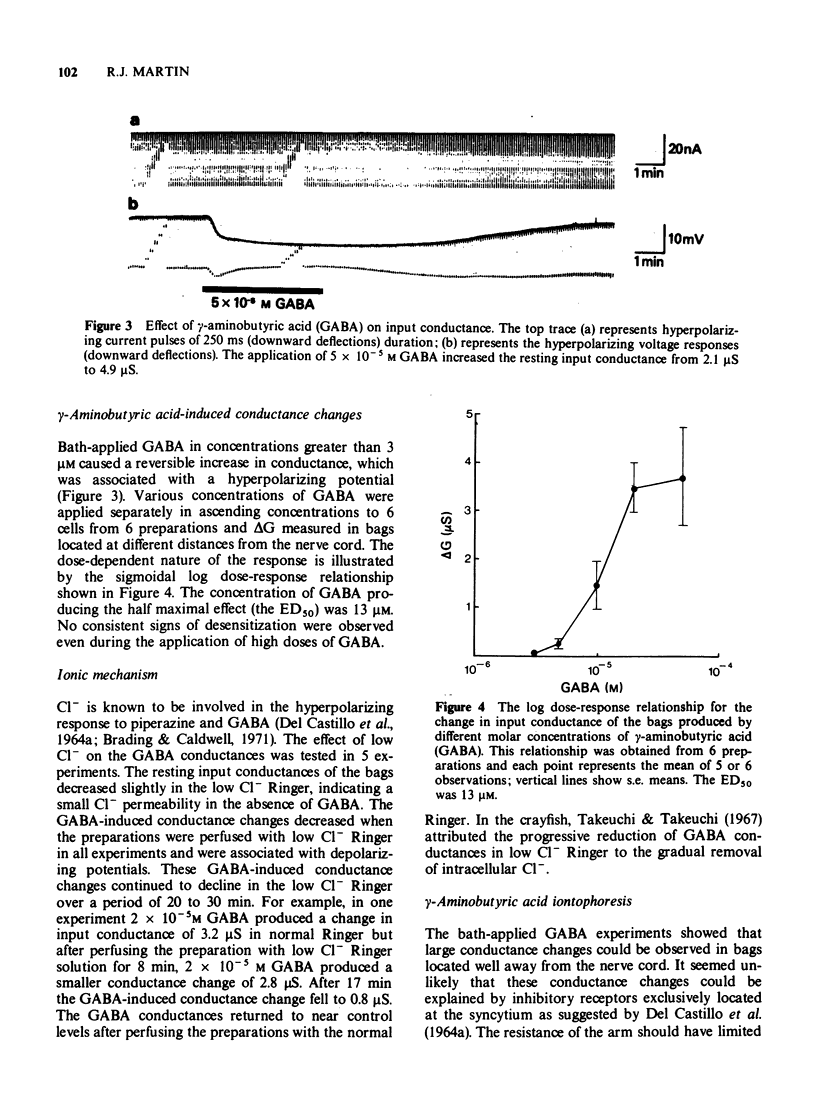
3 When the preparation was perfused with γ-aminobutyric acid (GABA) in concentrations greater than 3 μM, a dose-dependent increase in conductance associated with a hyperpolarizing potential was recorded. The log dose-response relationship obtained from 6 preparations was sigmoidal and had an ED50 of 13 μM.
4 When Cl- in the Ringer was replaced by SO42-, the GABA-induced conductance changes decreased and were associated with depolarizing potentials.
