Abstract
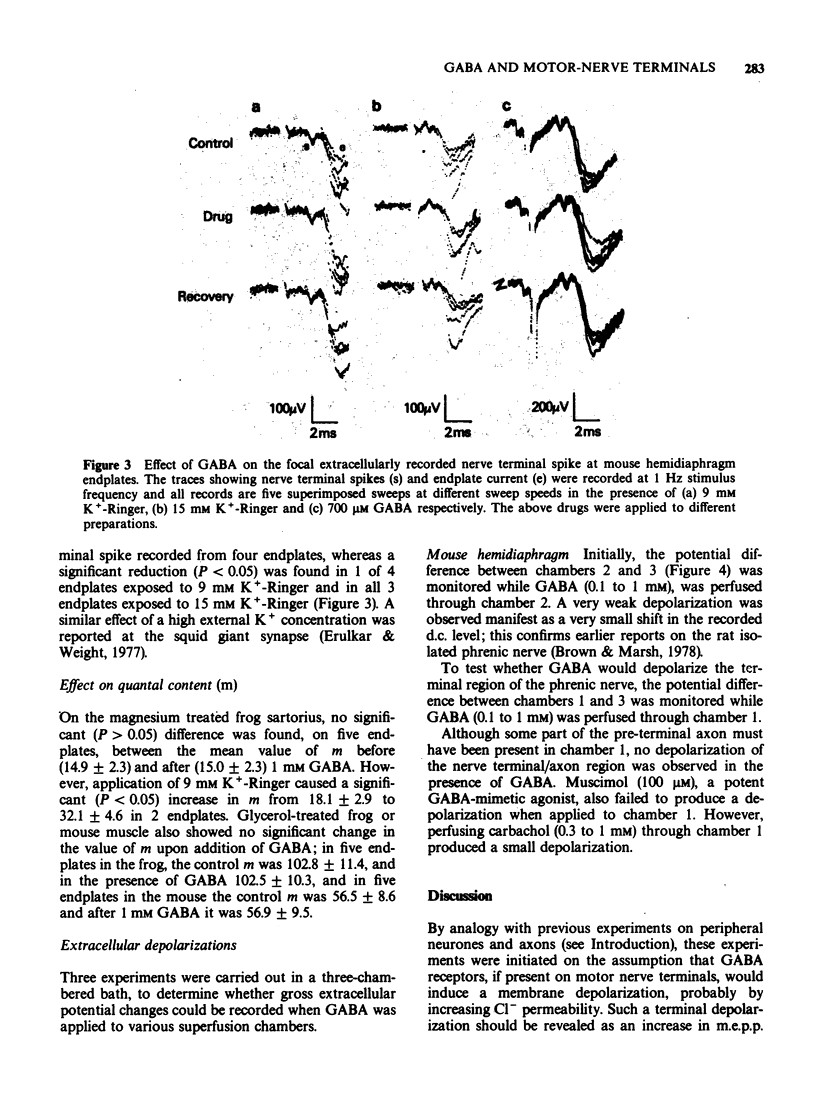
1 gamma-Aminobutyric acid (GABA, 0.1 to 1 mM) had no significant effect on the amplitude, rise time, half decay time or frequency of miniature endplate potentials (m.e.p.ps) at the frog or mouse neuromuscular junctions in vitro. 2 Addition of GABA (1 mM) to preparations previously treated with 11 mM K+-Ringer did not cause any further increase in m.e.pp. frequency. GABA also failed to increase the m.e.p.p. frequency in a low Cl--Ringer. 3 GABA (0.1 to 1 mM) did not reduce the high m.e.p.p. frequency induced by veratrine (20 to 40 mg/l). 4 GABA (0.5 to 1 mM) did not affect the amplitude of the extracellularly-recorded nerve terminal spike, whereas 15 mM [K+] reduced the spike. 5 The quantal content (m) of the evoked endplate potential was not significantly altered by GABA; 9 mM [K+] significantly increased m. 6 When external d.c. potential differences were recorded in a three-chambered bath, GABA (0.1 to 1 mM) produced a very small depolarization if applied to the phrenic nerve trunk, but not if applied to the pre-terminal axon/motor nerve terminal region. Carbachol (0.3 to 1 mM) evoked a small depolarization when applied to the nerve terminal chamber. 7 These results fail to provide evidence for the existence of GABA receptors on motor nerve terminals.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975 Aug;250(1):85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A. Depolarizing actions of gamma-aminobutyric acid and related compounds on rat superior cervical ganglia in vitro. Br J Pharmacol. 1974 Feb;50(2):205–218. doi: 10.1111/j.1476-5381.1974.tb08563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A., White R. D., Yamini G. [3H]gamma-Aminobutyric acid uptake into neuroglial cells of rat superior cervical sympathetic ganglia. J Physiol. 1979 Aug;293:51–74. doi: 10.1113/jphysiol.1979.sp012878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branisteanu D. D., Miyamoto M. D., Volle R. L. Effects of physiologic alterations on binomial transmitter release at magnesium-depressed neuromuscular junctions. J Physiol. 1976 Jan;254(1):19–37. doi: 10.1113/jphysiol.1976.sp011218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Higgins A. J. Presynaptic effects of gamma-aminobutyric acid in isolated rat superior cervical ganglia [proceedings]. Br J Pharmacol. 1979 May;66(1):108P–109P. [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Marsh S. Axonal GABA-receptors in mammalian peripheral nerve trunks. Brain Res. 1978 Nov 3;156(1):187–191. doi: 10.1016/0006-8993(78)90098-7. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., PHILLIS J. W., WATKINS J. C. The depression of spinal neurones by gamma-amino-n-butyric acid and beta-alanine. J Physiol. 1959 Apr 23;146(1):185–203. doi: 10.1113/jphysiol.1959.sp006188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat W. C. GABA-depolarization of a sensory ganglion: antagonism by picrotoxin and bicuculline. Brain Res. 1972 Mar 24;38(2):429–432. doi: 10.1016/0006-8993(72)90726-3. [DOI] [PubMed] [Google Scholar]
- Deschenes M., Feltz P., Lamour Y. A model for an estimate in vivo of the ionic basis of presynaptic inhibition: an intracellular analysis of the GABA-induced depolarization in rat dorsal root ganglia. Brain Res. 1976 Dec 24;118(3):486–493. doi: 10.1016/0006-8993(76)90318-8. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. S., Howell J. N., Vaughan P. C. The maintenance of resting potentials in glycerol-treated muscle fibres. J Physiol. 1971 May;215(1):95–102. doi: 10.1113/jphysiol.1971.sp009459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D., Weight F. F. Extracellular potassium and trasmitter release at the giant synapse of squid. J Physiol. 1977 Apr;266(2):209–218. doi: 10.1113/jphysiol.1977.sp011764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLOREY E., MCLENNAN H. The release of an inhibitory substance from mammalian brain, and its effect on peripheral synaptic transmission. J Physiol. 