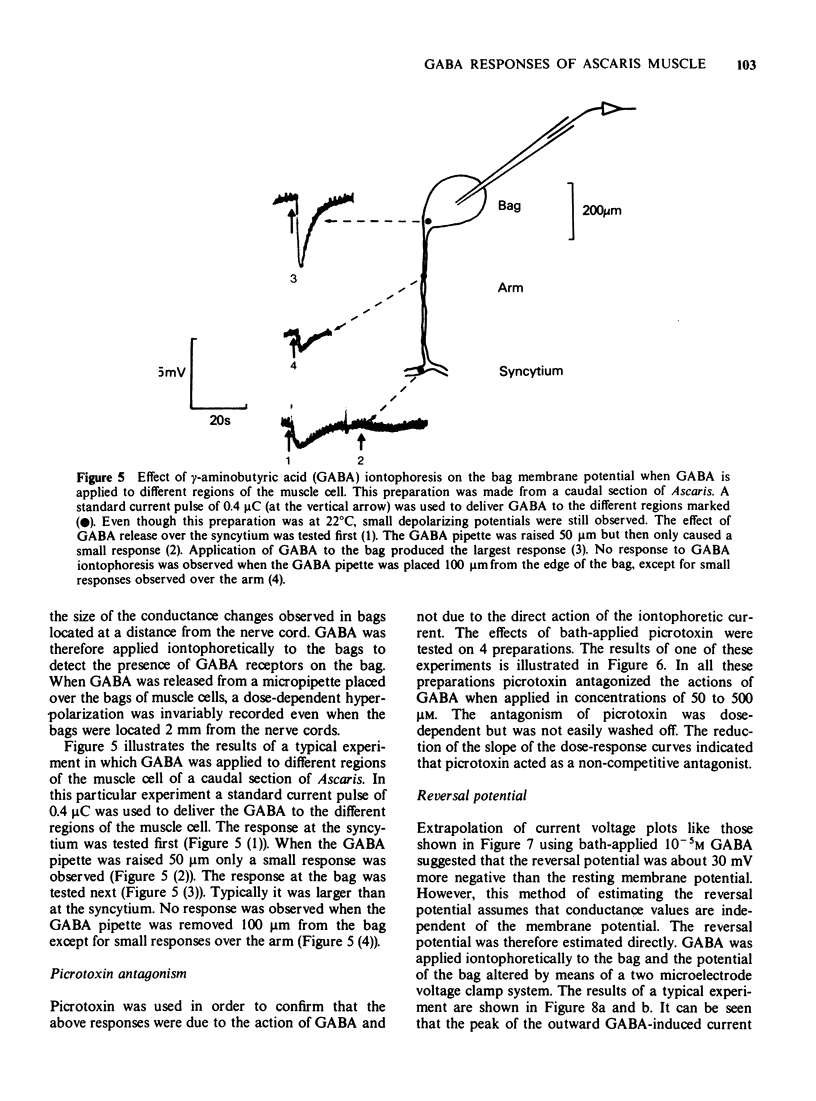
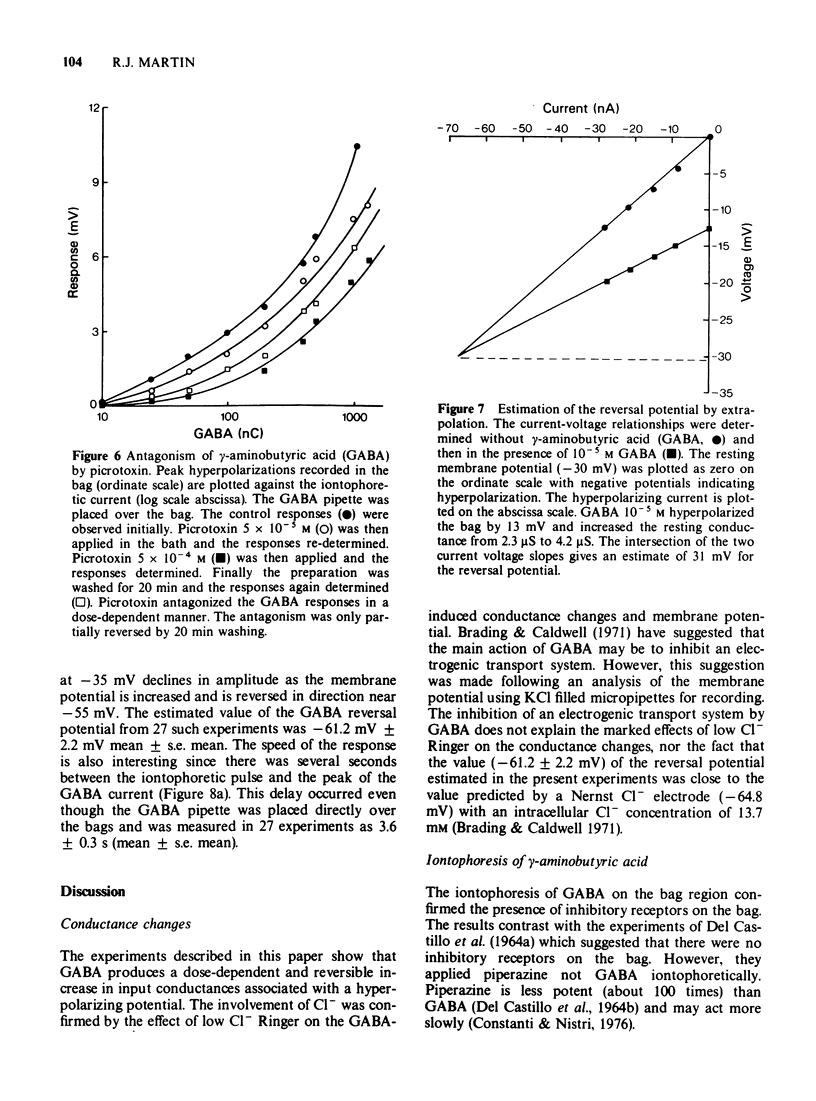
5 Voltage responses were recorded in the bag region during the iontophoretic application of GABA to different regions of the muscle cell. The largest responses were recorded when GABA was applied to the bag region. Smaller responses were recorded when GABA was applied to the arms and syncytial regions. The responses of the bags were dose-dependent and were antagonized by bath-applied picrotoxin.
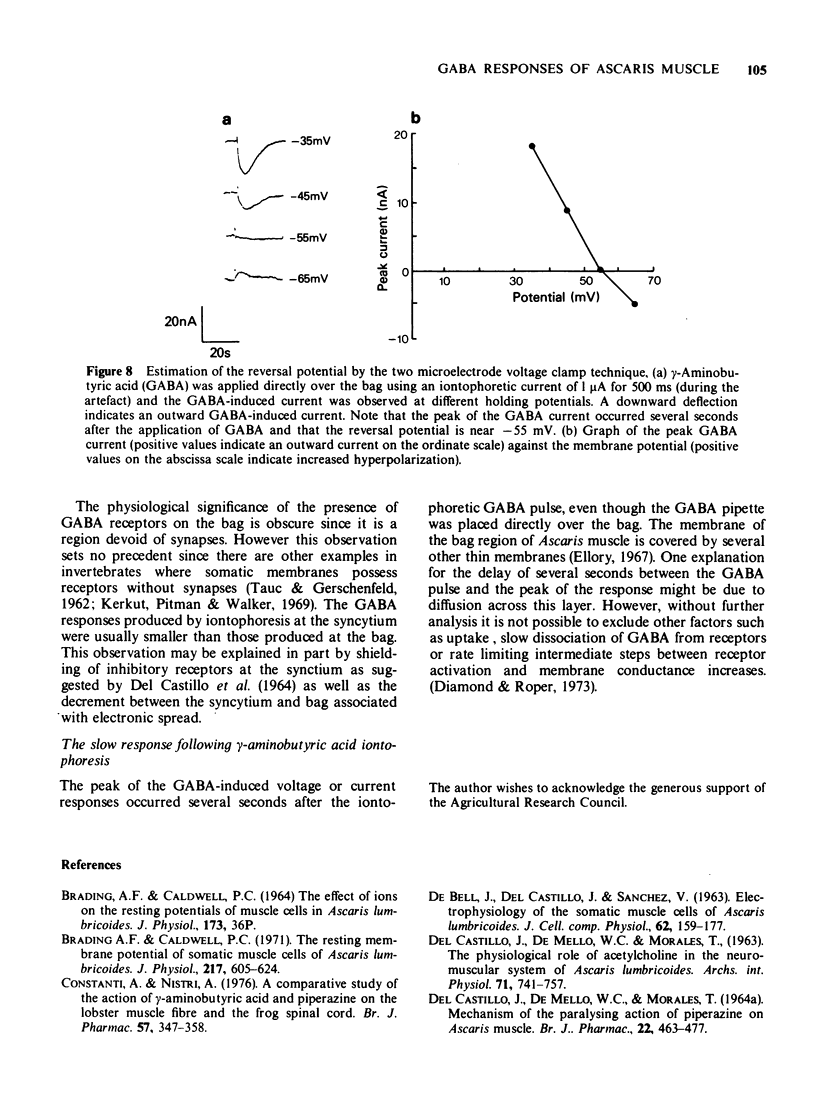
6 An extrapolation method using 10-5 M GABA suggested that the GABA reversal potential was about 30 mV more negative than the resting membrane potential. This was confirmed by means of a two microelectrode voltage clamp technique. The reversal potential was thus estimated as -61.2 ± 2.2 mV mean ± s.e. mean (n = 27).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brading A. F., Caldwell P. C. The resting membrane potential of the somatic muscle cells of Ascaris lumbricoides. J Physiol. 