1955 Aug 29;129(2):384–392. doi: 10.1113/jphysiol.1955.sp005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz P., Rasminsky M. A model for the mode of action of GABA on primary afferent terminals: depolarizing effects of GABA applied iontophoretically to neurones of mammalian dorsal root ganglia. Neuropharmacology. 1974 Jun;13(6):553–563. doi: 10.1016/0028-3908(74)90145-2. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Higashi H., Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J Physiol. 1978 Feb;275:263–282. doi: 10.1113/jphysiol.1978.sp012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L. On the presynaptic acetylcholine receptors in sympathetic ganglia of the frog. J Physiol. 1971 Jul;216(1):237–246. doi: 10.1113/jphysiol.1971.sp009521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFMANN W. W., FEIGEN G. A., GENTHER G. H. Effects of veratrine, nitrate ion and gamma-aminobutyric acid on mammalian miniature end-plate potentials. Nature. 1962 Jan 13;193:175–176. doi: 10.1038/193175a0. [DOI] [PubMed] [Google Scholar]
- Howell J. N. A lesion of the transverse tubules of skeletal muscle. J Physiol. 1969 May;201(3):515–533. doi: 10.1113/jphysiol.1969.sp008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Schmidt R. F., Yokota T. The effect of acetylcholine upon mammalian motor nerve terminals. J Physiol. 1965 Dec;181(4):810–829. doi: 10.1113/jphysiol.1965.sp007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Willis W. D. The effects of depolarization of motor nerve terminals upon the release of transmitter by nerve impulses. J Physiol. 1968 Feb;194(2):381–405. doi: 10.1113/jphysiol.1968.sp008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato E., Kuba K. Inhibition of transmitter release in bullfrog sympathetic ganglia induced by gamma-aminobutyric acid. J Physiol. 1980 Jan;298:271–283. doi: 10.1113/jphysiol.1980.sp013080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato E., Kuba K., Koketsu K. Presynaptic inhibition by gamma-aminobutyric acid in bullfrog sympathetic ganglion cells. Brain Res. 1978 Sep 22;153(2):398–402. doi: 10.1016/0006-8993(78)90422-5. [DOI] [PubMed] [Google Scholar]
- Koketsu K., Shoji T., Yamamoto K. Effects of GABA on presynaptic nerve terminals in bullfrog (Rana catesbiana) sympathetic ganglia. Experientia. 1974 Apr 15;30(4):382–383. doi: 10.1007/BF01921677. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M. D. Binomial analysis of quantal transmitter release at glycerol treated frog neuromuscular junctions. J Physiol. 1975 Aug;250(1):121–142. doi: 10.1113/jphysiol.1975.sp011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M. D. Estimates on magnitude of nonlinear summation of evoked potentials at motor end plate. J Neurophysiol. 1978 May;41(3):589–599. doi: 10.1152/jn.1978.41.3.589. [DOI] [PubMed] [Google Scholar]
- Miyamoto M. D., Volle R. L. Enhancement by carbachol of transmitter release from motor nerve terminals. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1489–1492. doi: 10.1073/pnas.71.4.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S., Minota S., Karczmar A. G. Primary afferent neurones: the ionic mechanism of GABA-mediated depolarization. Neuropharmacology. 1974 Mar;13(3):215–219. doi: 10.1016/0028-3908(74)90110-5. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Changes in potassium concentration around motor nerve terminals, produced by current flow, and their effects on neuromuscular transmission. J Physiol. 1961 Jan;155:46–58. doi: 10.1113/jphysiol.1961.sp006612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat W. C. The actions of gamma-aminobutyric acid and related amino acids on mammalian autonomic ganglia. J Pharmacol Exp Ther. 1970 Apr;172(2):384–396. [PubMed] [Google Scholar]