1971 Sep;217(3):605–624. doi: 10.1113/jphysiol.1971.sp009588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Nistri A. A comparative study of the action of gamma-aminobutyric acid and piperazine on the lobster muscle fibre and the frog spinal cord. Br J Pharmacol. 1976 Jul;57(3):347–358. doi: 10.1111/j.1476-5381.1976.tb07673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEBELL J. T., DELCASTILLO J., SANCHEZ V. ELECTROPHYSIOLOGY OF THE SOMATIC MUSCLE CELLS OF ASCARIS LUMBRICOIDES. J Cell Physiol. 1963 Oct;62:159–177. doi: 10.1159/000007808. [DOI] [PubMed] [Google Scholar]
- DELCASTILLO J., DEMELLO W. C., MORALES T. THE PHYSIOLOGICAL ROLE OF ACETYLCHOLINE IN THE NEUROMUSCULAR SYSTEM OF ASCARIS LUMBRICOIDES. Arch Int Physiol Biochim. 1963 Nov;71:741–757. [PubMed] [Google Scholar]
- Diamond J., Roper S. Analysis of Mauthner cell responses to iontophoretically delivered pulses of GABA, glycine and L-glutamate. J Physiol. 1973 Jul;232(1):113–128. doi: 10.1113/jphysiol.1973.sp010259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Gysels H. Immunoelectrophoresis of avian lens proteins. Experientia. 1964 Mar 15;20(3):145–146. doi: 10.1007/BF02150703. [DOI] [PubMed] [Google Scholar]
- JARMAN M. Electrical activity in the muscle cells of Ascaris lumbricoides. Nature. 1959 Oct 17;184(Suppl 16):1244–1244. doi: 10.1038/1841244a0. [DOI] [PubMed] [Google Scholar]
- Kerkut G. A., Pitman R. M., Walker R. J. Iontophoretic application of acetylcholine and GABA onto insect central neurones. Comp Biochem Physiol. 1969 Nov 15;31(4):611–633. doi: 10.1016/0010-406x(69)90063-2. [DOI] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. A cholinergic mechanism of inhibitory synaptic transmission in a molluscan nervous system. J Neurophysiol. 1962 Mar;25:236–262. doi: 10.1152/jn.1962.25.2.236. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. Anion permeability of the inhibitory post-synaptic membrane of the crayfish neuromuscular junction. J Physiol. 1967 Aug;191(3):575–590. doi: 10.1113/jphysiol.1967.sp008269. [DOI] [PMC free article] [PubMed] [Google Scholar]


